Analysis of Degradation Products of Biodegradable ZnMgY Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
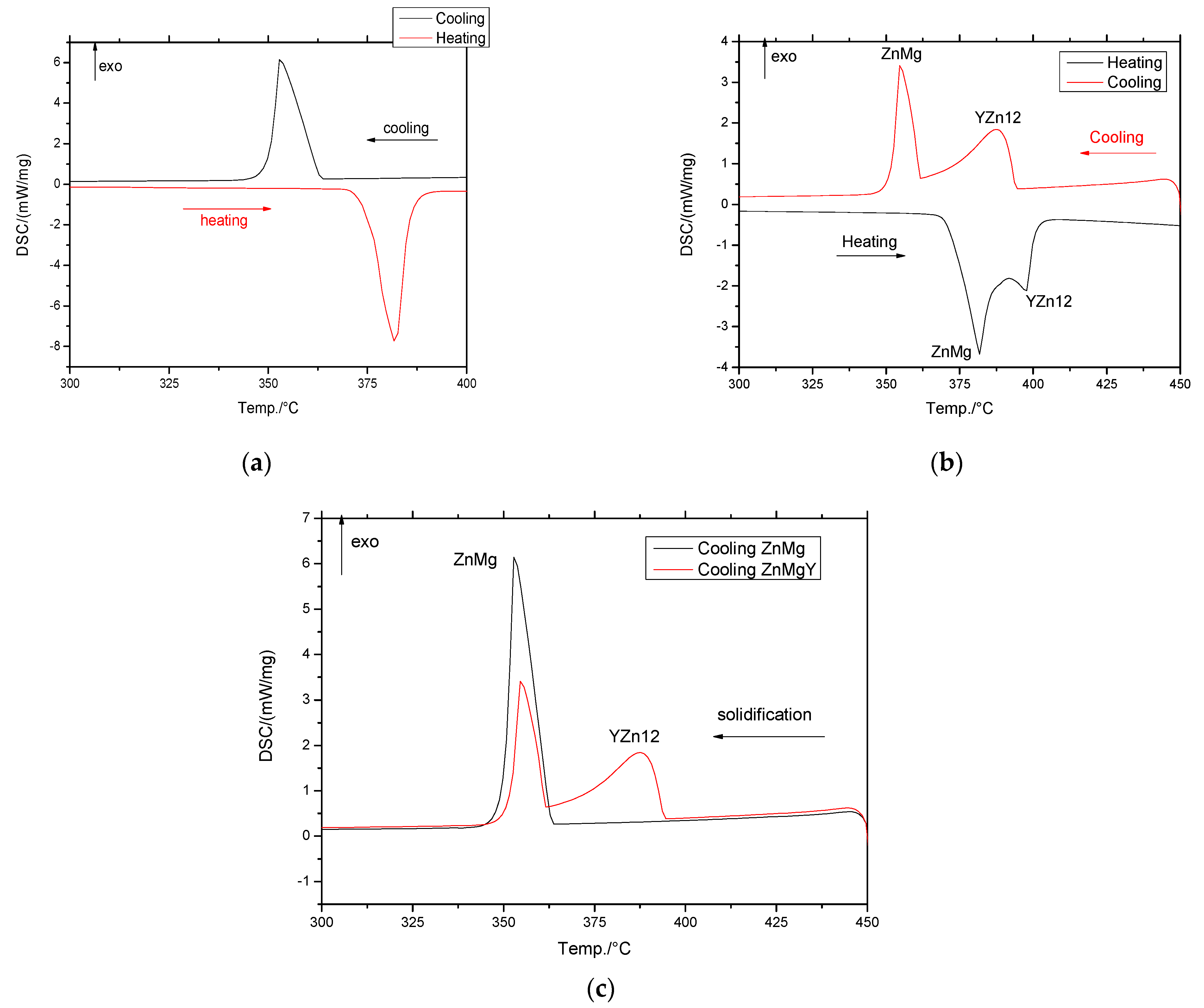
3.1. Differential Scanning Calorimetry (DSC) Analysis
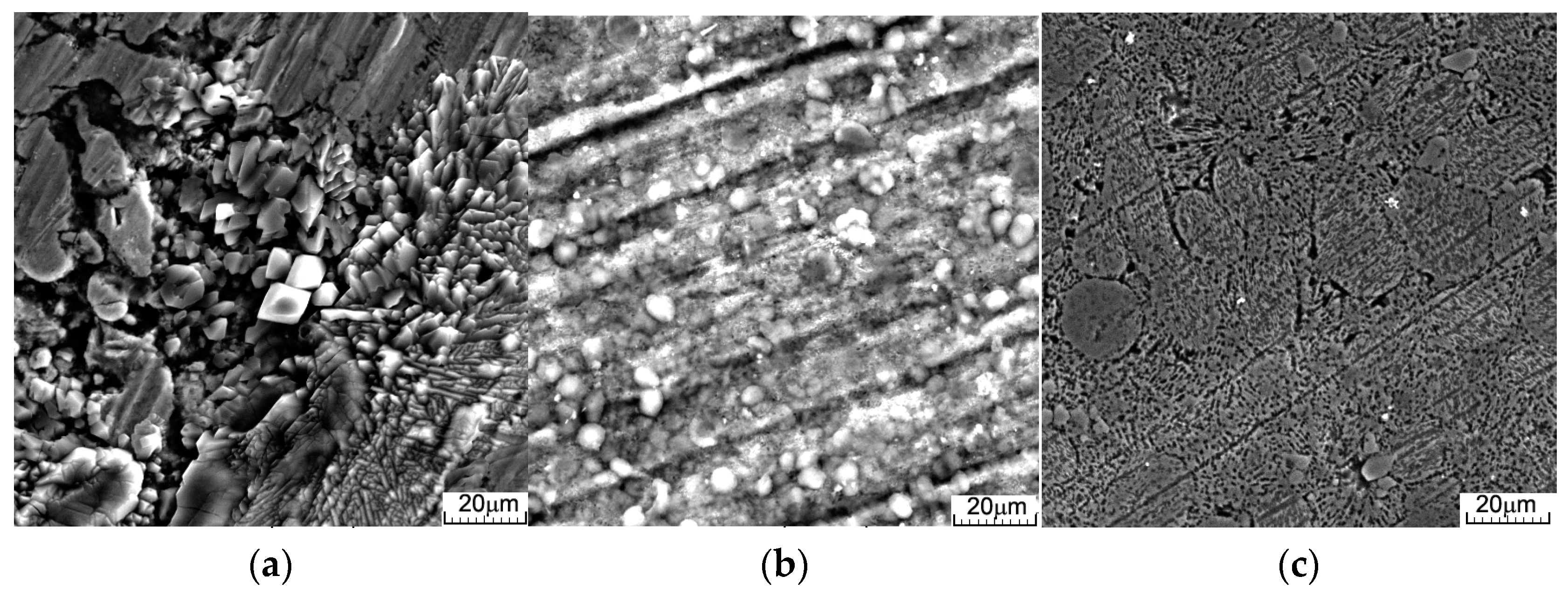
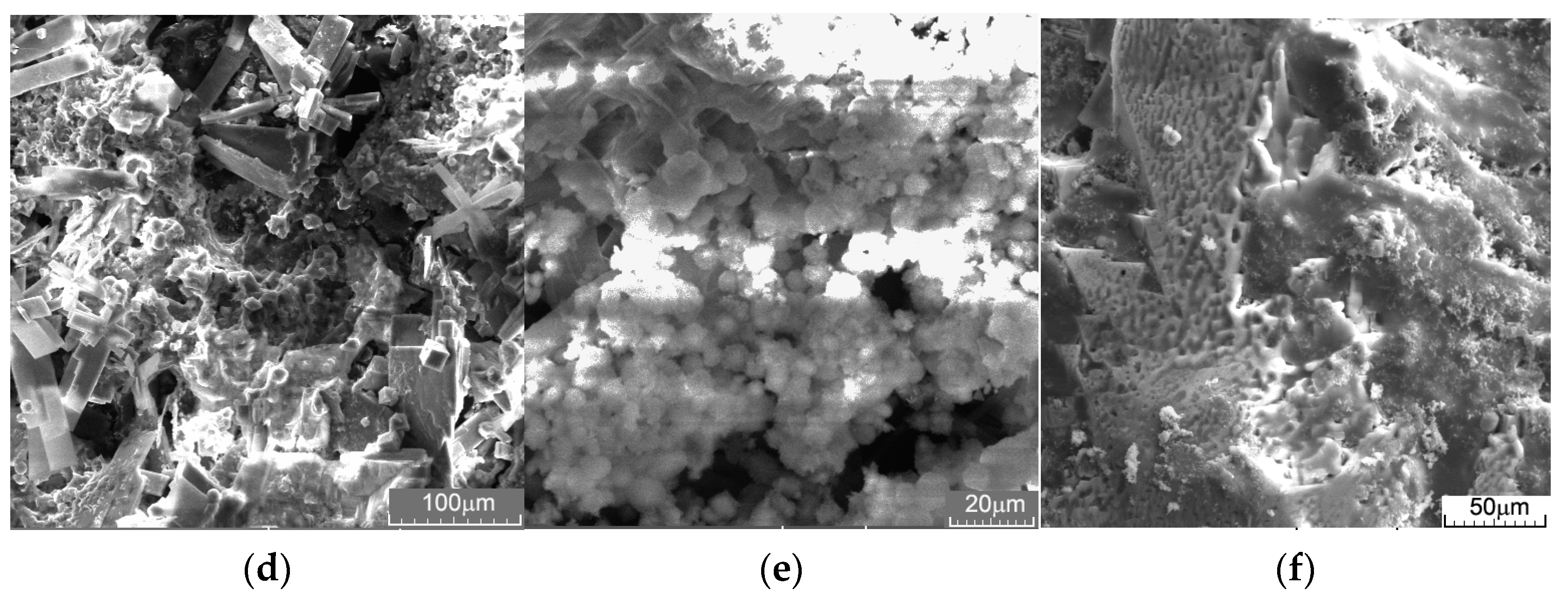
3.2. Immersion Tests
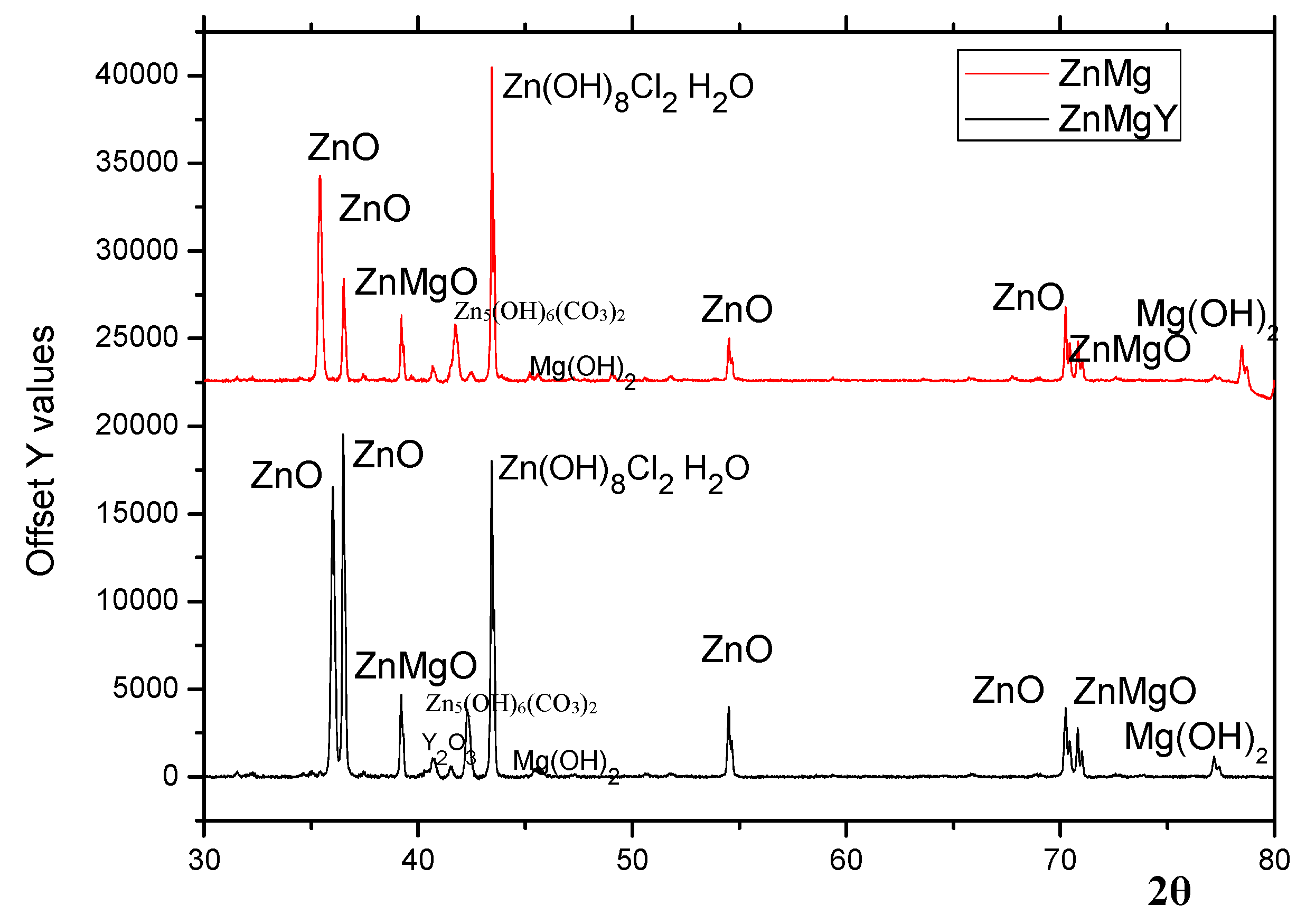
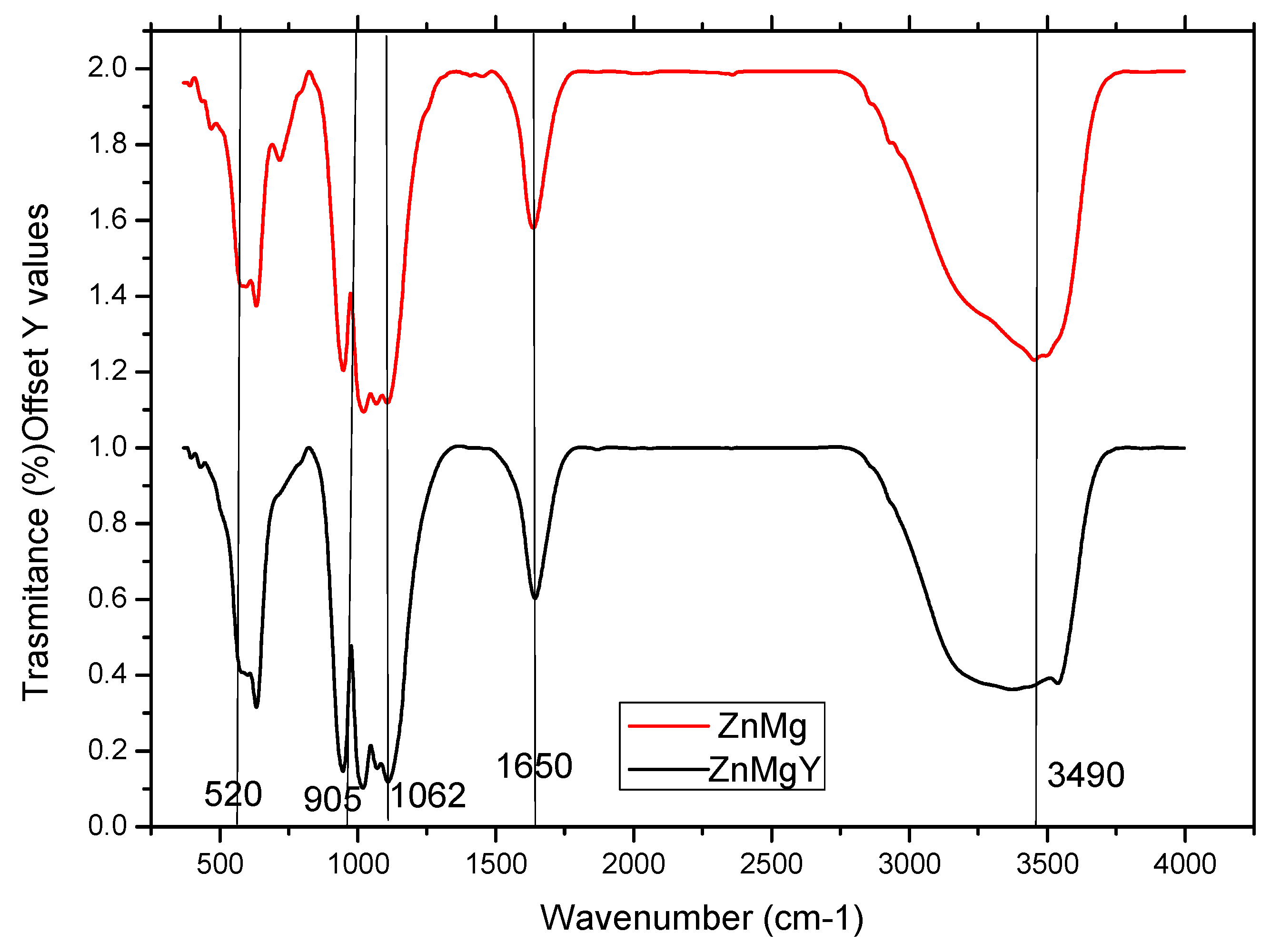
3.3. Phase Composition of the Corrosion Products
4. Conclusions
- –
- The main chemical degradation compounds are zincite, simonkolleite, skorpionite, hydrozincite, and some small traces of brucite;
- –
- Corrosion sites on the surfaces start from a few nanometers and can grow to micrometer sizes;
- –
- The degradation rate is similar for Ringer’s and SBF solutions and is much lower (10 times) in Dulbecco’s solution;
- –
- XRD and FTIR results of the corrosion compounds confirm the formation and detachment of zincite (ZnO) and complex compounds, such as hydrozincite or simonkolleite;
- –
- For a proper characterization of the corrosion compounds, it is suggested to perform daily tests from the first hour of immersion up to fourteen days, separately, for each type of immersion solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1. [Google Scholar] [CrossRef]
- Han, H.S.; Jun, I.; Seok, H.K.; Lee, K.S.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.C.; Glyn-Jones, S.; Edwards, J.R. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Deen, K.M.; Shabib, I.; Asselin, E.; Haider, W. Controlling the dissolution of iron through the development of nanostructured Fe-Mg for biomedical applications. Acta Biomater. 2020, 113, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, H.F.; Yin, Y.X.; Shi, Z.Z.; Li, T.; Feng, X.Y.; Zhang, J.W.; Song, C.X.; Cui, X.S.; Xu, K.L.; et al. Long-term in vivo study of biodegradable Zn-Cu stent: A 2-year implantation evaluation in porcine coronary artery. Acta Biomater. 2019, 97, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.Y.; Zhu, Y.J.; Qi, C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Wang, Y.; Venezuela, J.; Dargusch, M. Biodegradable shape memory alloys: Progress and prospects. Biomaterials 2021, 279, 121215. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.X.; Xu, L.; Gu, X.S.; Ma, X.L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable magnesium-based implants in orthopedics-A general review and perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Aghion, E.; Levy, G. The effect of Ca on the in vitro corrosion performance of biodegradable Mg-Nd-Y-Zr alloy. J. Mater. Sci. 2010, 45, 3096–3101. [Google Scholar] [CrossRef]
- Aghion, E.; Levy, G.; Ovadia, S. In vivo behavior of biodegradable Mg-Nd-Y-Zr-Ca alloy. J. Mater. Sci. Mater. Med. 2012, 23, 805–812. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, M.; Na, D.; Li, Y.D.; Tan, L.L.; Yang, K. Study on a biodegradable antibacterial Fe-Mn-C-Cu alloy as urinary implant material. Mater. Sci. Eng. C 2019, 103, 109718. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Zhao, M.; Liu, C.; Liu, L.; Gao, C.; Shuai, C.; Atrens, A. Study on Fe-xGO Composites Prepared by Selective Laser Melting: Microstructure, Hardness, Biodegradation and Cytocompatibility. JOM 2020, 72, 1163–1174. [Google Scholar] [CrossRef]
- Gorejová, R.; Haverová, L.; Oriňaková, R.; Oriňak, A.; Oriňak, M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2019, 54, 1913–1947. [Google Scholar] [CrossRef]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.V. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef]
- Buzatu, M.; Geanta, V.; Stefanoiu, R.; Butu, M.; Petrescu, M.I.; Buzatu, M.; Ghica, V.G.; Niculescu, F.; Iacob, G. Influence of the Tungsten Content on the Elastic Modulus of New Ti-15Mo-W Alloys Intended for Medical Applications. JOM 2019, 71, 2272–2279. [Google Scholar] [CrossRef]
- He, J.; He, F.; Li, D.; Liu, Y.; Liu, Y.; Ye, Y.; Yin, D. Advances in Fe-based biodegradable metallic materials. RSC Adv. 2016, 6, 112819–112838. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Spataru, M.C.; Verestiuc, L.; Balan, V.; Solcan, C.; Sandu, A.V.; Geanta, V.; Voiculescu, I.; Vizureanu, P. Design, Synthesis, and Preliminary Evaluation for Ti-Mo-Zr-Ta-Si Alloys for Potential Implant Applications. Materials 2021, 14, 6806. [Google Scholar] [CrossRef] [PubMed]
- Ulum, M.F.; Arafat, A.; Noviana, D.; Yusop, A.H.; Nasution, A.K.; Abdul, K.M.R.; Hermawan, H. In vitro and in vivo degradation evaluation of novel iron-bioceramic composites for bone implant applications. Mater. Sci. Eng. C 2014, 36, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, M.S.; Burduhos-Nergis, D.D.; Burduhos-Nergis, D.P.; Vizureanu, P. Advanced Metallic Biomaterials; Materials Research Forum LLC: Millersville, PA, USA, 2022; ISBN 978-1-64490-176-2. [Google Scholar]
- Bowen, P.K.; Shearier, E.R.; Zhao, S.; Guillory, R.J., II; Zhao, F.; Goldman, J.; Drelich, J.W. Biodegradable Metals for Cardiovascular Stents: From Clinical Concerns to Recent Zn-Alloys. Adv. Healthc. Mater. 2016, 5, 1121–1140. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zheng, Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Low, H.T.; Kasiri-Asgarani, M.; Farahany, S.; Akbari, E.; Cho, M.H. Fabrication of biodegradable Zn-AlMg alloy: Mechanical properties, corrosion behavior, cytotoxicity and antibacterial activities. Mater. Sci. Eng. C 2017, 73, 215. [Google Scholar] [CrossRef]
- Guillory, R.J.; Bowen, P.K.; Hopkins, S.P.; Shearier, E.R.; Earley, E.J.; Gillette, A.A.; Aghion, E.; Bocks, M.; Drelich, J.W.; Goldman, J. Corrosion Characteristics Dictate the Long-Term Inflammatory Profile of Degradable Zinc Arterial Implants. ACS Biomater. Sci. Eng. 2016, 2, 2355–2364. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Ren, Y.; Sun, S.; Zhang, E.; Yan, C.; Liu, Q.; Sun, X.; Shou, F.; Duan, J.; et al. Indirectly extruded biodegradable Zn-0.05wt%Mg alloy with improved strength and ductility: In vitro and in vivo studies. J. Mater. Sci. Technol. 2018, 34, 1618–1627. [Google Scholar] [CrossRef]
- Li, P.; Schille, C.; Schweizer, E.; Kimmerle-Müller, E.; Rupp, F.; Heiss, A.; Legner, C.; Klotz, U.E.; Geis-Gerstorfer, J.; Scheideler, L. Selection of extraction medium in fluences cytotoxicity of zinc and its alloys. Acta Biomater. 2019, 98, 235–245. [Google Scholar] [CrossRef]
- Lin, S.; Ran, X.; Yan, X.; Wang, Q.; Zhou, J.G.; Hu, T.; Wang, G. Systematical evolution on a Zn-Mg alloy potentially developed for biodegradable cardiovascular stents. J. Mater. Sci. Mater. Med. 2019, 30, 122. [Google Scholar] [CrossRef]
- ASTM. ASTM G59-97 Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Falk, T.; Svensson, J.E.; Johansson, L.G. The influence of CO2 and NaCl on the atmospheric corrosion of zinc; a laboratory study. J. Electrochem. Soc. 1998, 145, 2993–2999. [Google Scholar] [CrossRef]
- Kabir, K.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater. Des. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, D.H.; Lim, H.K.; Kim, D.H. Effects of Zn / Y ratio on microstructure and mechanical properties of Mg-Zn-Y alloys. Mater. Lett. 2005, 59, 3801–3805. [Google Scholar] [CrossRef]
- Ning, J.; Ma, Z.X.; Zhang, L.J.; Wang, D.P.; Na, S.J. Effects of magnesium on microstructure, properties and degradation behaviors of zinc-based alloys prepared by selective laser melting. Mater. Res. Express 2022, 9, 086511. [Google Scholar] [CrossRef]
- Panaghie, C.; Cimpoeșu, R.; Istrate, B.; Cimpoeșu, N.; Bernevig, M.A.; Zegan, G.; Roman, A.M.; Chelariu, R.; Sodor, A. New Zn3Mg-xY Alloys: Characteristics, Microstructural Evolution and Corrosion Behavior. Materials 2021, 14, 2505. [Google Scholar] [CrossRef]
- Panaghie, C.; Cimpoeșu, R.; Zegan, G.; Roman, A.M.; Ivanescu, M.C.; Aelenei, A.A.; Benchea, M.; Cimpoeșu, N.; Ioanid, N. In Vitro Corrosion Behavior of Zn3Mg0.7Y Biodegradable Alloy in Simulated Body Fluid (SBF). Appl. Sci. 2022, 12, 2727. [Google Scholar]
- Bernevig-Sava, M.A.; Darabont, D.C.; Lohan, M.; Mihalache, E.; Bejinariu, C. Selection and verification of personal protective equipment in the context of current legal requirements. Qual. Access Success 2019, 20, 109. [Google Scholar]
- Gröbner, J.; Kozlov, A.; Fang, X.Y.; Geng, J.; Nie, J.F.; Schmid-Fetzer, R. Phase equilibria and transformations in ternary Mg-rich Mg-Y-Zn alloys. Acta Mater. 2012, 60, 5948–5962. [Google Scholar] [CrossRef]
- Shao, G.; Varsani, V.; Fan, Z. Thermodynamic modelling of the Y-Zn and Mg-Zn-Y systems. Calphad 2006, 30, 286–295. [Google Scholar] [CrossRef]
- Ping, D.H.; Hono, K.; Kawamura, Y.; Inoue, A. Local chemistry of a nanocrystalline high-strength Mg97Y2Zn1 alloy. Philos. Mag. Lett. 2002, 82, 543–551. [Google Scholar] [CrossRef]
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; ASM International, Materials Park: Geauga County, OH, USA, 1985. [Google Scholar]
- Tsai, A.P.; Murakami, Y.; Niikura, A. The Zn-Mg-Y phase diagram involving quasicrystals. Philos. Mag. A 2000, 80, 1043–1054. [Google Scholar] [CrossRef]
- Singh, A.; Watanabe, M.; Kato, A.; Tsai, A.P. Formation of icosahedral-hexagonal H phase nano-composites in Mg-Zn-Y alloys. Scr. Mater. 2004, 51, 955–960. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Morton, A.J.; Nie, J.F. The 18R and 14H long-period stacking ordered structures in Mg-Y-Zn alloys. Acta Mater. 2010, 58, 2936–2947. [Google Scholar] [CrossRef]
- Pedezhnova, E.M.; Mel’nik, E.V.; Miliyevskiy, R.A.; Dobatkina, T.V.; Kinzhibalo, V.V. Investigation of the Mg-Zn-Y System. Russ. Metall. 1982, 4, 185–188. [Google Scholar]
- Matsuda, M.; Ii, S.; Kawamura, Y.; Ikuhara, Y.; Nishida, M. Variation of long-period stacking order structures in rapidly solidified Mg97Zn1Y2 alloy. Mater. Sci. Eng. A 2005, 393, 269–274. [Google Scholar] [CrossRef]
- Deng, D.W.; Kuo, K.H.; Luo, Z.P.; Miller, D.J.; Kramer, M.J.; Dennis, K.W. Crystal structure of the hexagonal Zn3MgY. J. Alloys Compd. 2004, 373, 156–160. [Google Scholar] [CrossRef]
- Takakura, H.; Sato, A.; Yamamoto, A.; Tsai, A.P. Crystal structure of a hexagonal phase and its relation to a quasicrystalline phase in Zn-Mg-Y alloy. Philos. Mag. Lett. 1998, 78, 263–270. [Google Scholar] [CrossRef]
- Luo, Z.P.; Zhang, S.Q. High-resolution electron microscopy on the X-Mg12ZnY phase in a high strength Mg-Zn-Zr-Y magnesium alloy. J. Mater. Sci. Lett. 2000, 19, 813–815. [Google Scholar] [CrossRef]
- Luo, Z.P.; Zhang, S.; Tang, Y.; Zhao, D. Quasicrystals in as-cast Mg-Zn-RE alloys. Scr. Metall. Mater. 1993, 28, 1513–1518. [Google Scholar] [CrossRef]
- Luo, Z.P.; Zhang, S.Q.; Tang, Y.L.; Zhao, D.S. On the stable quasicrystals in slowly cooled Mg-Zn-Y alloys. Scr. Metall. Mater. 1995, 32, 1411–1416. [Google Scholar] [CrossRef]
- Luo, Z.P.; Sui, H.X.; Zhang, S.Q. On the stable Mg-Zn-Y quasicrystals. Metall. Mater. Trans. A 1996, 27, 1779–1784. [Google Scholar] [CrossRef]
- Tsai, A.P.; Nijkura, A.; Inoue, A.; Masumoto, T. Stoichiometric icosahedral phase in the Zn-Mg-Y system. J. Mater. Res. 1997, 12, 1468–1471. [Google Scholar] [CrossRef]
- Abe, E.; Takakura, H.; Singh, H.; Tsai, A.P. Hexagonal superstructures in the Zn-Mg-rare-earth alloys. J. Alloys Compd. 1999, 283, 169–172. [Google Scholar] [CrossRef]
- Li, M.R.; Kuo, K.H. Intermetallic phases and phase reactions in Zn-Mg (<40 at.%)-Y (<20 at.%) region. J. Alloys Compd. 2007, 432, 81–89. [Google Scholar]
- Shao, G.; Varsani, V.; Wang, Y.; Qian, M.; Fan, Z. On the solidification microstructure of Mg-30Zn-2.5Y metal-intermetallic alloy. Intermetallics 2006, 14, 596–602. [Google Scholar] [CrossRef]
- Li, M.R.; Zou, X.D.; Kuo, K.H. A new hexagonal phase displaying pseudo-icosahedral symmetry in Zn-Mg-Y alloy. Scr. Mater. 2009, 60, 683–686. [Google Scholar] [CrossRef]
- Zhu, Z.; Pelton, A.D. Thermodynamic modeling of the Y-Mg-Zn, Gd-Mg-Zn, Tb-Mg-Zn, Dy-Mg-Zn, Ho-Mg-Zn, Er-Mg-Zn, Tm-Mg-Zn and Lu-Mg-Zn systems. J. Alloys Compd. 2015, 652, 426–443. [Google Scholar] [CrossRef]
- Dong, A.; Li, B.; Lu, Y.; Zhu, G.; Xing, H.; Shu, D.; Sun, B.; Wang, J. Effect of Mg on the microstructure and corrosion resistance of the continuously hot-dip galvanizing Zn-Mg coating. Materials 2017, 10, 980. [Google Scholar] [CrossRef] [Green Version]
- Törne, K.; Örnberg, A.; Weissenrieder, J. Influence of strain on the corrosion of magnesium alloys and zinc in physiological environments. Acta Biomater. 2017, 48, 541–550. [Google Scholar] [CrossRef]
- Zhao, S.; Seitz, J.M.; Eifler, R.; Maier, H.J.; Guillory II, R.J.; Earley, E.J.; Drelich, A.; Goldman, J.; Drelich, J.W. Zn-Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Mater. Sci. Eng. C 2017, 76, 301–312. [Google Scholar] [CrossRef]
- Čapek, J.; Jablonská, E.; Lipov, J.; Kubatík, T.F.; Vojtěch, D. Preparation and characterization of porous zinc prepared by spark plasma sintering as a material for biodegradable scaffolds. Mater. Chem. Phys. 2018, 203, 249–258. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Liu, Y.; Xiong, P.; Guo, H.; Huang, H.H.; Zheng, Y. Comparative Studies on Degradation Behavior of Pure Zinc in Various Simulated Body Fluids. JOM 2019, 71, 1414–1425. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Xiong, P.; Li, W.; Huang, H.H.; Zheng, Y. Comparative studies of Tris-HCl, HEPES and NaHCO3/CO2 buffer systems on the biodegradation behaviour of pure Zn in NaCl and SBF solutions. Corros. Sci. 2019, 157, 205–219. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Maitz, M.F.; Chen, M.; Zhang, H.; Mao, J.; Zhao, Y.; Huang, N.; Wan, G. Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline. Corros. Sci. 2016, 111, 541–555. [Google Scholar] [CrossRef]
- Volovitch, P.; Allely, C.; Ogle, K. Understanding corrosion via corrosion product characterization: I. Case study of the role of Mg alloying in Zn–Mg coating on steel. Corros. Sci. 2009, 51, 1251–1262. [Google Scholar] [CrossRef]
- Fazakas, E.; Varga, B.; Geanta, V.; Berecz, T.; Jenei, P.; Voiculescu, I.; Cosnita, M.; Stefanoiu, R. Microstructure, Thermal, and Corrosion Behavior of the AlAgCuNiSnTi Equiatomic Multicomponent Alloy. Materials 2019, 12, 926. [Google Scholar] [CrossRef] [Green Version]
- Nazarov, A.; Thierry, D. Probing the atmospheric corrosion of metals. Zinc. Prot. Met. 2006, 42, 437–451. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Lin, F.; Boccaccini, A.R.; Virtanen, S. Corrosion behavior of biodegradable metals in two different simulated physiological solutions: Comparison of Mg, Zn and Fe. Corros. Sci. 2021, 182, 109278. [Google Scholar] [CrossRef]
- Alves, M.M.; Prosek, T.; Santos, C.F.; Montemor, M.F. Evolution of the in vitro degradation of Zn–Mg alloys under simulated physiological conditions. RSC Adv. 2017, 7, 28224. [Google Scholar] [CrossRef] [Green Version]
- Roman, A.M.; Voiculescu, I.; Cimpoeșu, R.; Istrate, B.; Chelariu, R.; Cimpoeșu, N.; Zegan, G.; Panaghie, C.; Lohan, N.M.; Axinte, M.; et al. Microstructure, Shape Memory Effect, Chemical Composition and Corrosion Resistance Performance of Biodegradable FeMnSi-Al Alloy. Crystals 2023, 13, 109. [Google Scholar] [CrossRef]
- Habibi, M.; Karimi, B. Application of impregnation combustion method for fabrication of nanostructure CuO/ZnO composite oxide: XRD, FESEM, DRS and FTIR study. J. Ind. Eng. Chem. 2014, 20, 1566–1570. [Google Scholar] [CrossRef]
- Matos, C.R.S.; Xavier, M.J.; Barreto, L.S.; Costa, N.B., Jr.; Gimenez, I.F. Principal component analysis of X-ray diffraction patterns to yield morphological classification of brucite particles. Anal. Chem. 2007, 79, 2091–2095. [Google Scholar] [CrossRef]
- Vázquez, R.M.; Hernández, M.G.; Marure, A.L.; López-Camacho, P.Y.; Morales-Ramírez, A.J.; Beltrán-Conde, H.I. Soll-Gel Synthesis and Antioxidant Properties of Yttrium Oxide Nanocrystallites Incorporating P-123. Materials 2014, 7, 6768–6778. [Google Scholar] [CrossRef] [Green Version]
- Kubásek, J.; Dvorský, D.; Šedý, J.; Msallamová, S.; Levorová, J.; Foltán, R.; Vojtěch, D. The Fundamental Comparison of Zn–2Mg and Mg–4Y–3RE Alloys as a Perspective Biodegradable Materials. Materials 2019, 12, 3745. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Yang, K.; Li, D.; Zhang, Z.; Ma, J.; Ni, S.; Yang, X. The fabrication and characterization of Zn5(CO3)2(OH)6 as a new anode material for lithium ion batteries. Mater. Lett. 2016, 164, 148–151. [Google Scholar] [CrossRef]
- Prosek, T.; Bexell, A.U.; Thierry, D.; Serak, J. Corrosion mechanism of model zinc–magnesium alloys in atmospheric conditions. Corros. Sci. 2008, 50, 2216–2231. [Google Scholar] [CrossRef]
- Ishikawa, T.; Matsumoto, K.; Yasukawa, A.; Kandori, K.; Nakayama, T.; Tsubota, T. Influence of metal ions on the formation of artificial zinc rusts. Corros. Sci. 2003, 46, 329–342. [Google Scholar] [CrossRef]


| Chemical Composition [g/L] | NaCl | KCl | CaCl2 | KH2PO4 | Na2HPO4·7H2O | Na2SO4 | NaHCO3 | 1.0 M HCL | MgCl2·6H2O | K2HPO4·3H2O | ((HOCH2)3CNH2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBF | 8.03 | 0.22 | 0.29 | - | - | 0.07 | 0.35 | 39 | 0.31 | 0.23 | 6.11 |
| Dulbecco’s (DPBS) | 8.05 | 0.20 | - | 0.2 | 2.16 | - | - | - | - | - | - |
| Ringer’s | 6.5 | 0.42 | 0.25 | - | - | - | 0.20 | - | - | - | - |
| Sample | Melting | Solidification | YZn12 Compound | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Mass [mg] | Ms [°C] | M50 [°C] | Mf [°C] | DHheating [J/g] | Ss [°C] | S50 [°C] | Sf [°C] | Ss [°C] | S50 [°C] | Sf [°C] | DHcooling [J/g] | |
| Zn | 11.7 | 420.5 | 424.9 | 426.1 | −317.3 | 420.0 | 415.6 | 414.7 | - | - | - | - |
| Zn3Mg | 33.0 | 375.5 | 382.1 | 385.6 | −166.0 | 363.0 | 353.0 | 350.6 | - | - | - | - |
| ZnMgY_V | 50.0 | 370.9 | 387.1 | 403.8 | −170.1 | 356.7 | 345.8 | 339.2 | 382.9 | 373.5 | 357.0 | 134.2 |
| ZnMgY_IV | 37.7 | 378.2 | 392.1 | 396.5 | −168.9 | 343.5 | 346.6 | 359.4 | 361.7 | 363.0 | 372.6 | 128.8 |
| ZnMgY_III | 50.0 | 377.9 | 386.1 | 389.9 | −151.2 | 361.0 | 349.5 | 347.1 | 376.1 | 369.1 | 362.0 | 120.4 |
| ZnMgY_II | 38.2 | 394 | 398 | 400 | −157.1 | 361.4 | 354.8 | 352.1 | 394.0 | 387.5 | 374.3 | 142.1 |
| ZnMgY_I | 35.8 | 368.5 | 383.4 | 390.4 | −152.9 | 360.2 | 350.4 | 347.6 | 381.1 | 374.5 | 362.7 | 129.4 |
| Sample Zn3Mg0.4Y/Solution | Ringer’s | SBF | Dulbecco’s |
|---|---|---|---|
| Initial mass (mg) | 6739.4 | 15,607.1 | 4580.6 |
| After immersion (mg) | 6743.6 | 15,609.2 | 4589.3 |
| (+4.2) | (+2.1) | (+8.7) | |
| After ultrasound cleaning (mg) | 6734.7 | 15,595.7 | 4580.3 |
| (−4.7) | (−11.4) | (−0.3) | |
| Corrosion rate (mm/year) | 0.10 | 0.11 | 0.01 |
| Element/Sample | O% | C% | Na% | Cl% | Zn% | P% | K% | Mg% | Ca% | Y% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | |
| in Dulbecco’s | 29.29 | 59.82 | - | - | - | - | - | - | 61.42 | 30.69 | 8.22 | 8.67 | 0.26 | 0.21 | 0.3 | 0.40 | - | - | 0.52 | 0.19 |
| CpS (Dulbecco’s) | 32.78 | 48.79 | - | - | 25.63 | 26.55 | 20.51 | 13.77 | 12.62 | 6.18 | 6.18 | 4.75 | 1.91 | 1.16 | 0.39 | 0.38 | - | - | - | - |
| in Ringer’s | 23.54 | 45.96 | 8.21 | 21.35 | - | - | 0.37 | 0.33 | 66.96 | 31.99 | - | - | 0.05 | 0.05 | - | - | 0.13 | 0.1 | 0.87 | 0.31 |
| CpS (Ringer’s) | 20.38 | 21.18 | 46.92 | 64.93 | 5.86 | 4.24 | 10.31 | 4.83 | 12.8 | 3.26 | - | - | 2.1 | 0.89 | 0.01 | 0.01 | 1.55 | 0.64 | 0.07 | 0.01 |
| in SBF | 12.84 | 27.43 | 6.15 | 17.5 | 12.24 | 18.2 | 0.02 | 0.02 | 66.97 | 35.02 | 1.07 | 1.5 | 0.11 | 0.13 | 0.56 | 0.22 | ||||
| CpS (SBF) | 25.01 | 23.21 | 55.08 | 68.1 | 3.58 | 2.31 | 11.75 | 4.9 | 1.97 | 0.44 | 0.71 | 0.34 | 0.76 | 0.29 | - | - | 0.9 | 0.33 | 0.24 | 0.05 |
| EDS err.% | 1.5/5 | 3/4 | 0.35/1.5 | 1.5/1.5 | 0.15/0.2 | 0.05/1.2 | 0.07/0.2 | 0.04/0.1 | 0.05/0.1 | 0.1/0.2 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaghie, C.; Zegan, G.; Sodor, A.; Cimpoeșu, N.; Lohan, N.-M.; Istrate, B.; Roman, A.-M.; Ioanid, N. Analysis of Degradation Products of Biodegradable ZnMgY Alloy. Materials 2023, 16, 3092. https://doi.org/10.3390/ma16083092
Panaghie C, Zegan G, Sodor A, Cimpoeșu N, Lohan N-M, Istrate B, Roman A-M, Ioanid N. Analysis of Degradation Products of Biodegradable ZnMgY Alloy. Materials. 2023; 16(8):3092. https://doi.org/10.3390/ma16083092
Chicago/Turabian StylePanaghie, Cătălin, Georgeta Zegan, Alina Sodor, Nicanor Cimpoeșu, Nicoleta-Monica Lohan, Bogdan Istrate, Ana-Maria Roman, and Nicoleta Ioanid. 2023. "Analysis of Degradation Products of Biodegradable ZnMgY Alloy" Materials 16, no. 8: 3092. https://doi.org/10.3390/ma16083092







