Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
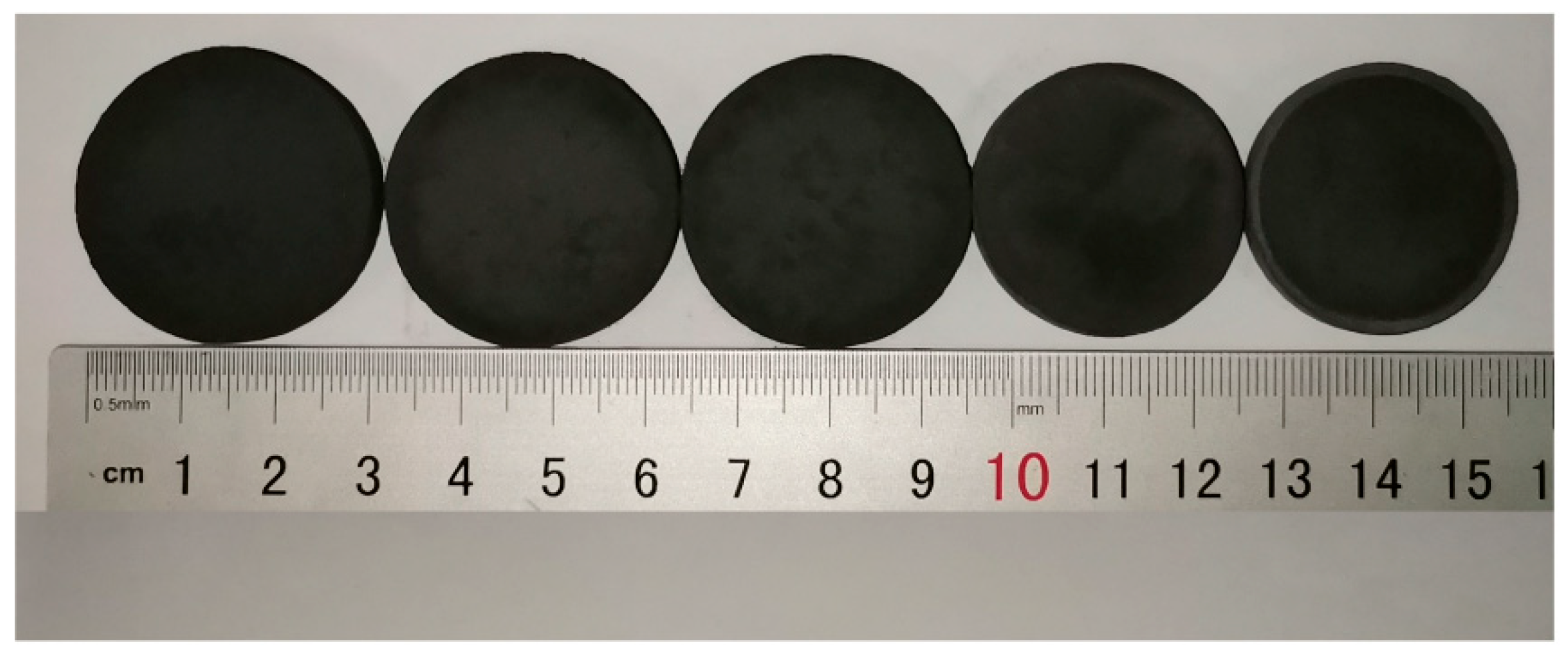
3.1. Macroscopic Comparison of Samples
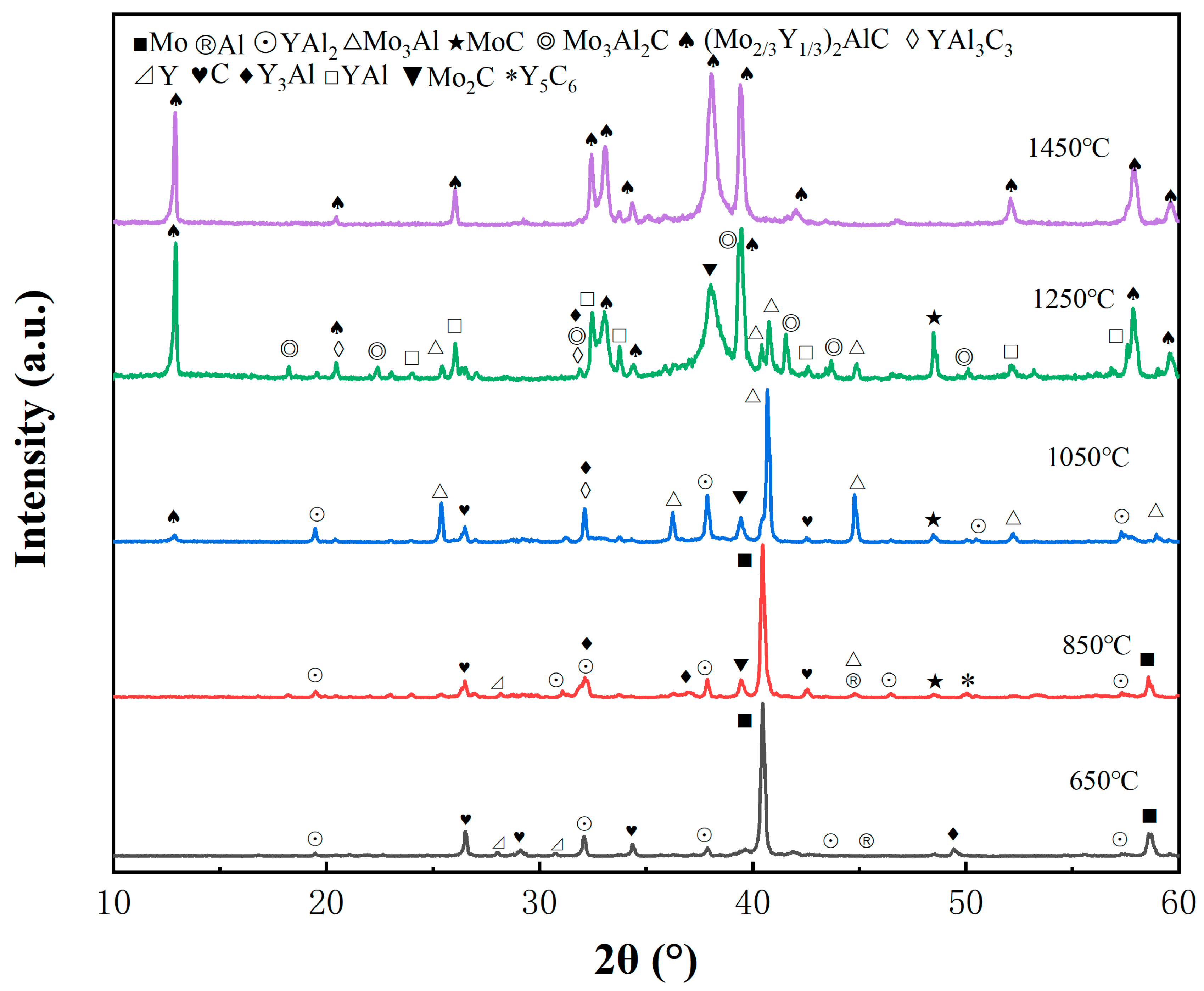
3.2. Effects of Sintering Temperature on the Synthesis of Porous (Mo2/3Y1/3)2AlC Ceramics
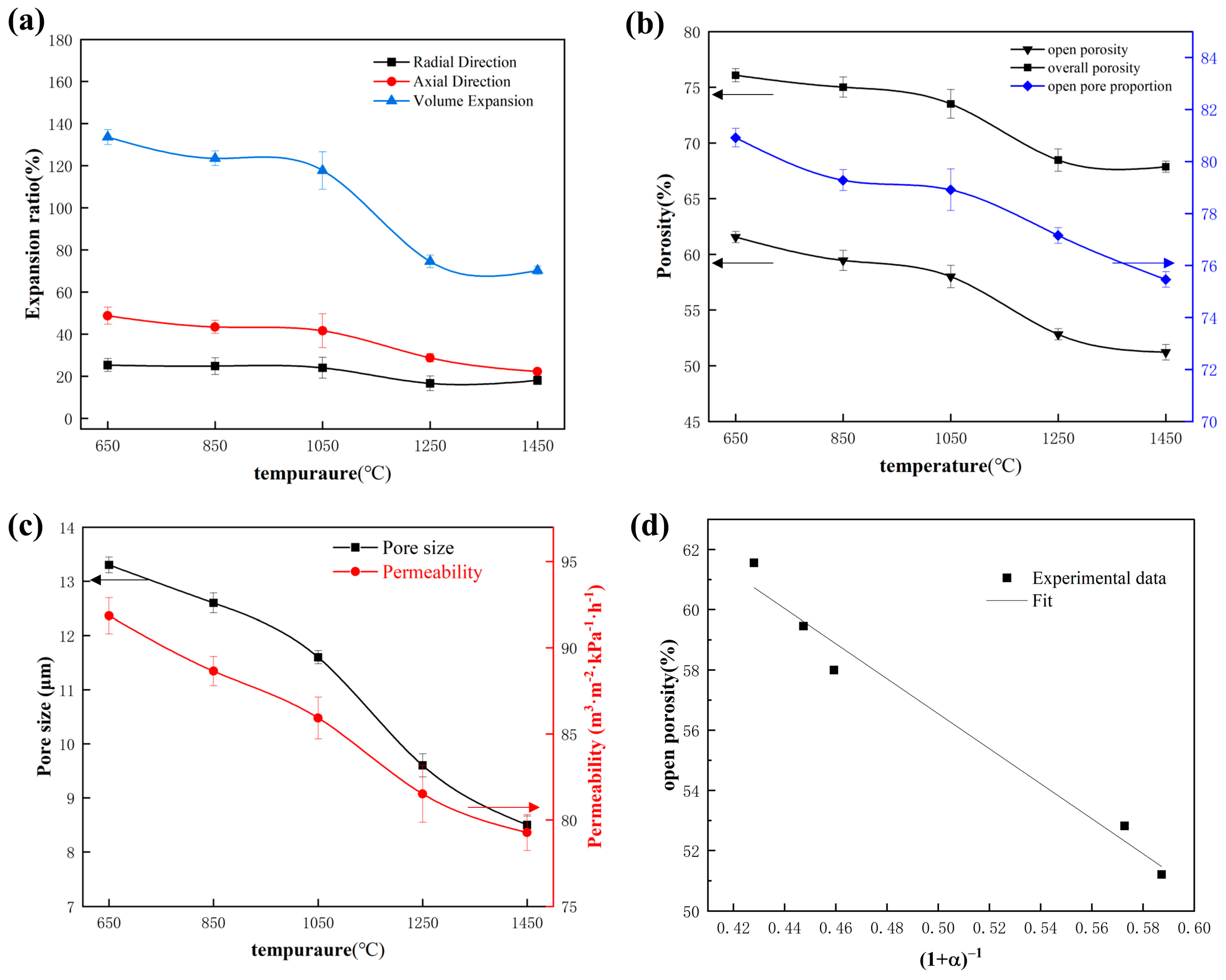
3.3. Effects of Temperature on Volume Evolution and Pore Structure Parameters
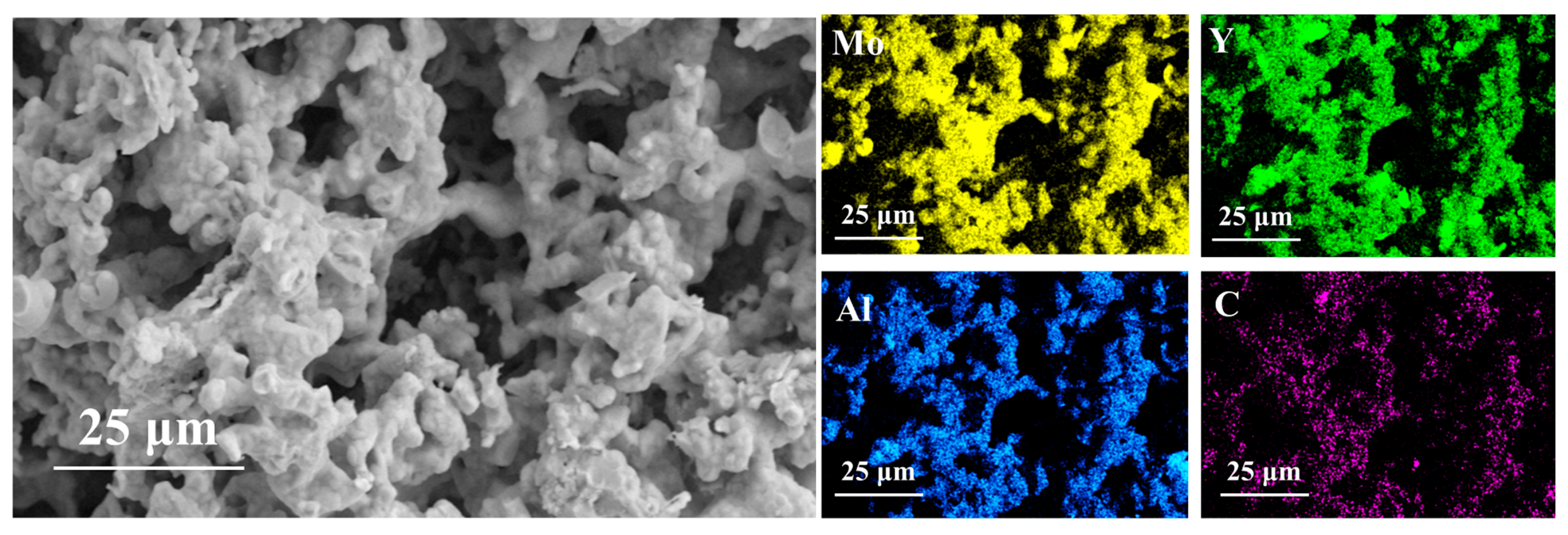
3.4. Micromorphology of Porous (Mo2/3Y1/3)2AlC Ceramics
3.5. Pore-Forming Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barsoum, M.W. The MN+1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Ingason, A.S.; Dahlqvist, M.; Rosen, J. Magnetic MAX phases from theory and experiments; a review. J. Phys. Condens. Matter 2016, 28, 433003. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M. Progress in research and development on MAX phases: A family of layered ternary compounds. Int. Mater. Rev. 2013, 56, 143–166. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Radovic, M. Elastic and Mechanical Properties of the MAX Phases. Annu. Rev. Mater. Res. 2011, 41, 195–227. [Google Scholar] [CrossRef]
- Ching, W.Y.; Mo, Y.; Aryal, S.; Rulis, P.; Zhou, Y. Intrinsic Mechanical Properties of 20 MAX-Phase Compounds. J. Am. Ceram. Soc. 2013, 96, 2292–2297. [Google Scholar] [CrossRef]
- Haftani, M.; Saeedi Heydari, M.; Baharvandi, H.R.; Ehsani, N. Studying the oxidation of Ti 2AlC MAX phase in atmosphere: A review. Int. J. Refract. Met. Hard Mater. 2016, 61, 51–60. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nishina, N.; Hirao, K.; Zhou, Y.; Hyuga, H.; Honda, S.; Iwamoto, Y. Formation mechanism of Ti2AlC under the self-propagating high-temperature synthesis (SHS) mode. Mater. Res. Bull. 2012, 47, 1164–1168. [Google Scholar] [CrossRef]
- Thomas, T.; Bowen, C.R. Effect of particle size on the formation of Ti2AlC using combustion synthesis. Ceram. Int. 2016, 42, 4150–4157. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Wang, X. Progress in machinable and electrically conductive laminated ternary ceramics (MAX Phases). Adv. Ceram. 2017, 38, 3–20. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Miloserdov, P.A.; Sachkova, N.V. High-Temperature Synthesis of Cast Materials Based on the MAX Phase Cr2AlC Using CaCrO4 + Al + C Mixtures. Inorg. Mater. 2020, 56, 321–327. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Luginina, M.A.; Vadchenko, S.G.; Konovalikhin, S.V.; Sychev, A.E.; Shchukin, A.S. Synthesis of a new MAX phase in the Ti–Zr–Al–C system. Mendeleev Commun. 2017, 27, 59–60. [Google Scholar] [CrossRef]
- Zhu, J.F.; Han, N.; Wang, K.; Wang, F. Fabrication of Ti2AlN by Mechanical Alloying and Hot Press Sintering. Adv. Mater. Res. 2011, 194–196, 425–428. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, J.; Jin, Y. Research Progress of Layered Cr2AlC Ternary Ceramic. China Ceram. Ind. 2017, 24, 22–29. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-Assisted Sintering Technology/Spark Plasma Sintering: Mechanisms, Materials, and Technology Developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Oh, H.-C.; Lee, S.-H.; Choi, S.-C. The reaction mechanism for the low temperature synthesis of Cr2AlC under electronic field. J. Alloys Compd. 2014, 587, 296–302. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.H.; Xu, N.P.; Zou, J.; Huang, B.Y.; Liu, C.T. Effects of the Al content on pore structures of porous Ti–Al alloys. Intermetallics 2008, 16, 327–332. [Google Scholar] [CrossRef]
- Hernandez, A. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method. J. Membr. Sci. 1996, 112, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Liao, C.; Wang, J.; Jiang, Y.; He, Y. Reactive synthesis for porous Ti3AlC2 ceramics through TiH2, Al and graphite powders. Ceram. Int. 2014, 40, 6739–6745. [Google Scholar] [CrossRef]
- Aliakbari, A.; Amiri, P.; Dezfuli, A.G. Stability and physical properties of yttrium-based new MAX phases Y2AX (A = Al, Si, Ga, and Ge; X = C and N): A first-principles prediction. Appl. Phys. A 2023, 129, 476. [Google Scholar] [CrossRef]
- ElMelegy, T.A.; Sokol, M.; Barsoum, M.W. Enhanced yield synthesis of bulk dense (M2/3Y1/3)2AlC (M = Cr, W, Mo) in-plane chemically ordered quaternary atomically laminated i-MAX phases and oxidation of (Cr2/3Y1/3)2AlC and (Mo2/3Y1/3)2AlC. J. Alloys Compd. 2021, 867, 158930. [Google Scholar] [CrossRef]
- Watson, R.E.; Weinert, M.; Alatalo, M. Transition-metal aluminide formation: The 4d aluminides. Phys. Rev. B 2001, 65, 014103. [Google Scholar] [CrossRef]
- Chen, M.R.; Jiang, Y.; He, Y.H.; Lin, L.W.; Huang, B.Y.; Liu, C.T. Pore evolution regulation in synthesis of open pore structured Ti–Al intermetallic compounds by solid diffusion. J. Alloys Compd. 2012, 521, 12–15. [Google Scholar] [CrossRef]


| Temperature | Phase | Main Phase |
|---|---|---|
| 650 °C | Mo, Y, Al, C, YAl2 | element powder |
| 850 °C | Mo, Y, Al, C, YAl2, Y3Al, Mo3Al, Y5C6, Mo2C, MoC | element powder |
| 1050 °C | Mo3Al, YAl2, Y3Al, Mo2C, MoC, YAl3C3, (Mo2/3Y1/3)2AlC, C | Mo3Al, YAl2 |
| 1250 °C | (Mo2/3Y1/3)2AlC, YAl, Mo2C, MoC, YAl3C3, Mo3Al2C, Y3Al, Mo3Al | YAl, Mo2C, (Mo2/3Y1/3)2AlC |
| 1450 °C | (Mo2/3Y1/3)2AlC, Mo3Al2C, YAl3C3, Y3Al, YAl, Mo2C, MoC, Mo3Al | (Mo2/3Y1/3)2AlC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Xiao, G.; Wang, B.; Yu, K.; Li, J.; Jiang, W.; Zhang, H.; Yang, X.; Yang, J. Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders. Materials 2024, 17, 3272. https://doi.org/10.3390/ma17133272
Tan S, Xiao G, Wang B, Yu K, Li J, Jiang W, Zhang H, Yang X, Yang J. Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders. Materials. 2024; 17(13):3272. https://doi.org/10.3390/ma17133272
Chicago/Turabian StyleTan, Siwei, Gan Xiao, Baogang Wang, Kui Yu, Jie Li, Wenkai Jiang, Heng Zhang, Xuejin Yang, and Junsheng Yang. 2024. "Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders" Materials 17, no. 13: 3272. https://doi.org/10.3390/ma17133272
APA StyleTan, S., Xiao, G., Wang, B., Yu, K., Li, J., Jiang, W., Zhang, H., Yang, X., & Yang, J. (2024). Reactive Synthesis for Porous (Mo2/3Y1/3)2AlC Ceramics through Mo, Y, Al and Graphite Powders. Materials, 17(13), 3272. https://doi.org/10.3390/ma17133272





