Microstructure and Strengthening Effect of Coated Diamond Particles on the Porous Aluminum Composites
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of Diamond-Reinforced Porous Al Composites
2.3. Characterization and Testing
3. Results and Discussion
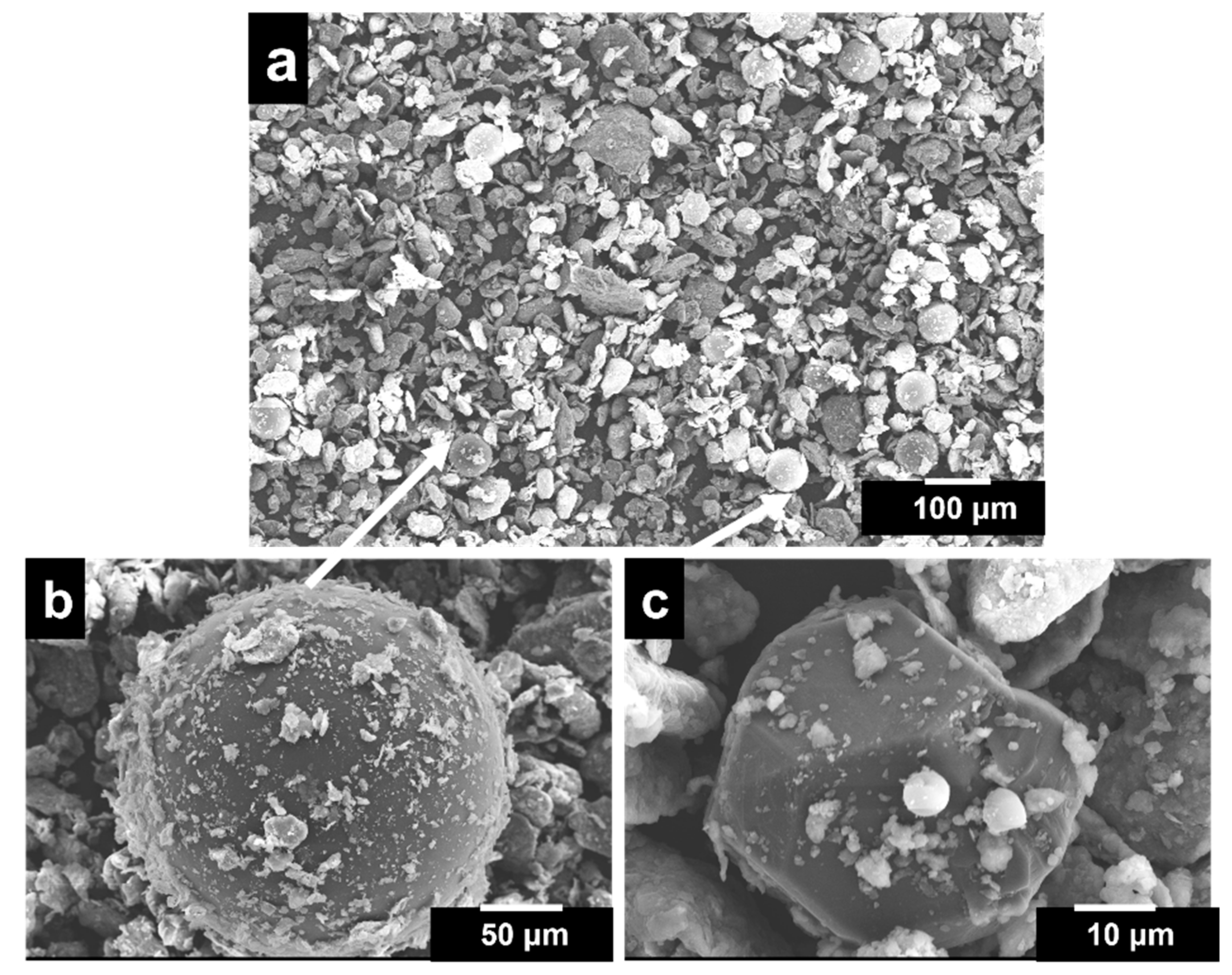
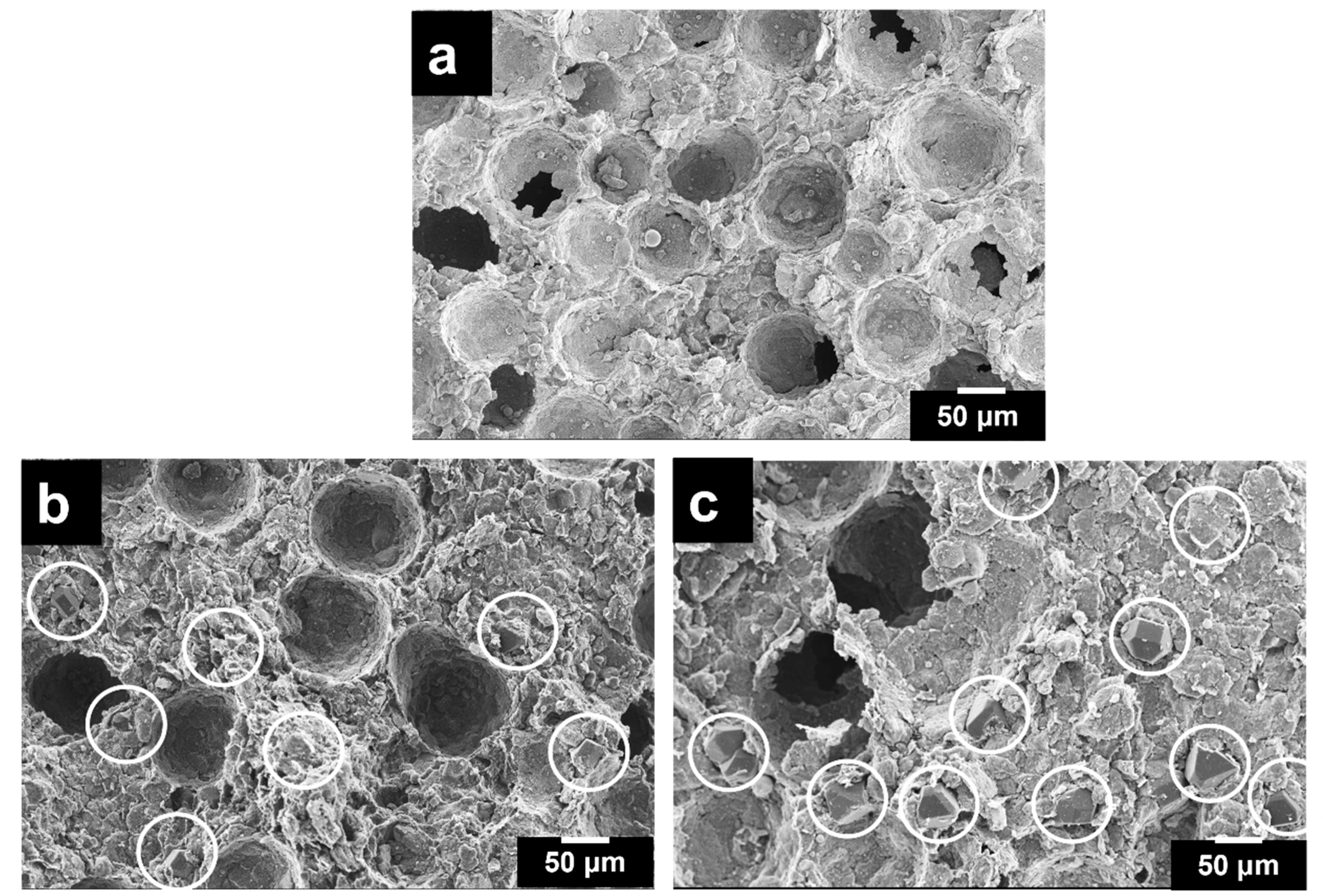
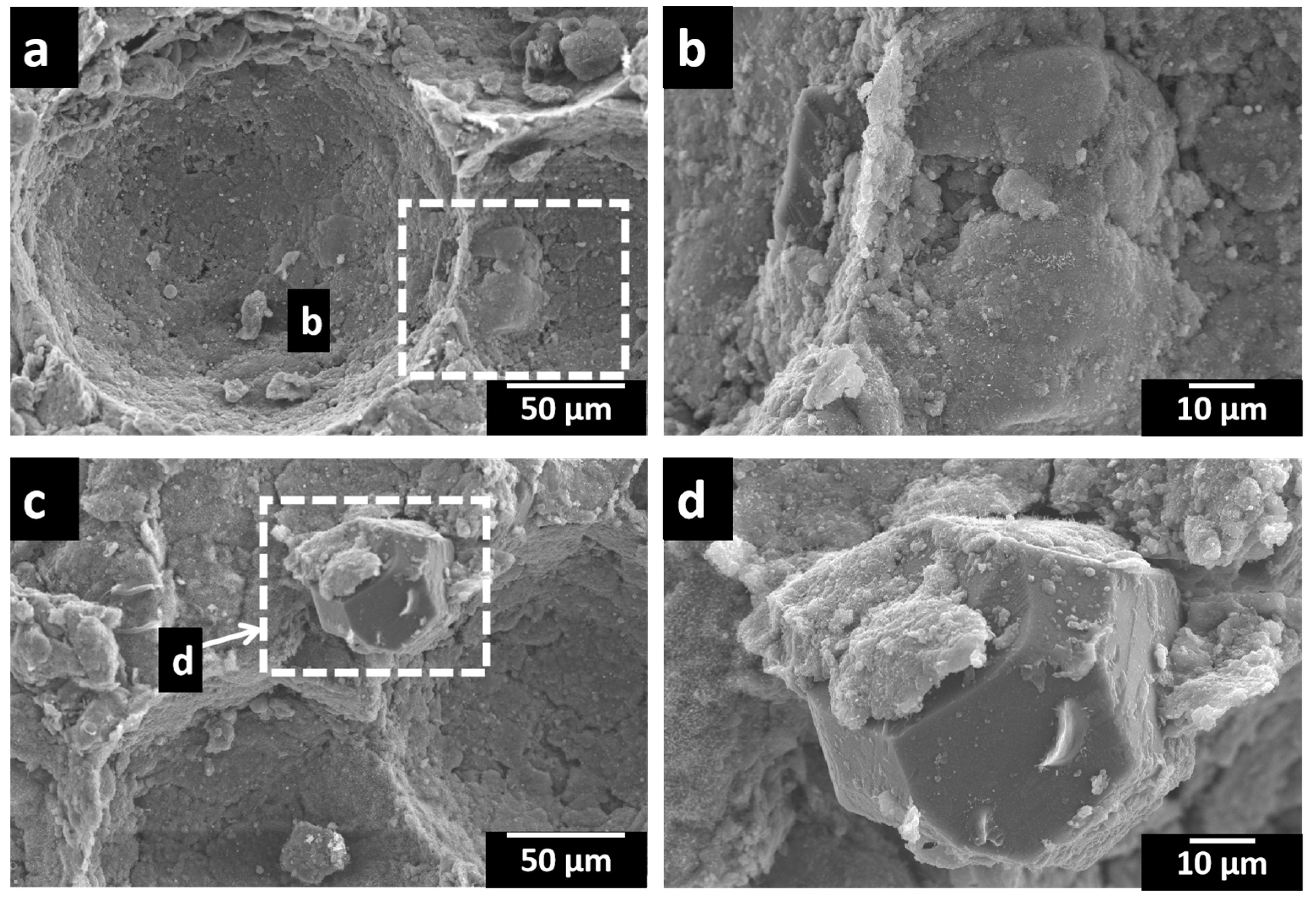
3.1. Microstructural Analysis
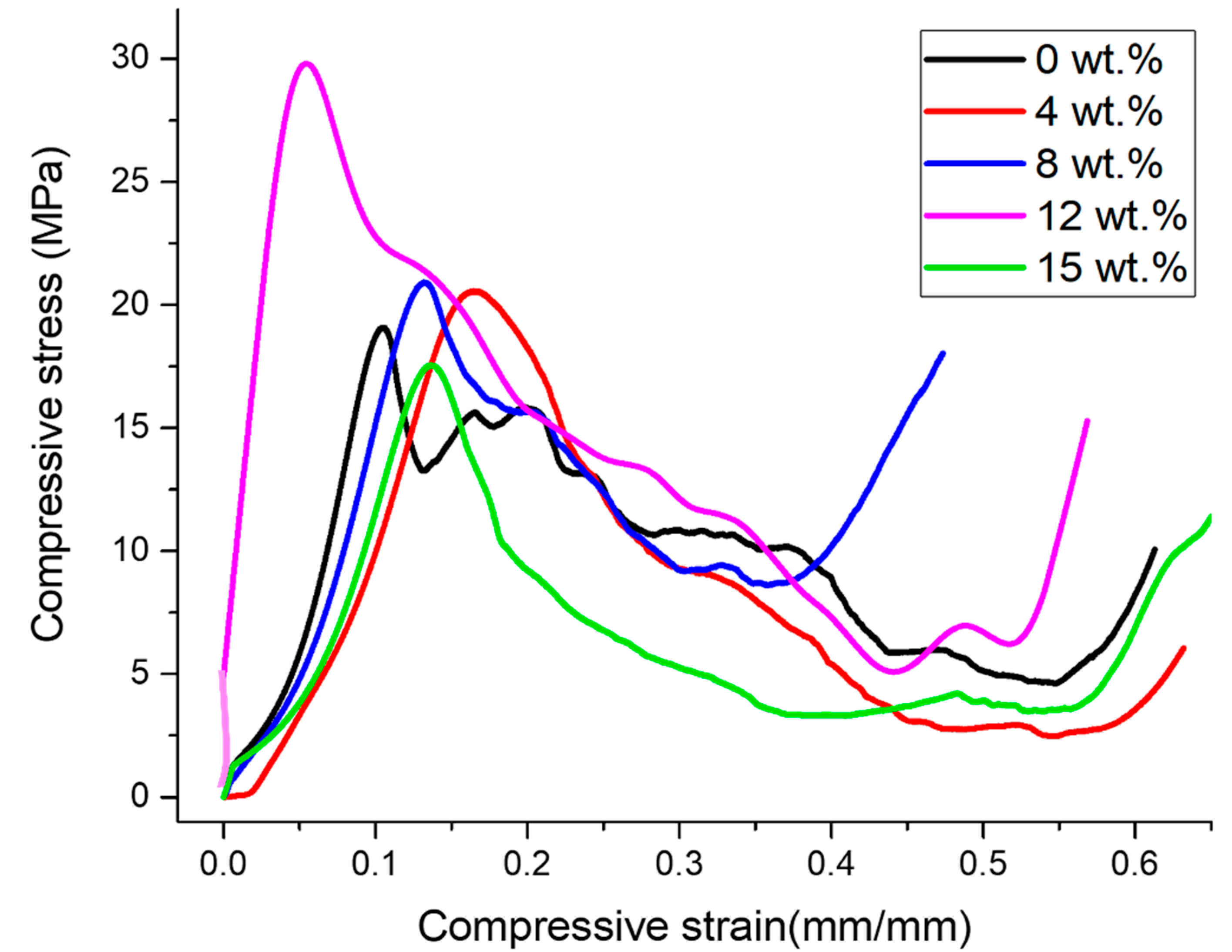
3.2. Compressive Behavior
4. Conclusions
- (1)
- The microstructure of the porous Al composites revealed a uniformly distributed porous structure with less formation of micro-pores and cracks. The pore morphology resembled that of space holders and can thus be controlled. The spherical porosities improve the properties. The porous composites up to 12 wt.% exhibited a homogeneous distribution of pores and diamond particles.
- (2)
- The porosity and relative densities were found to be maximum for porous Al composites with a 12 wt.% diamond content due to the better interfacial bonding between the Al alloy matrix and diamond particles as a result of the intermetallic and Ti-coating on diamond particles.
- (3)
- The maximum plateau stress and energy absorption capacity were found to depend on the relative density, and their values were 31.51 MPa and 7.46 MJ/m3, respectively, for 12 wt.% diamond.
- (4)
- Therefore, better microstructural and compressive behavior was obtained for porous Al composites with 12 wt.% of diamond particles; beyond this, the agglomeration of diamond particles occurred.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parveez, B.; Jamal, N.A.; Maleque, A.; Yusof, F.; Jamadon, N.H.; Adzila, S. Review on advances in porous Al composites and the possible way forward. J. Mater. Res. Technol. 2021, 14, 2017–2038. [Google Scholar] [CrossRef]
- Parveez, B.; Jamal, N.A.; Anuar, H.; Ahmad, Y.; Aabid, A.; Baig, M. Microstructure and Mechanical Properties of Metal Foams Fabricated via Melt Foaming and Powder Metallurgy Technique: A Review. Materials 2022, 15, 5302. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, O.; Ipekoglu, M.; Altintas, S. Effects of alumina (Al2O3) addition on the cell structure and mechanical properties of 6061 foams. J. Mater. Res. 2013, 28, 2509–2519. [Google Scholar] [CrossRef]
- Almajid, A.A. High-temperature deformation of naturally aged 7010 aluminum alloy. Metals 2021, 11, 581. [Google Scholar] [CrossRef]
- Attia, M.S.; Meguid, S.A.; Tan, K.T.; Yeo, S.C. Influence of cellular imperfections on mechanical response of metallic foams. Int. J. Crashworthiness 2010, 15, 357–367. [Google Scholar] [CrossRef]
- Mu, Y.; Yao, G.; Liang, L.; Luo, H.; Zu, G. Deformation mechanisms of closed-cell aluminum foam in compression. Scr. Mater. 2010, 63, 629–632. [Google Scholar] [CrossRef]
- Said, M.R.; Tan, C.F. The response of aluminium foams under quasi-static loading. Chiang Mai J. Sci. 2008, 35, 241–249. [Google Scholar]
- Hamdi, A.A. Effect Of Magnesium and Tin As Sintering Additives on Microstructure and Compressive Properties of Porous Aluminum. Master’s Thesis, UUM Malaysia, Bukit Kayu Hitam, Malaysia, 2018. [Google Scholar]
- Sercombe, T.B. Non-Conventional Sintered Aluminium Powder Alloys. Ph.D. Thesis, University of Queensland, St Lucia, QLD, Australia, 1998. [Google Scholar]
- Ali, S.; Rani, A.M.A.; Altaf, K.; Baig, Z. Investigation of Boron addition and compaction pressure on the compactibility, densification and microhardness of 316L Stainless Steel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 344, 012023. [Google Scholar] [CrossRef]
- Sánchez de la Muela, A.M.; García Cambronero, L.E.; Malheiros, L.F.; Ruiz-Román, J.M. New Aluminum Syntactic Foam: Synthesis and Mechanical Characterization. Materials 2022, 15, 5320. [Google Scholar] [CrossRef]
- Li, Y.G.; Wei, Y.H.; Hou, L.F.; Guo, C.L.; Yang, S.Q. Fabrication and compressive behaviour of an aluminium foam composite. J. Alloys Compd. 2015, 649, 76–81. [Google Scholar] [CrossRef]
- Yang, K.; Yang, X.; He, C.; Liu, E.; Shi, C.; Ma, L.; Li, Q.; Li, J.; Zhao, N. Damping characteristics of Al matrix composite foams reinforced by in-situ grown carbon nanotubes. Mater. Lett. 2017, 209, 68–70. [Google Scholar] [CrossRef]
- Yang, X.; Hu, Q.; Li, W.; Song, H.; Zou, T.; Zong, R.; Sha, J.; He, C.; Zhao, N. Compression-compression fatigue performance of aluminium matrix composite foams reinforced by carbon nanotubes. Fatigue Fract. Eng. Mater. Struct. 2020, 43, 744–756. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Zhang, M.; Li, J.; Shi, C.; Zhao, N.; Zou, T. A novel approach to obtain in-situ growth carbon nanotube reinforced aluminum foams with enhanced properties. Mater. Lett. 2015, 161, 763–766. [Google Scholar] [CrossRef]
- Alizadeh, M.; Mirzaei-Aliabadi, M. Compressive properties and energy absorption behavior of Al-Al2O3 composite foam synthesized by space-holder technique. Mater. Des. 2012, 35, 419–424. [Google Scholar] [CrossRef]
- Chung, C.Y.; Chu, C.H.; Lee, M.T.; Lin, C.M.; Lin, S.J. Effect of titanium addition on the thermal properties of diamond/Cu-Ti composites fabricated by pressureless liquid-phase sintering technique. Sci. World J. 2014, 2014, 713537. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Che, Z.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Combining Cr pre-coating and Cr alloying to improve the thermal conductivity of diamond particles reinforced Cu matrix composites. J. Alloys Compd. 2018, 749, 1098–1105. [Google Scholar] [CrossRef]
- Che, Z.; Li, J.; Wang, L.; Qi, Y.; Zhang, Y.; Zhang, H.; Wang, X.; Wang, J.; Kim, M.J. Effect of diamond surface chemistry and structure on the interfacial microstructure and properties of Al/diamond composites. RSC Adv. 2016, 6, 67252–67259. [Google Scholar] [CrossRef]
- Yang, B.; Yu, J.K.; Chen, C. Microstructure and thermal expansion of Ti coated diamond/Al composites. Trans. Nonferrous Met. Soc. China 2009, 19, 1167–1173. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Z.; Wang, R.; Peng, C.; Qiu, K.; Wang, N. Microstructure and thermal properties of Al/W-coated diamond composites prepared by powder metallurgy. Mater. Des. 2016, 95, 39–47. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. Mo 2 C Coating on Diamond: Different Effects on Thermal Conductivity of Diamond/Al and Diamond/Cu Composites; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 402, ISBN 4520647482879. [Google Scholar]
- Suleiman, M.J.; Ahmad, M.A.; Mohamed, N.; Mohamad Junus, Y.; Ibrahim, R.; Mohamad, M.; Abdul Kadir, M.R.; Abu Kassim, N.H.; Awang, R.; Muhammad, S. Effect of Atmosphere on the Sintered Titanium Alloy Produced By Metal Injection Molding (Mim) Technique. J. Ind. Technol. 2013, 21, 9–17. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, R.; He, X.; Ye, Z.; Qu, X. Fabrication, and infiltration kinetics analysis of Ti-coated diamond/copper composites with near-net-shape by pressureless infiltration. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 1524–1530. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Z.; Wang, R.; Peng, C.; Feng, Y. Enhancing densification capacity and properties of Al/diamond composites by partial liquid hot pressing. Surf. Coat. Technol. 2017, 313, 347–354. [Google Scholar] [CrossRef]
- Abyzov, A.M.; Kruszewski, M.J.; Ciupiński, Ł.; Mazurkiewicz, M.; Michalski, A.; Kurzydłowski, K.J. Diamond-tungsten based coating-copper composites with high thermal conductivity produced by Pulse Plasma Sintering. Mater. Des. 2015, 76, 97–109. [Google Scholar] [CrossRef]
- Parveez, B.; Jamal, N.A.; Aabid, A.; Baig, M.; Yusof, F. Experimental Analysis and Parametric Optimization on Compressive Properties of Diamond-Reinforced Porous Al Composites. Materials 2023, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, O.; Cizek, J.; Yin, S.; Lupoi, R.; Janovska, M.; Cech, J.; Capek, J.; Siegl, J.; Chraska, T. Mechanical and Fatigue Properties of Diamond-Reinforced Cu and Al Metal Matrix Composites Prepared by Cold Spray. J. Therm. Spray Technol. 2022, 31, 217–233. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; He, L.; Meng, Q.; Liu, B.C.; Gao, K.; Wu, J. Enhanced bending strength and thermal conductivity in diamond/Al composites with B4C coating. Sci. Rep. 2018, 8, 11104. [Google Scholar] [CrossRef]
- Almajid, A.; Walter, R.; Kroos, T.; Junaidi, H.; Gurka, M.; Khalil, K.A. Development of polypropylene/polyethylene terephthalate microfibrillar composites filament to support waste management. Polymers 2021, 13, 233. [Google Scholar] [CrossRef]
- Almajid, A.; Walter, R.; Kroos, T.; Junaedi, H.; Gurka, M.; Khalil, K.A. The multiple uses of polypropylene/polyethylene terephthalate microfibrillar composite structures to support waste management—composite processing and properties. Polymers 2021, 13, 1296. [Google Scholar] [CrossRef]
- Aboraia, M.; Sharkawi, R.; Doheim, M.A. Production of aluminium foam and the effect of calcium carbonate as a foaming agent. J. Eng. Sci. 2011, 39, 441–451. [Google Scholar] [CrossRef]
- Nakamura, T.; Gnyloskurenko, S.V.; Sakamoto, K.; Byakova, A.V.; Ishikawa, R. Development of new foaming agent for metal foam. Mater. Trans. 2002, 43, 1191–1196. [Google Scholar] [CrossRef]
- Mukherjee, M.; García-Moreno, F.; Jiménez, C.; Rack, A.; Banhart, J. Microporosity in aluminium foams. Acta Mater. 2017, 131, 156–168. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, Z.; Zhao, N. Effect of pore size and relative density on the mechanical properties of open cell aluminum foams. Scr. Mater. 2007, 56, 169–172. [Google Scholar] [CrossRef]
- Mohd Razali, R.N.; Abdullah, B.; Ismail, M.H.; Ahmad, U.K.; Idham, M.F.; Rasmli, A. Mechanical properties of aluminium foam by conventional casting combined with NaCL space holder. Appl. Mech. Mater. 2013, 393, 156–160. [Google Scholar] [CrossRef]
- Krommenhoek, M.; Shamma, M.; Morsi, K. Processing, characterization, and properties of aluminum–carbon nanotube open-cell foams. J. Mater. Sci. 2017, 52, 3927–3935. [Google Scholar] [CrossRef]
- Jamal, N.A.; Maizatul, O.; Anuar, H.; Yusof, F.; Nor, Y.A.; Khalid, K.; Zakaria, M.N. Preliminary development of porous aluminum via powder metallurgy technique. Mater. Werkstofftech 2018, 49, 460–466. [Google Scholar] [CrossRef]
- Tan, P.P.; Mohamad, H.; Anasyida, A.S. Properties of Porous Magnesium Using Polymethyl Methacrylate (PMMA) as a Space Holder. J. Phys. Conf. Ser. 2018, 1082, 012063. [Google Scholar] [CrossRef]
- Li, B.Q.; Wang, C.Y.; Lu, X. Effect of pore structure on the compressive property of porous Ti produced by powder metallurgy technique. Mater. Des. 2013, 50, 613–619. [Google Scholar] [CrossRef]
- Bi, Y.; Zheng, Y.; Li, Y. Microstructure and mechanical properties of sintered porous magnesium using polymethyl methacrylate as the space holder. Mater. Lett. 2015, 161, 583–586. [Google Scholar] [CrossRef]
- Baumeister, J.; Banhart, J.; Weber, M. Aluminium foams for transport industry. Mater. Des. 1997, 18, 217–220. [Google Scholar] [CrossRef]
- Showaiter, N.; Youseffi, M. Compaction, sintering and mechanical properties of elemental 6061 Al powder with and without sintering aids. Mater. Des. 2008, 29, 752–762. [Google Scholar] [CrossRef]
- Kang, B.; Kong, T.; Dan, N.H.; Phuong, D.D.; Ryu, H.J.; Hong, S.H. Effect of boron addition to microstructure and mechanical properties of refractory Al 0.1 CrNbVMo high-entropy alloy. Int. J. Refract. Met. Hard Mater. 2021, 100, 105636. [Google Scholar] [CrossRef]
- Dixit, M.; Srivastava, R. The effect of copper granules on interfacial bonding and properties of the copper-graphite composite prepared by flake powder metallurgy. Adv. Powder Technol. 2019, 30, 3067–3078. [Google Scholar] [CrossRef]
- Miyoshi, T.; Hara, S.; Mukai, T.; Higashi, K. Development of a closed cell aluminum alloy foam with enhancement of the compressive strength. Mater. Trans. 2001, 42, 2118–2123. [Google Scholar] [CrossRef]
- German, R.M.; Rabin, B.H. Enhanced sintering through second phase additions. Powder Metall. 1985, 28, 7–12. [Google Scholar] [CrossRef]
- Hassani, A.; Bagherpour, E.; Qods, F. Influence of pores on workability of porous Al/SiC composites fabricated through powder metallurgy + mechanical alloying. J. Alloys Compd. 2014, 591, 132–142. [Google Scholar] [CrossRef]
- Bekoz, N.; Oktay, E. Effects of carbamide shape and content on processing and properties of steel foams. J. Mater. Process. Technol. 2012, 212, 2109–2116. [Google Scholar] [CrossRef]
- Yang, K.; Yang, X.; Liu, E.; Shi, C.; Ma, L.; He, C.; Li, Q.; Li, J.; Zhao, N. Elevated temperature compressive properties and energy absorption response of in-situ grown CNT- reinforced Al composite foams. Mater. Sci. Eng. A 2017, 690, 294–302. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y.; Morisada, Y.; Sugioka, M.; Tanaka, M.; Takeuchi, T.; Tani, J.I.; Kawahara, M.; Makino, Y. Processing of diamond particle dispersed aluminum matrix composites in continuous solid-liquid co-existent state by SPS and their thermal properties. Compos. Part B Eng. 2011, 42, 825–831. [Google Scholar] [CrossRef]
- Parveez, B.; Jamal, N.A.; Raeez, M. Investigation of Morphology and Compressive Properties of Diamond Reinforced Porous Aluminium Composites. Asian J. Fundam. Appl. Sci. 2023, 4, 12–17. [Google Scholar]
- Kwon, H.; Leparoux, M.; Heintz, J.M.; Silvain, J.F.; Kawasaki, A. Fabrication of single crystalline diamond reinforced aluminum matrix composite by powder metallurgy route. Met. Mater. Int. 2011, 17, 755–763. [Google Scholar] [CrossRef]
- Yang, X.; Zou, T.; Shi, C.; Liu, E.; He, C.; Zhao, N. Effect of carbon nanotube (CNT) content on the properties of in-situ synthesis CNT reinforced Al composites. Mater. Sci. Eng. A 2016, 660, 11–18. [Google Scholar] [CrossRef]
- Amore, S.; Ricci, E.; Borzone, G.; Novakovic, R. Wetting behaviour of lead-free Sn-based alloys on Cu and Ni substrates. Mater. Sci. Eng. A 2008, 495, 108–112. [Google Scholar] [CrossRef]
- Lasky, R.C. Copper-Tin intermetallics: Their importance, growth rate, and nature. In Proceedings of the SMTA International 2018 Conference Brochure, Rosemont, IL, USA, 14–18 October 2018. [Google Scholar]
- Jiang, M.G.; Xu, C.; Nakata, T.; Yan, H.; Chen, R.S.; Kamado, S. Development of dilute Mg-Zn-Ca-Mn alloy with high performance via extrusion. J. Alloys Compd. 2016, 668, 13–21. [Google Scholar] [CrossRef]
- Mota, J.M.; Martinez, M.A.; Velasco, F.; Criado, A.J. Preparation of aluminium boride by powder technology. Ceram. Int. 2004, 30, 301–306. [Google Scholar] [CrossRef]
- Kayikci, R.; Kurtulus, O.; Gurbuz, R. The formation and growth behavior of aluminium boride crystals in an Al-B alloy. Solid State Phenom. 2009, 144, 140–144. [Google Scholar] [CrossRef]
- Ebhota, W.S.; Jen, T.-C. Intermetallics Formation and Their Effect on Mechanical Properties of Al-Si-X Alloys. Intermet. Compd.-Form. Appl. 2018, 21–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Jiang, S.; Wu, J. Thermo-physical properties of Ti-coated diamond/Al composites prepared by pressure infiltration. Mater. Sci. Forum 2010, 654–656, 2572–2575. [Google Scholar] [CrossRef]
- Al-Dabbagh, J.B.; Tahar, R.M.; Ishak, M.; Harun, S.A. Structural and phase formation of TiAl alloys synthesized by mechanical alloying and heat treatment. Int. J. Nanoelectron. Mater. 2015, 8, 23–32. [Google Scholar]
- Pal, U.; Sandoval, A.; Madrid, S.I.U.; Corro, G.; Sharma, V.; Mohanty, P. Mixed titanium, silicon, and aluminum oxide nanostructures as novel adsorbent for removal of rhodamine 6G and methylene blue as cationic dyes from aqueous solution. Chemosphere 2016, 163, 142–152. [Google Scholar] [CrossRef]
- Jamal, N.A.; Tan, A.W.; Yusof, F.; Katsuyoshi, K.; Hisashi, I.; Singh, S.; Anuar, H. Fabrication and compressive properties of low to medium porosity closed-cell porous Aluminum using PMMA space holder technique. Materials 2016, 9, 254. [Google Scholar] [CrossRef]
- Linul, E.; Marsavina, L.; Ková, J. Collapse mechanisms of metal foam matrix composites under static and dynamic loading conditions. Mater. Sci. Eng. A 2017, 690, 214–224. [Google Scholar] [CrossRef]
- Salur, E.; Nazik, C.; Acarer, M.; Şavklıyıldız, İ.; Akdoğan, E.K. Ultrahigh hardness in Y2O3 dispersed ferrous multicomponent nanocomposites. Mater. Today Commun. 2021, 28, 102637. [Google Scholar] [CrossRef]
- Aldoshan, A.; Khanna, S. Effect of relative density on the dynamic compressive behavior of carbon nanotube reinforced aluminum foam. Mater. Sci. Eng. A 2017, 689, 17–24. [Google Scholar] [CrossRef]
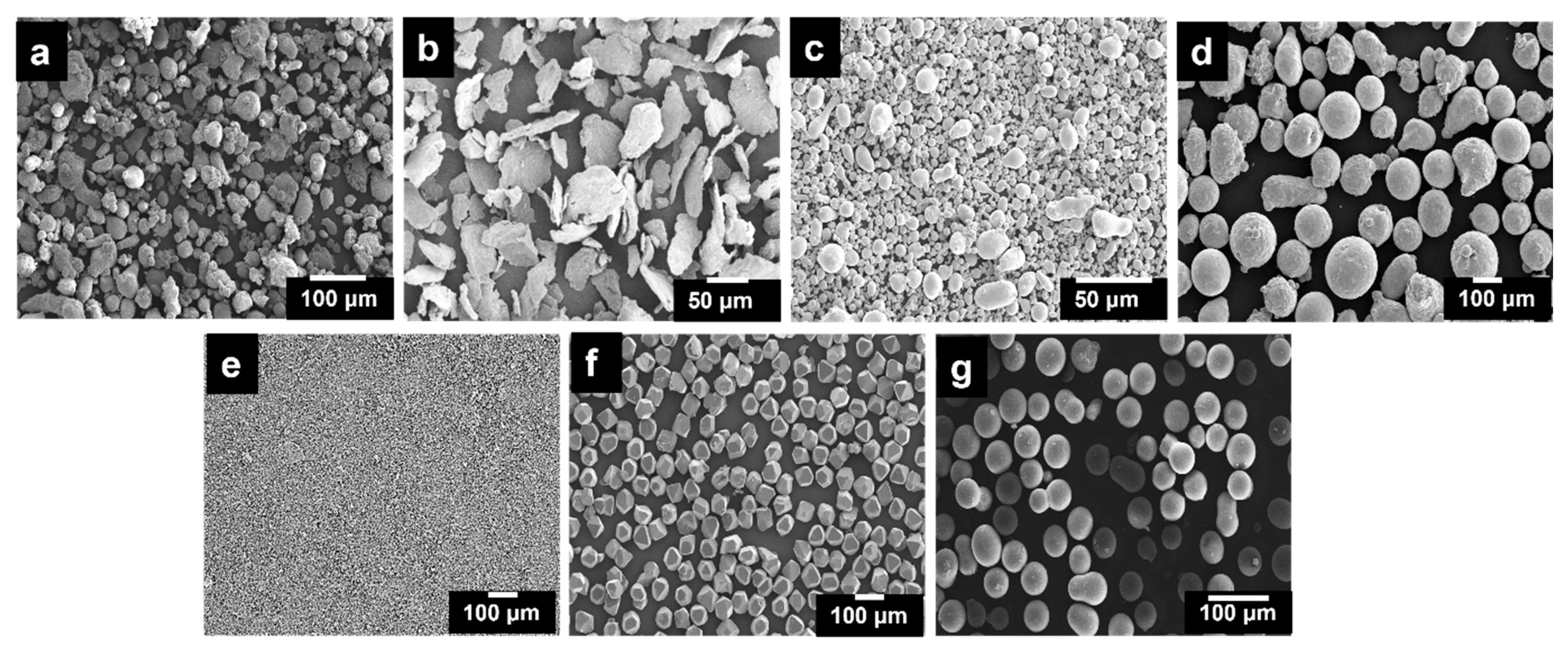

| Constituents | Material | Average Particle Size (µm) | Purity (%) | Wt.% |
|---|---|---|---|---|
| Al | 45 | 99.9 | 94 | |
| Mg | 10 | 99.9 | 1 | |
| Alloy Matrix | Sn | 45 | 99.5 | 2 |
| Cu | 50 | 99.5 | 2 | |
| B | 10 | 99.5 | 1 | |
| Reinforcement | Ti-coated diamond | 45 | 99.5 | 0, 4, 8, 12 and 15 |
| Space holder | PMMA | 150 | 99.9 | 25 |
| wt.% | Porosity (%) | Relative Density (%) |
|---|---|---|
| 0 | 18 | 0.71 |
| 4 | 20 | 0.65 |
| 8 | 24 | 0.63 |
| 12 | 26 | 0.69 |
| 15 | 35 | 0.60 |
| wt.% | Plateau Stress (MPa) | Energy Absorption Capacity (Mj/m3) |
|---|---|---|
| 0 | 19.08 | 4.25 |
| 4 | 20.50 | 4.69 |
| 8 | 20.86 | 5.43 |
| 12 | 31.51 | 7.46 |
| 15 | 17.56 | 3.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveez, B.; Jamal, N.A.; Aabid, A.; Baig, M. Microstructure and Strengthening Effect of Coated Diamond Particles on the Porous Aluminum Composites. Materials 2023, 16, 3240. https://doi.org/10.3390/ma16083240
Parveez B, Jamal NA, Aabid A, Baig M. Microstructure and Strengthening Effect of Coated Diamond Particles on the Porous Aluminum Composites. Materials. 2023; 16(8):3240. https://doi.org/10.3390/ma16083240
Chicago/Turabian StyleParveez, Bisma, Nur Ayuni Jamal, Abdul Aabid, and Muneer Baig. 2023. "Microstructure and Strengthening Effect of Coated Diamond Particles on the Porous Aluminum Composites" Materials 16, no. 8: 3240. https://doi.org/10.3390/ma16083240






