CO2 Sorption on Ti-, Zr-, and [Ti,Zr]-Pillared Montmorillonites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Analysis
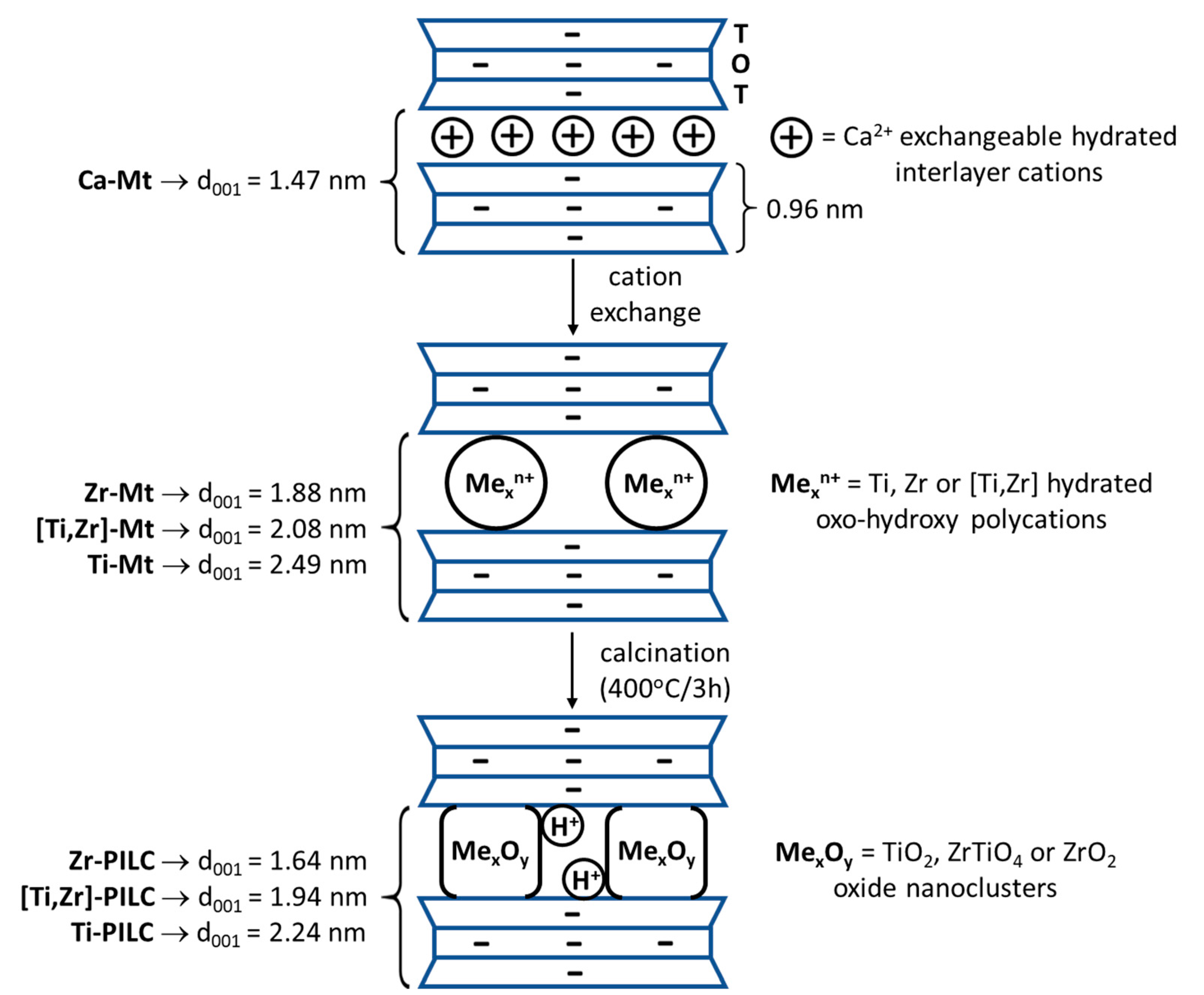
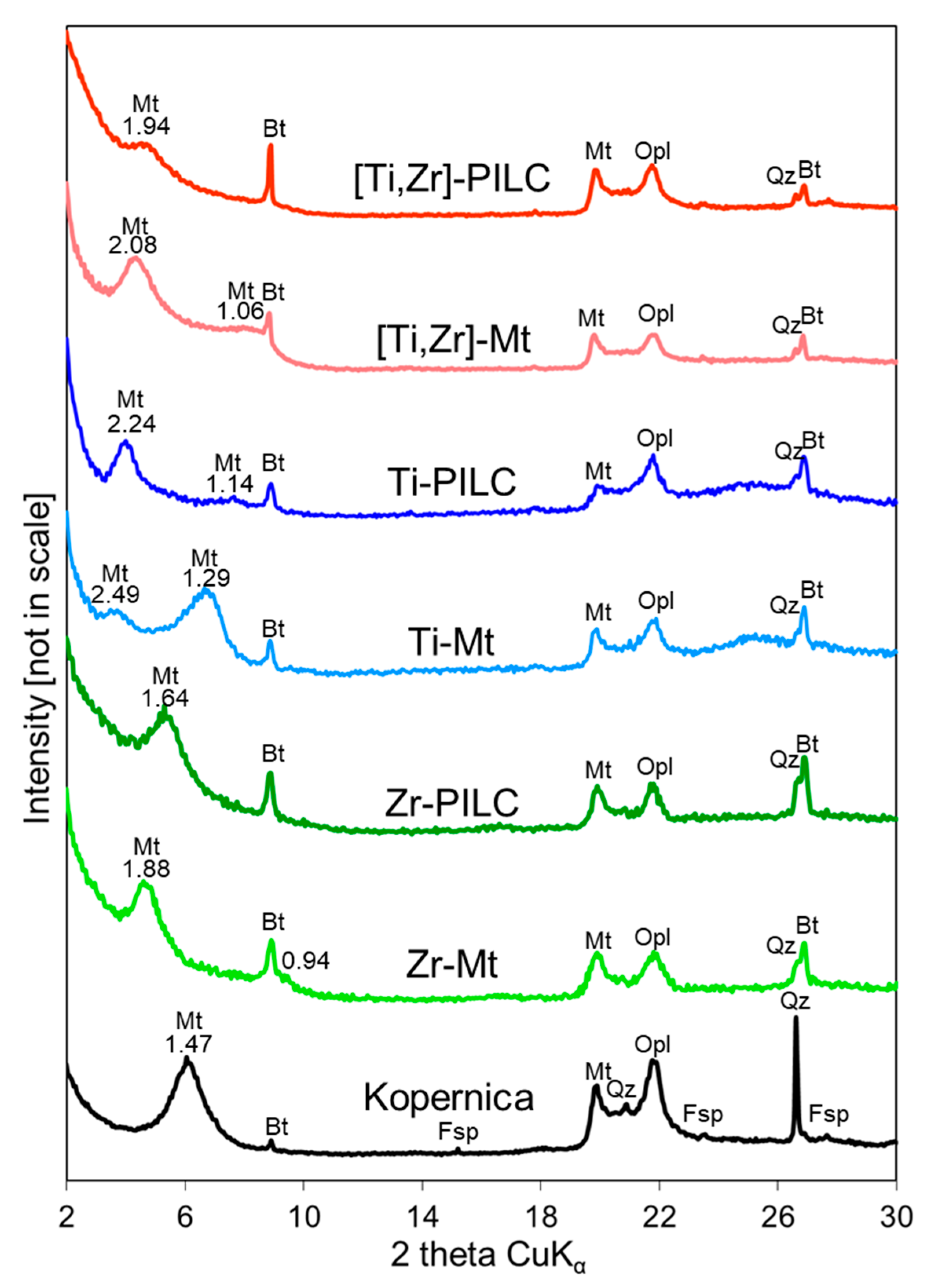
3.2. XRD Analysis
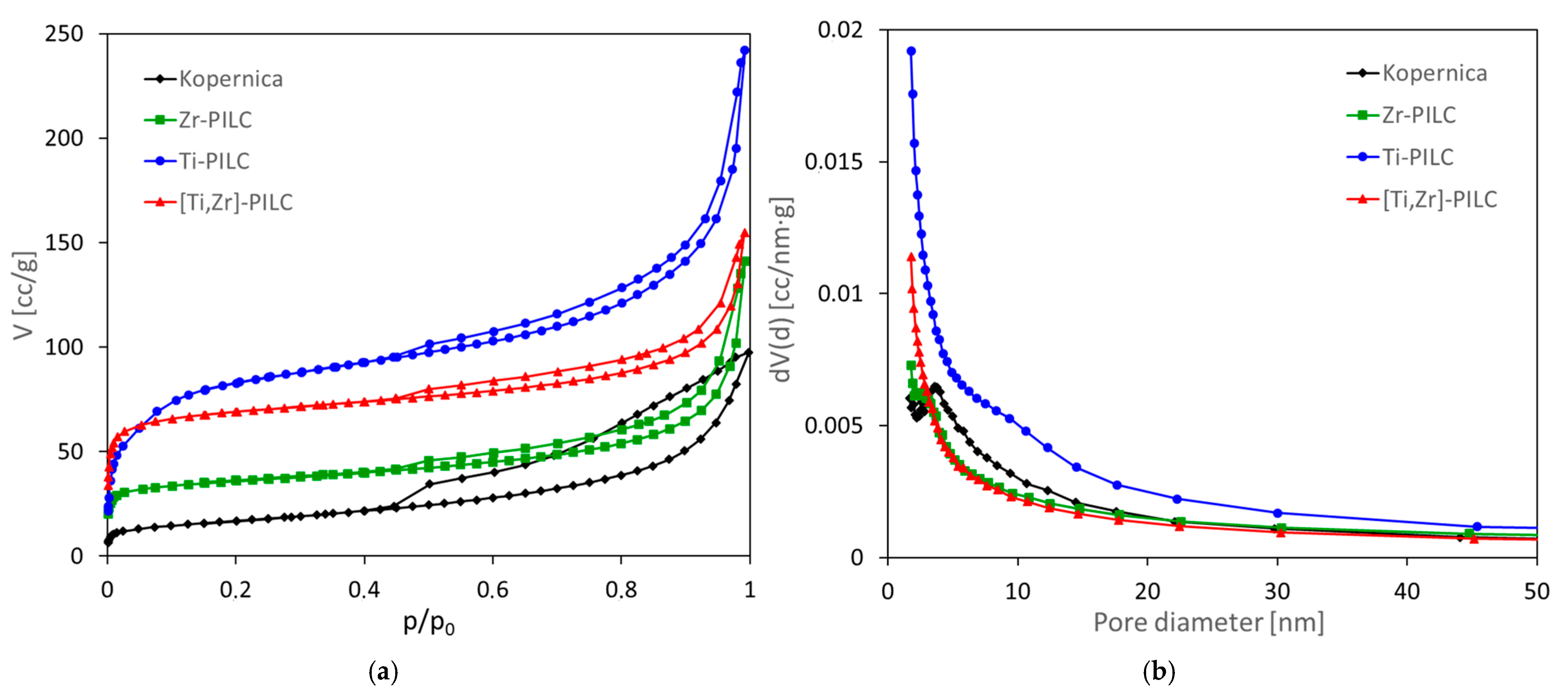
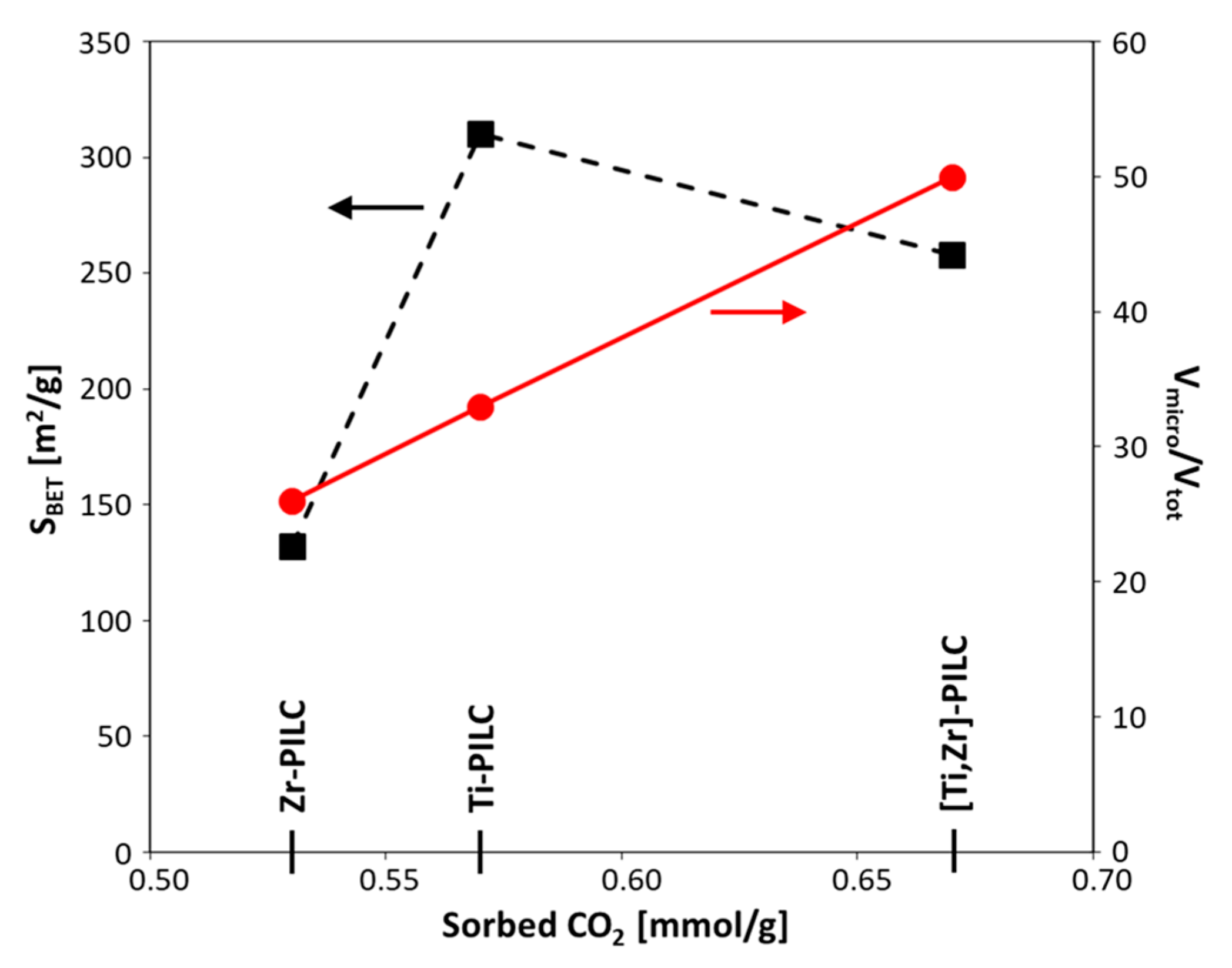
3.3. Textural Analysis
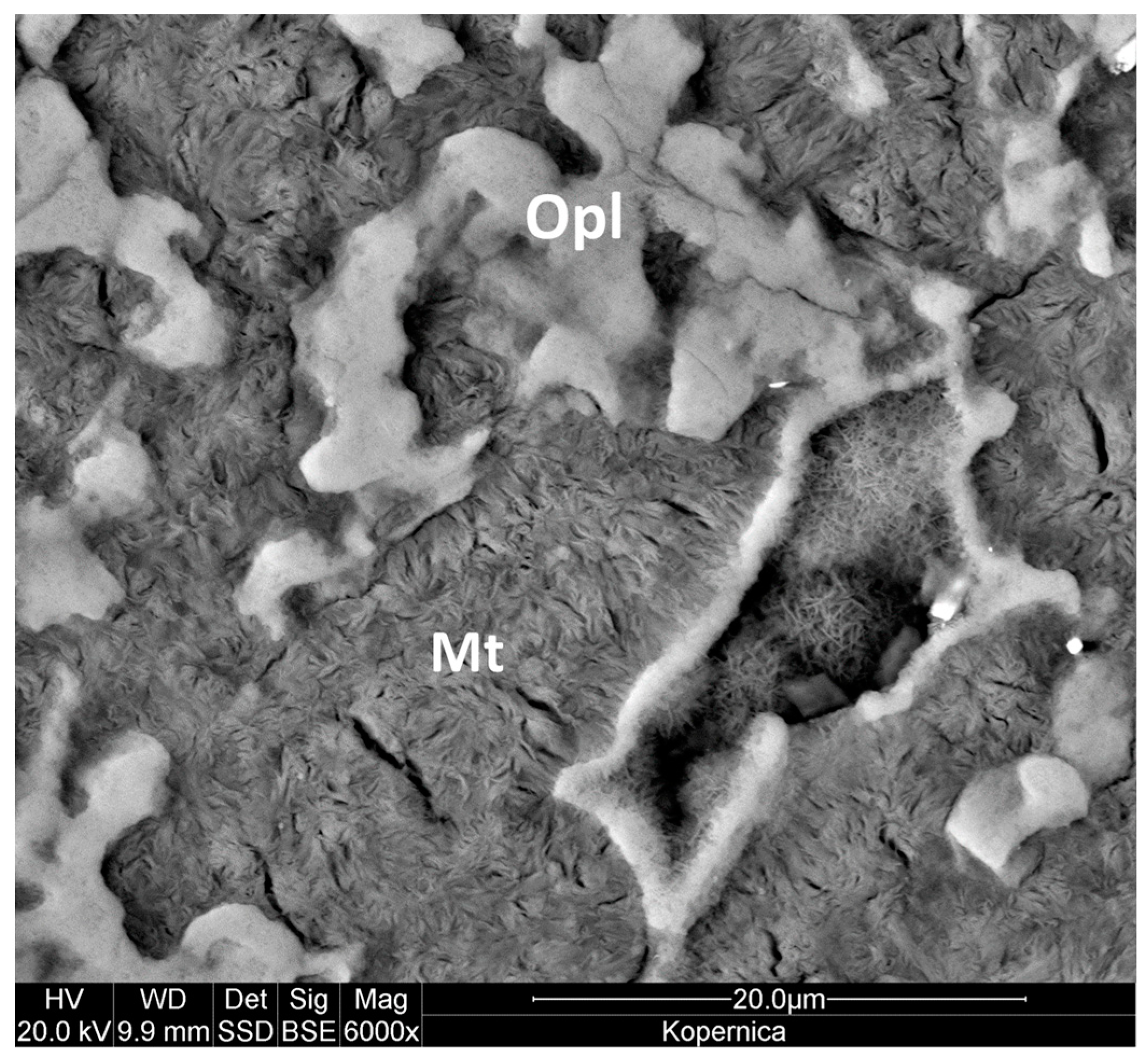
3.4. FESEM Analysis
3.5. CO2 Sorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergaya, F.; Lagaly, G. (Eds.) General Introduction: Clays, Clay Minerals, and Clay Science. In Developments in Clay Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5A, pp. 1–19. [Google Scholar]
- Borah, D.; Nath, H.; Saikia, H. Modification of bentonite clay & its applications: A review. Rev. Inorg. Chem. 2022, 42, 265–282. [Google Scholar]
- Starý, J.; Jirásek, J.; Pticen, F.; Zahradník, J.; Sivek, M. Review of production, reserves, and processing of clays (including bentonite) in the Czech Republic. Appl. Clay Sci. 2021, 205, 106049. [Google Scholar] [CrossRef]
- Abdulkareem, M.A.; Al-Kaabi, F.S.; Muhsin, N.A.; Badr, N.D.; Radhi, D.J. Activation of calcium bentonite for use as a drilling fluid: Physical and chemical methods evaluation. Case Stud. Chem. Environ. Eng. 2024, 9, 100587. [Google Scholar] [CrossRef]
- Vicente, M.A.; Gil, A.; Bergaya, F. Pillared Clays and Clay Minerals. In Developments in Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 523–667. [Google Scholar]
- Gil, A.; Vicente, M.A. Progress and perspectives on pillared clays applied in energetic and environmental remediation processes. Curr. Opin. Green Sustain. Chem. 2020, 21, 56–63. [Google Scholar] [CrossRef]
- Baloyi, J.; Ntho, T.; Moma, J. Synthesis and application of pillared clay heterogeneous catalysts for wastewater treatment: A review. RSC Adv. 2018, 8, 5197–5211. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Util. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for Direct Capture of CO2 from Ambient Air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Chemically modified carbonaceous adsorbents for enhanced CO2 capture: A review. J. Clean. Prod. 2021, 290, 125776. [Google Scholar] [CrossRef]
- Halliday, C.; Hatton, T.A. Sorbents for the Capture of CO2 and Other Acid Gases: A Review. Ind. Eng. Chem. Res. 2021, 60, 9313–9346. [Google Scholar] [CrossRef]
- Chang, R.; Wu, X.; Cheung, O.; Liu, W. Synthetic solid oxide sorbents for CO2 capture: State-of-the art and future perspectives. J. Mater. Chem. A 2022, 10, 1682. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 capture materials: A review of current trends and future challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Volzone, C. Retention of pollutant gases: Comparison between clay minerals and their modified products. Appl. Clay Sci. 2007, 36, 191–196. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.J. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation. Chem. Eng. J. 2021, 420, 130421. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Baksh, M.S.A.; Yang, R.T. Unique adsorption properties and potential energy profiles of microporous pillared clays. AIChE J. 1992, 38, 1357–1368. [Google Scholar] [CrossRef]
- Molinard, A.; Vansant, E.F. Controlled gas adsorption properties of various pillared clays. Adsorption 1995, 1, 49–59. [Google Scholar] [CrossRef]
- Pereira, P.R.; Pires, J.; Brotas de Carvalho, M. Zirconium pillared clays for carbon dioxide/methane separation. 1. Preparation of adsorbent materials and pure gas adsorption. Langmuir 1998, 14, 4584–4588. [Google Scholar] [CrossRef]
- Gil, A.; Gandía, L.M. Microstructure and quantitative estimation of the micropore-size distribution of an alumina-pillared clay from nitrogen adsorption at 77K and carbon dioxide adsorption at 273K. Chem. Eng. Sci. 2003, 58, 3059–3075. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, J.; Cao, D. Adsorption of carbon dioxide of 1-site and 3-site models in pillared clays: A Gibbs ensemble Monte Carlo simulation. J. Coll. Interface Sci. 2007, 310, 391–401. [Google Scholar] [CrossRef]
- Garcés-Polo, S.I.; Villarroel-Rocha, J.; Sapag, K.; Korili, S.A.; Gil, A. A comparative study of CO2 diffusion from adsorption kinetic measurements on microporous materials at low pressures and temperatures. Chem. Eng. J. 2016, 302, 278–286. [Google Scholar] [CrossRef]
- Wang, K.; Yan, X.; Komarneni, S. CO2 Adsorption by Several Types of Pillared Montmorillonite Clays. Appl. Petrochem. Res. 2018, 8, 173–177. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghhaier, S.; Chlendi, M.; Duro, F.I.F.; Castellon, E.R.; Bagane, M. CO2 adsorption of materials synthesized from clay minerals: A review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Wu, K.; Ye, Q.; Wu, R.; Dai, H. Alkali metal-promoted aluminum-pillared montmorillonites: High-performance CO2 adsorbents. J. Solid State Chem. 2020, 291, 121585. [Google Scholar] [CrossRef]
- Wu, K.; Ye, Q.; Wu, R.; Chen, S.; Dai, H. Carbon dioxide adsorption behaviors of aluminum-pillared montmorillonite-supported alkaline earth metals. J. Environ. Sci. 2020, 98, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Qian, X.; Zhou, Y.; Cheng, H. Research progress of clay minerals in carbon dioxide capture. Renew. Sustain. Energy Rev. 2022, 164, 112536. [Google Scholar] [CrossRef]
- Wal, K.; Rutkowski, P.; Stawinski, W. Application of clay minerals and their derivatives in adsorption from gaseous phase. Appl. Clay Sci. 2021, 215, 106323. [Google Scholar] [CrossRef]
- Tingelinhas, J.; Saragoça, C.; Al Mohtar, A.; Mateus, M.; Pinto, M.L. Pillared clays as cost-efective adsorbents for carbon capture by pressure swing adsorption processes in the cement industry. Ind. Eng. Chem. Res. 2023, 62, 5613–5623. [Google Scholar] [CrossRef]
- Aneja, R.; Chauhan, A.; Chauhan, T.; Vyas, R.; Saini, V.K. Understanding adsorption selectivity in zirconium-pillared clays for biogas upgradation: The role of metal/clay ratio. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Bahranowski, K.; Włodarczyk, W.; Wisła-Walsh, E.; Gaweł, A.; Matusik, J.; Klimek, A.; Gil, B.; Michalik-Zym, A.; Dula, R.; Socha, R.P.; et al. [Ti,Zr]-pillared montmorillonite-A new quality with respect to Ti- and Zr-pillared clays. Micropor. Mesopor. Mater. 2015, 202, 155–164. [Google Scholar] [CrossRef]
- Michalik-Zym, A.; Dula, R.; Duraczyńska, D.; Kryściak-Czerwenka, J.; Machej, T.; Socha, R.P.; Włodarczyk, W.; Gaweł, A.; Matusik, J.; Bahranowski, K.; et al. Active, selective and robust Pd and/or Cr catalysts supported on Ti-, Zr- or [Ti,Zr]-pillared montmorillonites for destruction of chlorinated volatile organic compounds. Appl. Catal. B Environ. 2015, 174–175, 293–307. [Google Scholar] [CrossRef]
- Bahranowski, K.; Klimek, A.; Gaweł, A.; Górniak, K.; Michalik-Zym, A.; Serwicka-Bahranowska, E. Structural transformations of hydrolysates obtained from Ti-, Zr-, and Ti, Zr-solutions used for clay pillaring: Towards understanding of the mixed pillars nature. Materials 2018, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Górniak, K.; Szydłak, T.; Gaweł, A.; Klimek, A.; Tomczyk, A.; Sulikowski, B.; Olejniczak, Z.; Motyka, J.; Serwicka, E.M.; Bahranowski, K. Commercial bentonite from the Kopernica deposit (Tertiary, Slovakia): A petrographic and mineralogical approach. Clay Miner. 2016, 51, 97–122. [Google Scholar] [CrossRef]
- González, B.; Pérez, A.H.; Trujillano, R.; Gil, A.; Vicente, M.A. Microwave-Assisted Pillaring of a Montmorillonite with Al-Polycations in Concentrated Media. Materials 2017, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Pacula, A.; Bielanska, E.; Gaweł, A.; Bahranowski, K.; Serwicka, E. Textural effects in powdered montmorillonite induced by freeze-drying and ultrasound pretreatment. Appl. Clay Sci. 2006, 32, 64–72. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, J.; Xing, W.; Xue, Q.; Yan, Z.; Zhuo, S.; Qiao, S.Z. Critical role of small micropores in high CO2 uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523–2529. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Johnston, C.T.; Bergaya, F. Clay minerals and their surfaces. In Surface and Interface Chemistry of Clay Minerals; Schoonheydt, R., Johnston, C.T., Bergaya, F., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–21. [Google Scholar]
- Cecilia, J.A.; Vilarrasa-García, E.; Cavalcante, C.L.; Azevedo, D.C.S.; Franco, F.; Rodríguez-Castellón, E. Evaluation of two fibrous clay minerals (sepiolite and palygorskite) for CO2 capture. J. Environ. Chem. Eng. 2018, 6, 4573–4587. [Google Scholar] [CrossRef]
- Elkhalifah, A.E.I.; Maitra, S.; Bustam, M.A.; Murugesan, T. Effects of exchanged ammonium cations on structure characteristics and CO2 adsorption capacities of bentonite clay. Appl. Clay Sci. 2013, 83–84, 391–398. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Bielańska, E.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Sulikowski, B.; Socha, R.P.; Gaweł, A.; Bahranowski, K.; Olejniczak, Z.; et al. New insight into the preferred valency of interlayer anions in hydrotalcite-like compounds: The effect of Mg/Al ratio. Appl. Clay Sci. 2018, 155, 84–94. [Google Scholar] [CrossRef]
- Li, M.; Tumuluri, U.; Wu, Z.; Dai, S. Effect of Dopants on the Adsorption of Carbon Dioxide on Ceria Surfaces. ChemSusChem 2015, 8, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Yuan, M.; Su, Y.; Heynderickx, P.M.; Memon, A. A review on pillared clay-based catalysts for low-temperature selective catalytic reduction of NOx with hydrocarbons. Appl. Clay Sci. 2023, 233, 106847. [Google Scholar] [CrossRef]



| Sample | SiO2 [wt.%] | Al2O3 [wt.%] | MgO [wt.%] | Fe2O3 [wt.%] | CaO [wt.%] | Na2O [wt.%] | K2O [wt.%] | TiO2 [wt.%] | ZrO2 [wt.%] |
|---|---|---|---|---|---|---|---|---|---|
| Kopernica | 70.95 | 21.04 | 2.71 | 2.37 | 2.09 | 0.06 | 0.58 | 0.16 | 0.05 |
| Zr-PILC | 59.95 | 19.30 | 2.02 | 2.01 | 0.04 | 0.05 | 0.40 | 0.13 | 16.08 |
| Ti-PILC | 43.63 | 13.31 | 1.36 | 1.42 | 0.07 | 0.04 | 0.28 | 39.76 | 0.03 |
| [Ti,Zr]-PILC | 53.54 | 17.33 | 1.86 | 1.78 | 0.04 | 0.06 | 0.29 | 9.41 | 15.64 |
| Sample | 001 Reflection Maximum [° 2θ] | 001 Reflection FWHM [° 2θ] | d001 [nm] |
|---|---|---|---|
| Kopernica | 6.03 | 1.20 | 1.47 |
| Zr-Mt | 4.70 | 0.94 | 1.88 |
| Zr-PILC | 5.38 | 1.17 | 1.64 |
| Ti-Mt | 3.55 | 0.92 | 2.49 |
| Ti-PILC | 3.94 | 0.87 | 2.24 |
| [Ti,Zr]-Mt | 4.25 | 1.00 | 2.08 |
| [Ti,Zr]-PILC | 4.55 | 1.07 | 1.94 |
| Sample | SBET [m2/g] | Vtot [cm3/g] | Vmicro [cm3/g] | Vmicro/Vtot | Vmeso [cm3/g] | Vmeso/Vtot | Vmacro [cm3/g] | Vmacro/Vtot | Dav [nm] | d001 [nm] | CO2 [mmol/g] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kopernica | 52 | 0.17 | 0.02 | 0.12 | 0.11 | 0.65 | 0.04 | 0.23 | 13.2 | 1.47 | 0.16 |
| Zr-PILC | 132 | 0.21 | 0.06 | 0.26 | 0.09 | 0.45 | 0.06 | 0.29 | 6.3 | 1.65 | 0.53 |
| Ti-PILC | 310 | 0.36 | 0.12 | 0.33 | 0.17 | 0.46 | 0.07 | 0.20 | 4.7 | 2.24 | 0.57 |
| [Ti,Zr]-PILC | 258 | 0.23 | 0.12 | 0.50 | 0.09 | 0.37 | 0.03 | 0.12 | 3.6 | 1.94 | 0.67 |
| Pillared Clay | Clay Provenience/ Pretreatment | CO2 Adsorption Capacity (mmol CO2/g) | Reference |
|---|---|---|---|
| Al-PILC | Montmorillonite Tsukinuno, Japan/ purified | 1.0 | [23] |
| Al-PILC | Montmorillonite Kunipia, Japan/ Na-exchanged | 0.8 | [24] |
| Al-PILC | Montmorillonite Wyoming, USA/ Na-exchanged | 0.6 | [24] |
| Zr-PILC | Montmorillonite Tsukinuno, Japan/ purified | 0.8 | [23] |
| Zr-PILC | Bentonite Benavila-Alentejo, Portugal/ purified, Na-exchanged | 1.5 | [20] |
| Zr-PILC | Montmorillonite Wyoming, USA/ Na-exchanged | 0.5 | [24] |
| (TiO2–SiO2)-PILC | Montmorillonite Wyoming, USA/ Na-exchanged | 1.2 | [24] |
| Zr-PILC | Industrial bentonite Kopernica, Slovakia/ as received | 0.5 | This work |
| Ti-PILC | Industrial bentonite Kopernica, Slovakia/ as received | 0.6 | This work |
| [Ti,Zr]-PILC | Industrial bentonite Kopernica, Slovakia/ as received | 0.7 | This work |
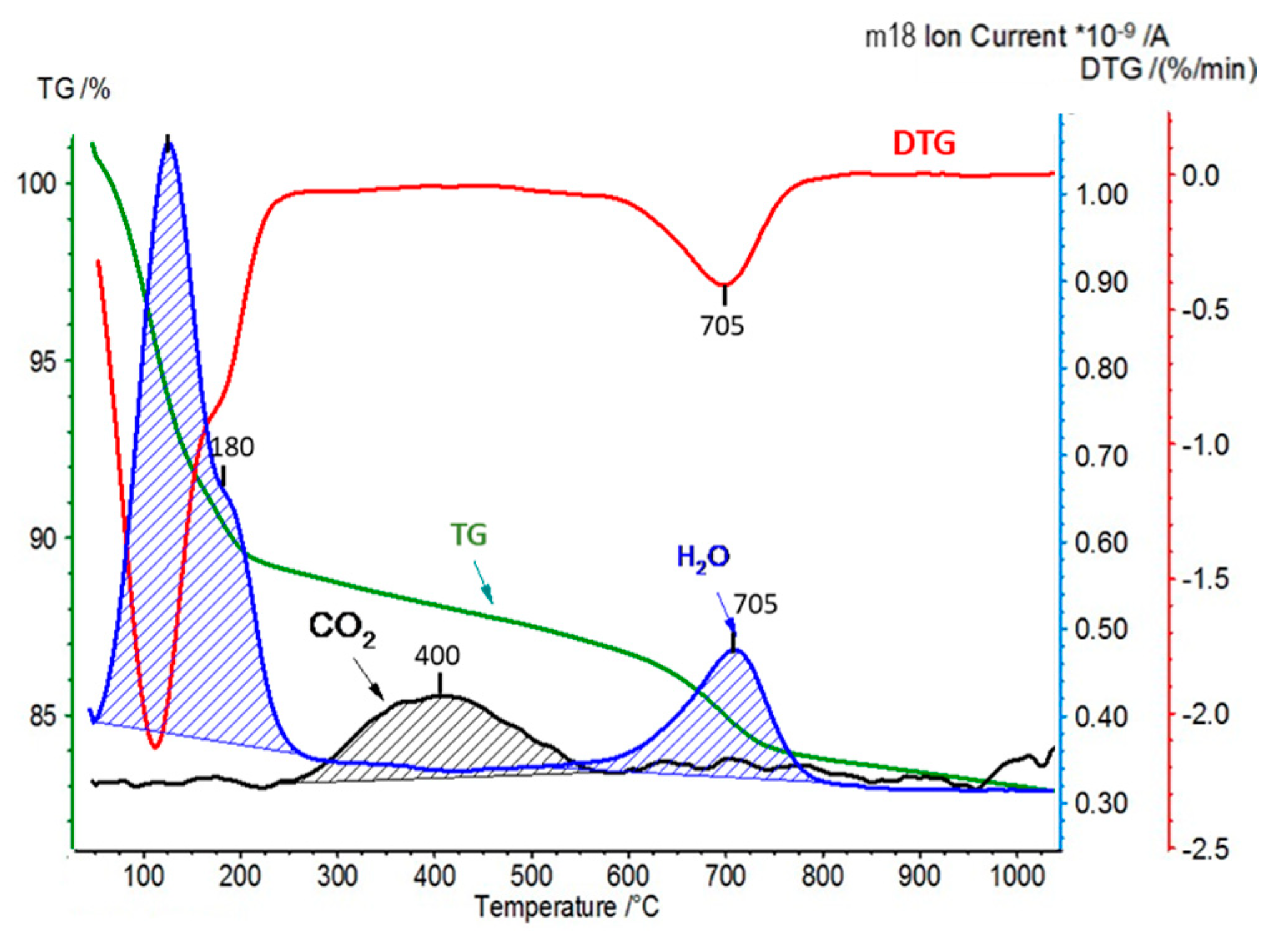
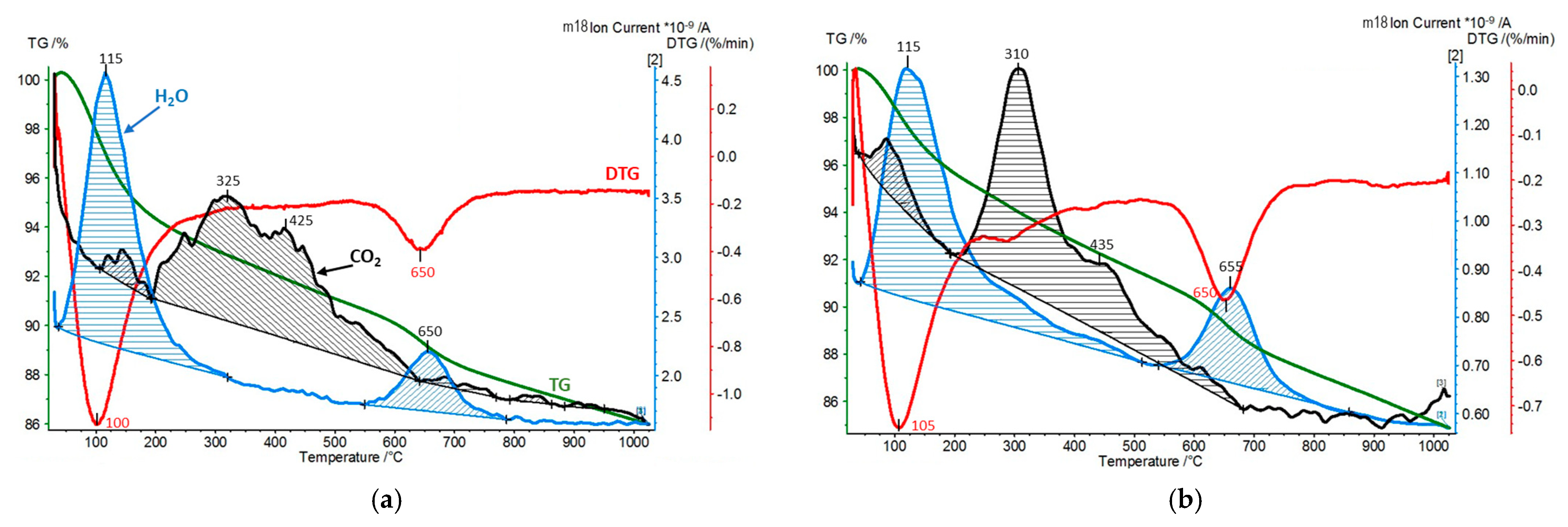
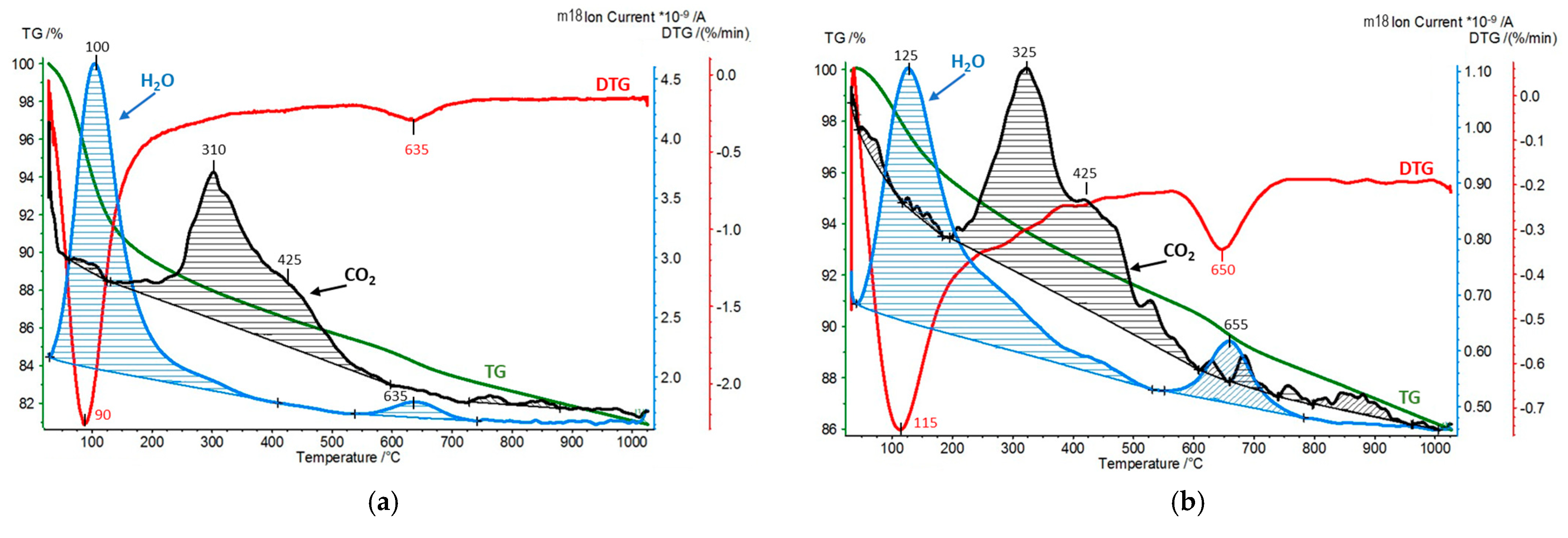
| Sample | CO2 Desorption/QMS [mmol/g] | H2O Hydration/TG [wt.%] | H2O Dehydroxylation/TG [wt.%] |
|---|---|---|---|
| Kopernica | 0.10 | 12.0 | 4.0 |
| Zr-PILC | 0.04 | 6.3 | 2.9 |
| Zr-PILC exposed to dry CO2 | 0.03 | 5.0 | 4.0 |
| Ti-PILC | 0.04 | 11.1 | 2.4 |
| Ti-PILC exposed to dry CO2 | 0.02 | 5.1 | 3.0 |
| [Ti,Zr]-PILC | 0.05 | 10.8 | 3.1 |
| [Ti,Zr]-PILC exposed to dry CO2 | 0.01 | 5.0 | 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek, A.; Gaweł, A.; Górniak, K.; Tomczyk-Chmiel, A.; Serwicka, E.M.; Bahranowski, K. CO2 Sorption on Ti-, Zr-, and [Ti,Zr]-Pillared Montmorillonites. Materials 2024, 17, 4036. https://doi.org/10.3390/ma17164036
Klimek A, Gaweł A, Górniak K, Tomczyk-Chmiel A, Serwicka EM, Bahranowski K. CO2 Sorption on Ti-, Zr-, and [Ti,Zr]-Pillared Montmorillonites. Materials. 2024; 17(16):4036. https://doi.org/10.3390/ma17164036
Chicago/Turabian StyleKlimek, Agnieszka, Adam Gaweł, Katarzyna Górniak, Anna Tomczyk-Chmiel, Ewa M. Serwicka, and Krzysztof Bahranowski. 2024. "CO2 Sorption on Ti-, Zr-, and [Ti,Zr]-Pillared Montmorillonites" Materials 17, no. 16: 4036. https://doi.org/10.3390/ma17164036






