Induction of Chirality in Atomically Thin ZnSe and CdSe Nanoplatelets: Strengthening of Circular Dichroism via Different Coordination of Cysteine-Based Ligands on an Ultimate Thin Semiconductor Core
Abstract
1. Introduction
2. Materials and Methods
3. Results
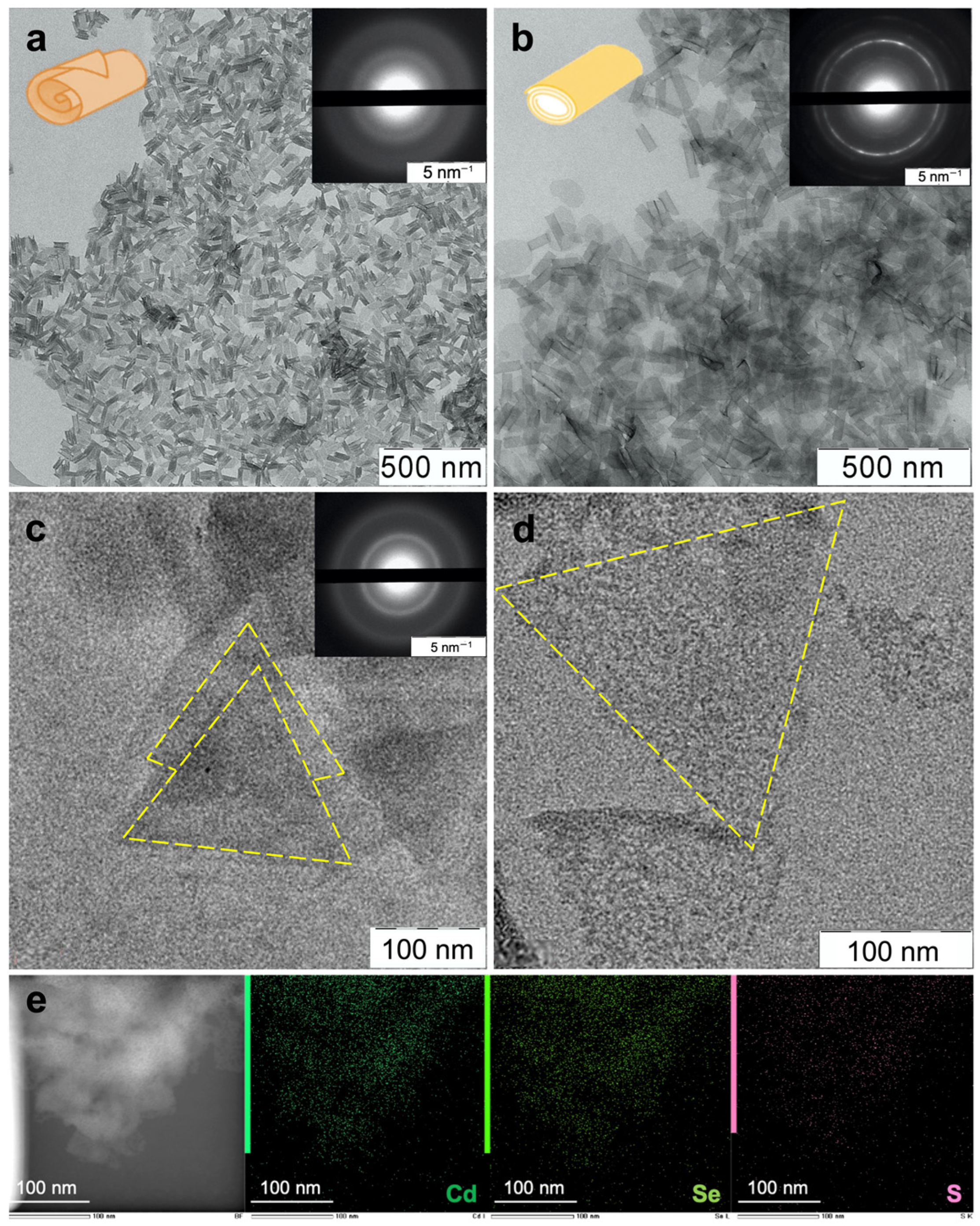
3.1. Atomically Thin Nanoplatelet Growth and Ligand Exchange
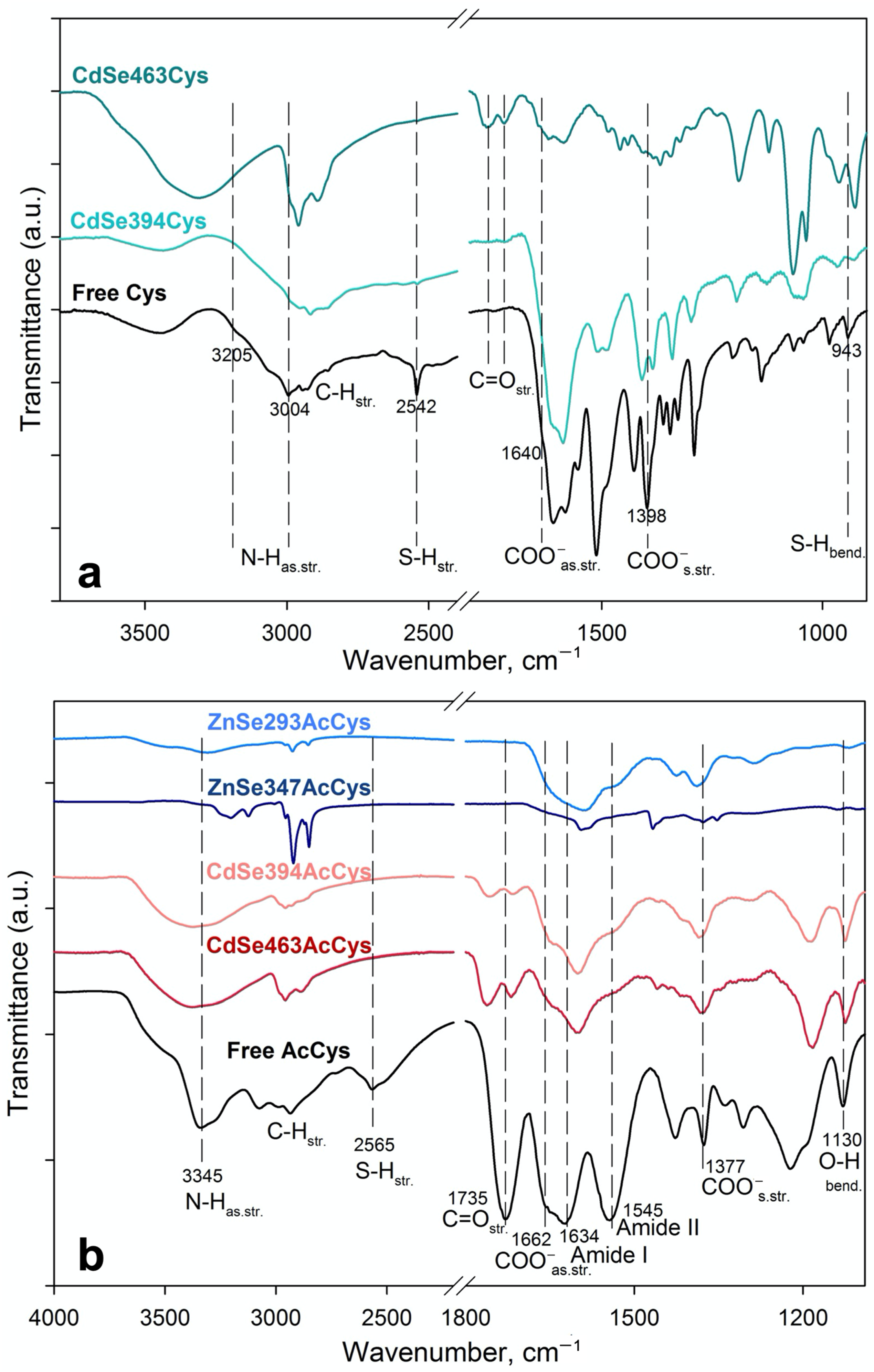
3.2. FTIR Analysis of Ligand Coordination
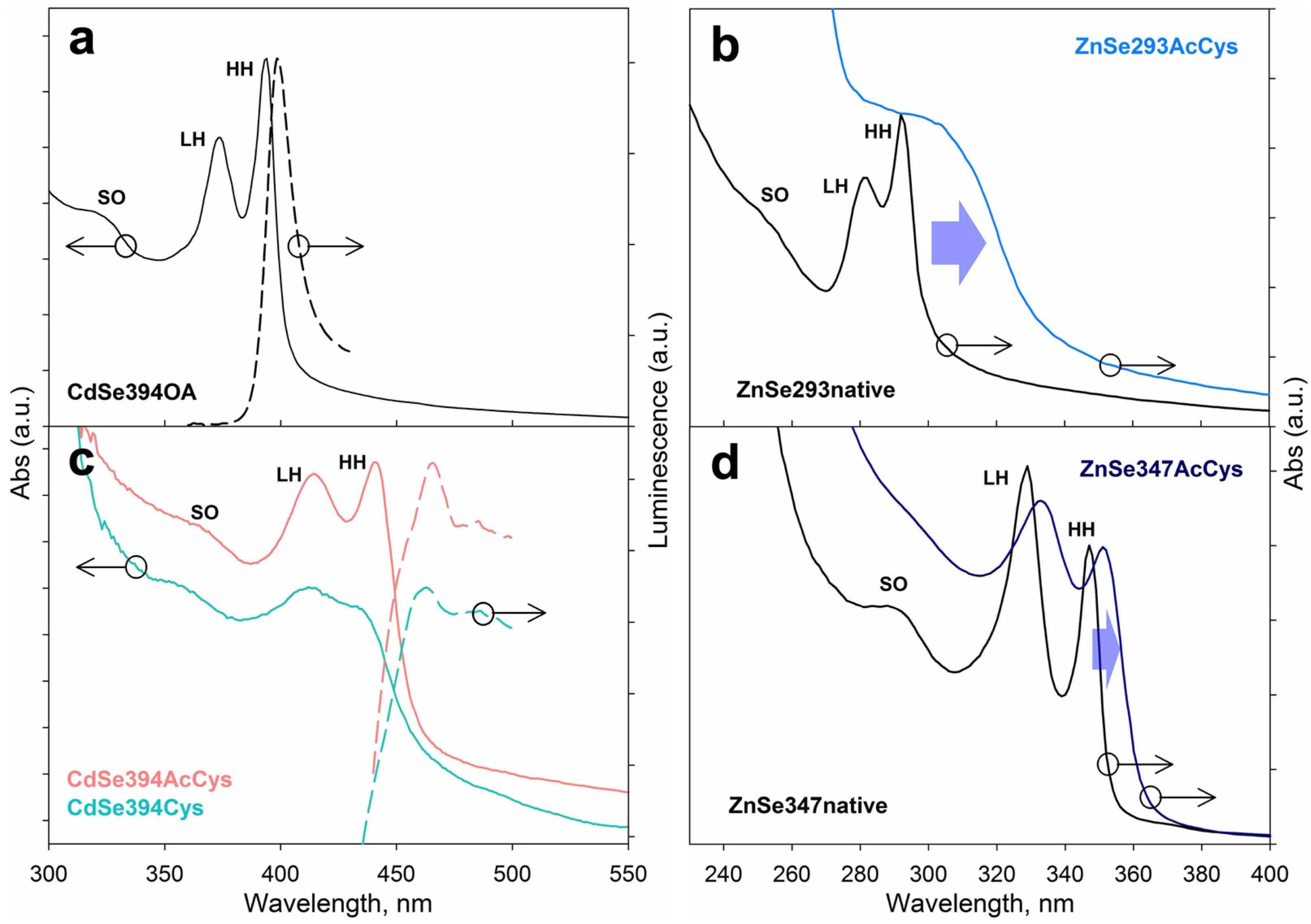
3.3. Analysis of Optical and Chiroptical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, W.; Xu, L.; de Moura, A.F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral Inorganic Nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef] [PubMed]
- Banerjee-Ghosh, K.; Ben Dor, O.; Tassinari, F.; Capua, E.; Yochelis, S.; Capua, A.; Yang, S.H.; Parkin, S.S.P.; Sarkar, S.; Kronik, L.; et al. Separation of enantiomers by their enantiospecific interaction with achiral magnetic substrates. Science 2018, 360, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Warning, L.A.; Miandashti, A.R.; McCarthy, L.A.; Zhang, Q.; Landes, C.F.; Link, S. Nanophotonic Approaches for Chirality Sensing. ACS Nano 2021, 15, 15538–15566. [Google Scholar] [CrossRef] [PubMed]
- Naaman, R.; Paltiel, Y.; Waldeck, D.H. Chiral Molecules and the Electron Spin. Nat. Rev. Chem. 2019, 3, 250–260. [Google Scholar] [CrossRef]
- Chen, C.; Gao, L.; Gao, W.; Ge, C.; Du, X.; Li, Z.; Yang, Y.; Niu, G.; Tang, J. Circularly Polarized Light Detection Using Chiral Hybrid Perovskite. Nat. Commun. 2019, 10, 1927. [Google Scholar] [CrossRef]
- Kim, Y.H.; Zhai, Y.; Lu, H.; Pan, X.; Xiao, C.; Gaulding, E.A.; Harvey, S.P.; Berry, J.J.; Vardeny, Z.V.; Luther, J.M.; et al. Chiral-Induced Spin Selectivity Enables a Room-Temperature Spin Light-Emitting Diode. Science 2021, 371, 1129–1133. [Google Scholar] [CrossRef]
- Ben-Moshe, A.; Govorov, A.O.; Markovich, G. Enantioselective Synthesis of Intrinsically Chiral Mercury Sulfide Nanocrystals. Angew. Chem. Int. Ed. 2013, 52, 1275–1279. [Google Scholar] [CrossRef]
- Moloney, M.P.; Gun’ko, Y.K.; Kelly, J.M. Chiral Highly Luminescent CdS Quantum Dots. Chem. Commun. 2007, 38, 3900–3902. [Google Scholar] [CrossRef]
- Elliott, S.D.; Moloney, M.P.; Gun’ko, Y.K. Chiral Shells and Achiral Cores in CdS Quantum Dots. Nano Lett. 2008, 8, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Kobayashi, Y.; Kawai, T. Optical Activity and Chiral Memory of Thiol-Capped CdTe Nanocrystals. J. Am. Chem. Soc. 2009, 131, 10342–10343. [Google Scholar] [CrossRef]
- Gallagher, S.A.; Moloney, M.P.; Wojdyla, M.; Quinn, S.J.; Kelly, J.M.; Gun’ko, Y.K. Synthesis and Spectroscopic Studies of Chiral CdSe Quantum Dots. J. Mater. Chem. 2010, 20, 8350–8355. [Google Scholar] [CrossRef]
- Tohgha, U.; Varga, K.; Balaz, M. Achiral CdSe Quantum Dots Exhibit Optical Activity in the Visible Region Upon Post-Synthetic Ligand Exchange with D- or L-Cysteine. Chem. Commun. 2013, 49, 1844–1846. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Ferry, V.E. Circular Dichroism of CdSe Nanocrystals Bound by Chiral Carboxylic Acids. ACS Nano 2017, 11, 12240–12246. [Google Scholar] [CrossRef] [PubMed]
- Ben Moshe, A.; Szwarcman, D.; Markovich, G. Size Dependence of Chiroptical Activity in Colloidal Quantum Dots. ACS Nano 2011, 5, 9034–9043. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, X.; Deng, K.; Han, B.; Zhao, L.; Wu, M.; Shi, L.; Lv, J.; Tang, Z. Excitonic Circular Dichroism of Chiral Quantum Rods. J. Am. Chem. Soc. 2017, 139, 8734–8739. [Google Scholar] [CrossRef]
- Yang, G.; Kazes, M.; Oron, D. Chiral 2D Colloidal Semiconductor Quantum Wells. Adv. Funct. Mater. 2018, 28, 1802012. [Google Scholar] [CrossRef]
- Kurtina, D.A.; Garshev, A.V.; Vasil’eva, I.S.; Shubin, V.V.; Gaskov, A.M.; Vasiliev, R.B. Atomically-Thin Population of Colloidal CdSe Nanoplatelets: Growth of Rolled-up Nanosheets and Strong Circular Dichroism Induced by Ligand Exchange. Chem. Mater. 2019, 31, 9652. [Google Scholar] [CrossRef]
- Kuznetsova, V.A.; Mates-Torres, E.; Prochukhan, N.; Marcastel, M.; Purcell-Milton, F.; O’Brien, J.; Visheratina, A.K.; Martinez-Carmona, M.; Gromova, Y.; Garcia-Melchor, M.; et al. Effect of Chiral Ligand Concentration and Binding Mode on Chiroptical Activity of CdSe/CdS Quantum Dots. ACS Nano 2019, 13, 13560–13572. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Zhao, L.; Huang, P.; Han, B.; Lv, J.; Qiu, X.; Wei, S.H.; Tang, Z. Distinct Excitonic Circular Dichroism between Wurtzite and Zincblende CdSe Nanoplatelets. Nano Lett. 2018, 18, 6665–6671. [Google Scholar] [CrossRef]
- Cunningham, P.D.; Coropceanu, I.; Mulloy, K.; Cho, W.; Talapin, D.V. Quantized Reaction Pathways for Solution Synthesis of Colloidal ZnSe Nanostructures: A Connection between Clusters, Nanowires, and Two-Dimensional Nanoplatelets. ACS Nano 2020, 14, 3847–3857. [Google Scholar] [CrossRef]
- Vasiliev, R.B.; Lazareva, E.P.; Karlova, D.A.; Garshev, A.V.; Yao, Y.; Kuroda, T.; Gaskov, A.M.; Sakoda, K. Spontaneous folding of CdTe nanosheets induced by ligand exchange. Chem. Mater. 2018, 30, 1710–1717. [Google Scholar] [CrossRef]
- Shlenskaya, N.N.; Yao, Y.; Mano, T.; Kuroda, T.; Garshev, A.V.; Kozlovskii, V.F.; Gaskov, A.M.; Vasiliev, R.B.; Sakoda, K. Scroll-like alloyed CdSxSe1-x nanoplatelets: Facile synthesis and detailed analysis of tunable optical properties. Chem. Mater. 2017, 29, 579–586. [Google Scholar] [CrossRef]
- Li, L.S.; Pradhan, N.; Wang, Y.; Peng, X. High quality ZnSe and ZnS nanocrystals formed by activating zinc carboxylate precursors. Nano Lett. 2004, 4, 2261–2264. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Wayman, V.L.; Gibbons, P.C.; Loomis, R.A.; Buhro, W.E. Origin of High Photoluminescence Efficiencies in CdSe Quantum Belts. Nano Lett. 2010, 10, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Noda, Y.; Takegoshi, K. Capping Structure of Ligand–Cysteine on CdSe Magic-Sized Clusters. ACS Omega 2019, 4, 3476–3483. [Google Scholar] [CrossRef]
- Shindo, H.; Brown, T.L. Infrared Spectra of Complexes of L- Cysteine and Related Compounds with Zinc(II), Cadmium(II), Mercury(II), and Lead(II). J. Am. Chem. Soc. 1965, 87, 1904–1909. [Google Scholar] [CrossRef]
- Pawlukojć, A.; Leciejewicz, J.; Ramirez-Cuesta, A.J.; Nowicka-Scheibe, J. L-Cysteine: Neutron Spectroscopy, Raman, IR and Ab Initio Study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 2474–2481. [Google Scholar] [CrossRef]
- Wei, S.; Guo, C.; Wang, L.; Xu, J.; Dong, H. Bacterial synthesis of PbS nanocrystallites in one-step with L-cysteine serving as both sulfur source and capping ligand. Sci. Rep. 2021, 11, 1216. [Google Scholar] [CrossRef]
- Saha, A.; Rani, B.; Kanti, M. Au–Ag core–shell composite nanoparticles as a selective and sensitive plasmonic chemical probe for L-cysteine detection in Lens culinaris (lentils). RSC Adv. 2021, 11, 20380. [Google Scholar] [CrossRef]
- Li, L.; Liao, L.; Ding, L.; Zeng, H. Dithizone-etched CdTe nanoparticles-based fluorescence sensor for the off–on detection of cadmium ion in aqueous media. RSC Adv. 2017, 7, 10361. [Google Scholar] [CrossRef]
- Picquart, M.; Abedinzadeh, Z.; Grajcar, L.; Baron, M.H. Spectroscopic study of N-acetylcysteine and N-acetylcystinerhydrogen peroxide complexation. Chem. Phys. 1998, 228, 279–291. [Google Scholar] [CrossRef]
- Boeckx, B.; Ramaekers, R.; Maes, G. A theoretical and matrix-isolation FT-IR investigation of the conformational landscape of N-acetylcysteine. J. Mol. Spectrosc. 2010, 261, 73–81. [Google Scholar] [CrossRef]
- Du, W.; Liao, L.; Yang, L. Aqueous synthesis of functionalized copper sulfide quantum dots as near-infrared luminescent probes for detection of Hg2+, Ag+ and Au3+. Sci. Rep. 2017, 7, 11451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Fang, Y.; Wanga, H.; He, Z. Synthesis and characterization of high-quality water-soluble CdTe: Zn2+ quantum dots capped by N-acetyl-L-cysteine via hydrothermal method. J. Mater. Chem. 2011, 21, 13365. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Cui, E.; Liu, R. Spectroscopic Investigations on the Effect of N-Acetyl-L-cysteine-Capped CdTe Quantum Dots on Catalase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.P. Medical Nutritional and Biochemical Role of N-Acetyl-L-Cysteine and its Spectrophotometric Determination by Complexion 09 with RU (III) and Characterization by Elemental Analysis, FTIR, ESR, NMR, TGA, DTA Proposed Structure of the Complex. J. Chem. Appl. Biochem. 2017, 4, 122. [Google Scholar]
- Ithurria, S.; Tesier, M.D.; Mahler, B.; Lobo, R.P.S.M.; Dubertret, B.; Efros, A.L. Colloidal nanoplatelets with two-dimensional electronic structure. Nat. Mater. 2011, 10, 936–941. [Google Scholar] [CrossRef]
- Vasiliev, R.B.; Lebedev, A.I.; Lazareva, E.P.; Shlenskaya, N.N.; Zaytsev, V.B.; Vitukhnovsky, A.G.; Yao, Y.; Sakoda, K. High-energy exciton transitions in quasi-two-dimensional cadmium chalcogenide nanoplatelets. Phys. Rev. B 2017, 95, 165414. [Google Scholar] [CrossRef]
- Choi, J.K.; Haynie, B.E.; Tohgha, U.; Pap, L.; Elliott, K.W.; Leonard, B.M.; Dzyuba, S.V.; Varga, K.; Kubelka, J.; Balaz, M. Chirality Inversion of CdSe and CdS Quantum Dots without Changing the Stereochemistry of the Capping Ligand. ACS Nano 2016, 10, 3809–3815. [Google Scholar] [CrossRef]
- Purcell-Milton, F.; Visheratina, A.K.; Kuznetsova, V.A.; Ryan, A.; Orlova, A.O.; Gun’ko, Y.K. Impact of Shell Thickness on Photoluminescence and Optical Activity in Chiral CdSe/CdS Core/Shell Quantum Dots. ACS Nano 2017, 11, 9207–9214. [Google Scholar] [CrossRef]

| Sample | Ligand | 𝜆CD, nm | g-Factor (×10−3) |
|---|---|---|---|
| CdSe394 | AcCys | 403 | 1.30 |
| 428 | −1.85 | ||
| Cys | 402 | −0.37 | |
| 425 | 0.52 | ||
| CdSe463 | AcCys | 453 | 1.13 |
| 483 | −1.2 | ||
| Cys | 443 | −0.13 | |
| 473 | 0.35 | ||
| ZnSe293 | AcCys | 309 | 3.22 |
| ZnSe347 | AcCys | 330 | −0.77 |
| 348 | −0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurtina, D.A.; Grafova, V.P.; Vasil’eva, I.S.; Maksimov, S.V.; Zaytsev, V.B.; Vasiliev, R.B. Induction of Chirality in Atomically Thin ZnSe and CdSe Nanoplatelets: Strengthening of Circular Dichroism via Different Coordination of Cysteine-Based Ligands on an Ultimate Thin Semiconductor Core. Materials 2023, 16, 1073. https://doi.org/10.3390/ma16031073
Kurtina DA, Grafova VP, Vasil’eva IS, Maksimov SV, Zaytsev VB, Vasiliev RB. Induction of Chirality in Atomically Thin ZnSe and CdSe Nanoplatelets: Strengthening of Circular Dichroism via Different Coordination of Cysteine-Based Ligands on an Ultimate Thin Semiconductor Core. Materials. 2023; 16(3):1073. https://doi.org/10.3390/ma16031073
Chicago/Turabian StyleKurtina, Daria A., Valeria P. Grafova, Irina S. Vasil’eva, Sergey V. Maksimov, Vladimir B. Zaytsev, and Roman B. Vasiliev. 2023. "Induction of Chirality in Atomically Thin ZnSe and CdSe Nanoplatelets: Strengthening of Circular Dichroism via Different Coordination of Cysteine-Based Ligands on an Ultimate Thin Semiconductor Core" Materials 16, no. 3: 1073. https://doi.org/10.3390/ma16031073
APA StyleKurtina, D. A., Grafova, V. P., Vasil’eva, I. S., Maksimov, S. V., Zaytsev, V. B., & Vasiliev, R. B. (2023). Induction of Chirality in Atomically Thin ZnSe and CdSe Nanoplatelets: Strengthening of Circular Dichroism via Different Coordination of Cysteine-Based Ligands on an Ultimate Thin Semiconductor Core. Materials, 16(3), 1073. https://doi.org/10.3390/ma16031073







