Effect of Electrodeposition Conditions on Adsorption and Photocatalytic Properties of ZnO
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Electrodeposition of ZnO Nanostructured Coatings
2.3. Characterization
2.4. Photocatalytic Measurements
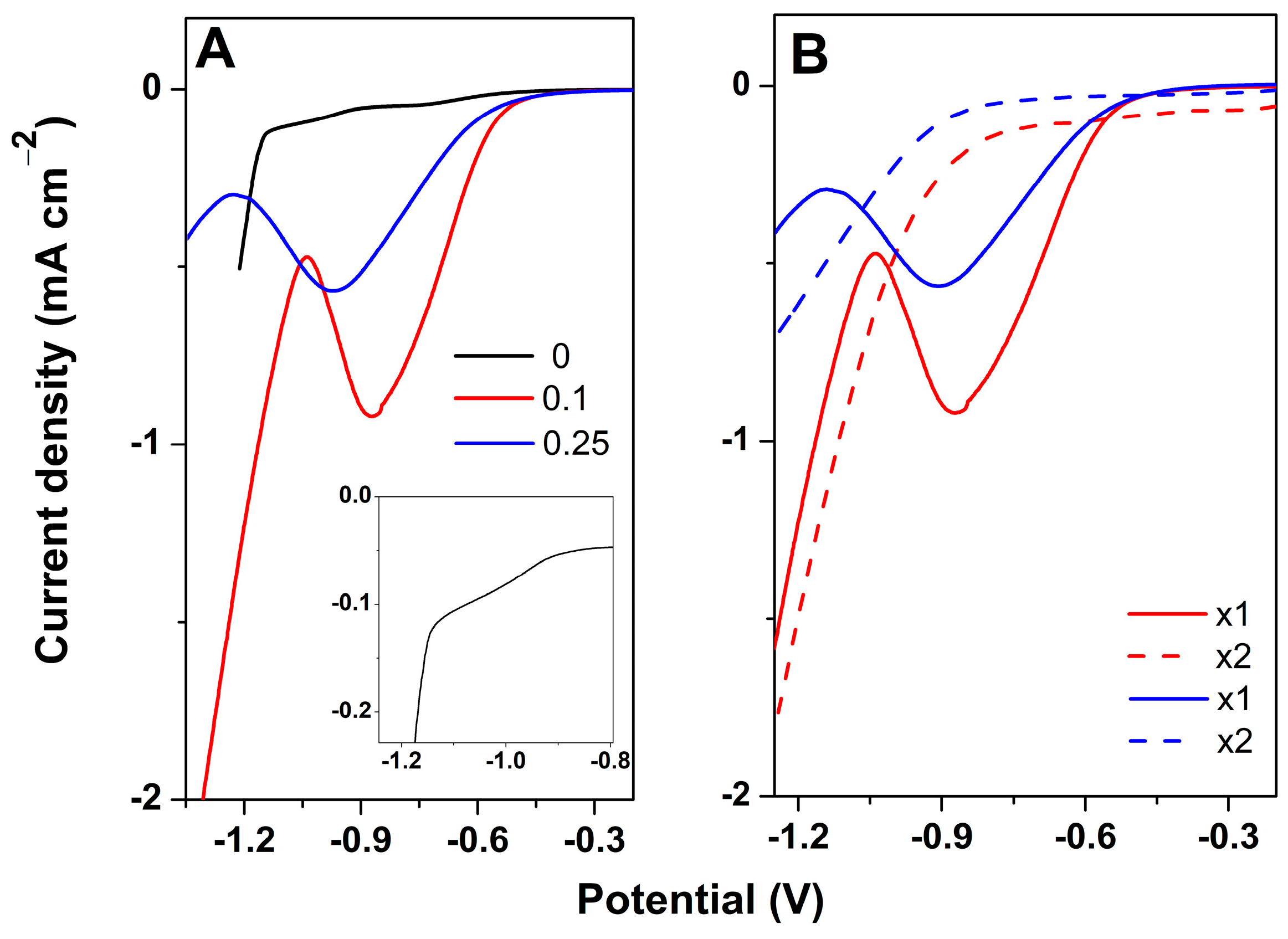
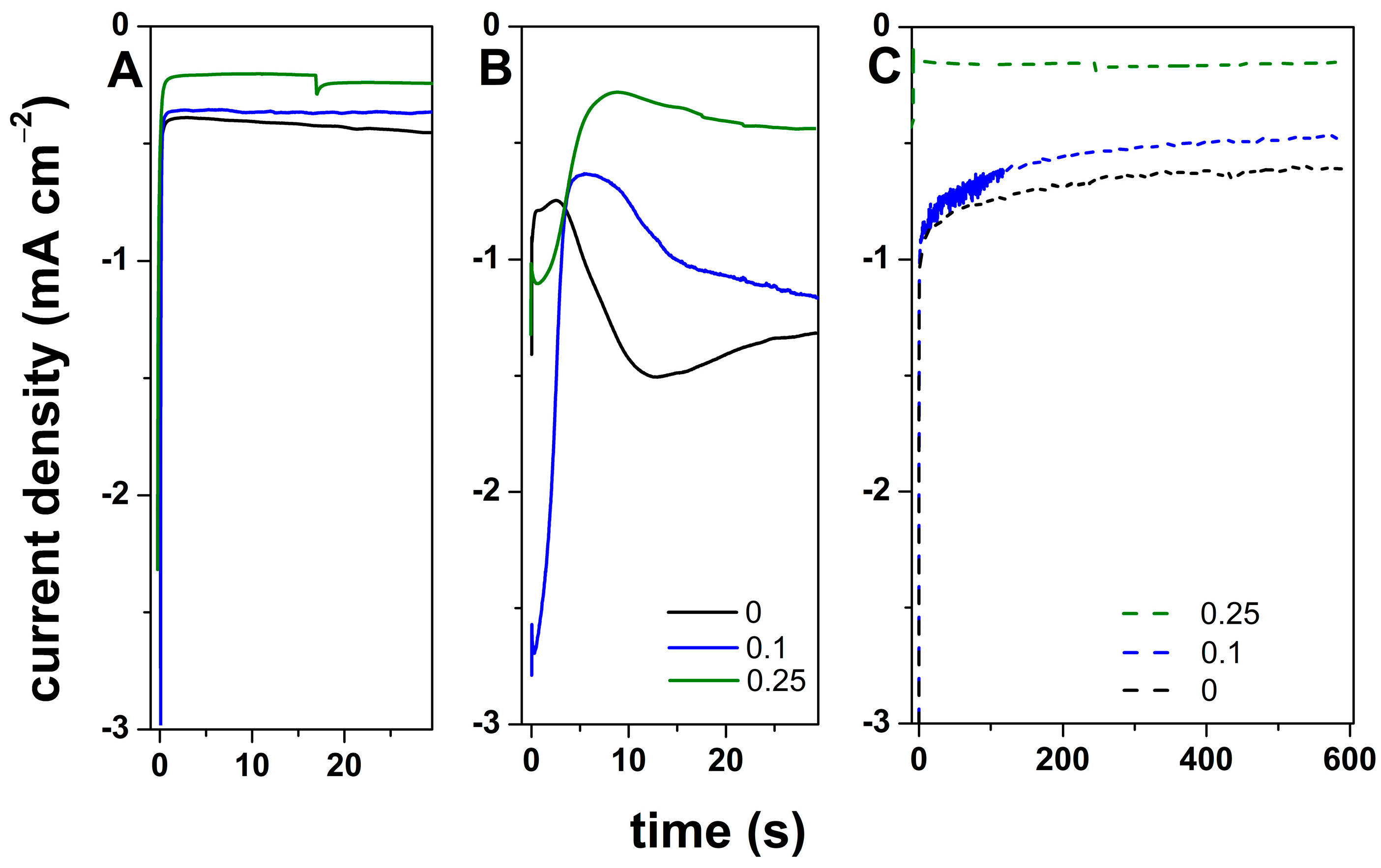
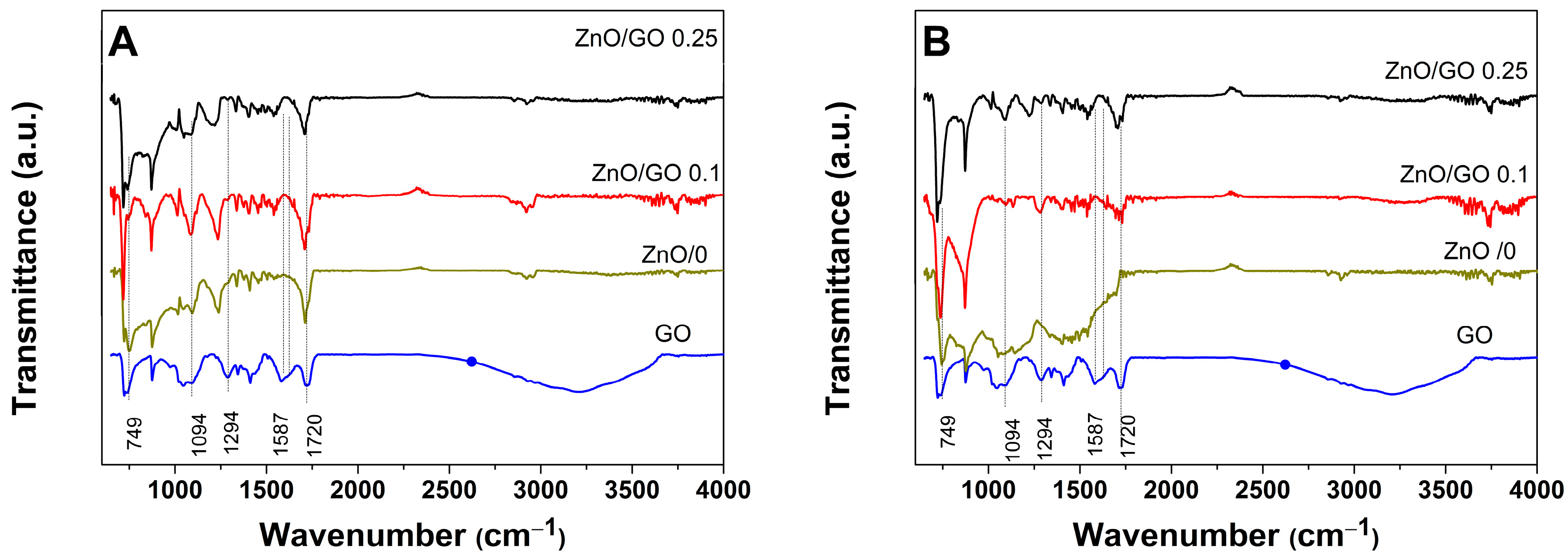
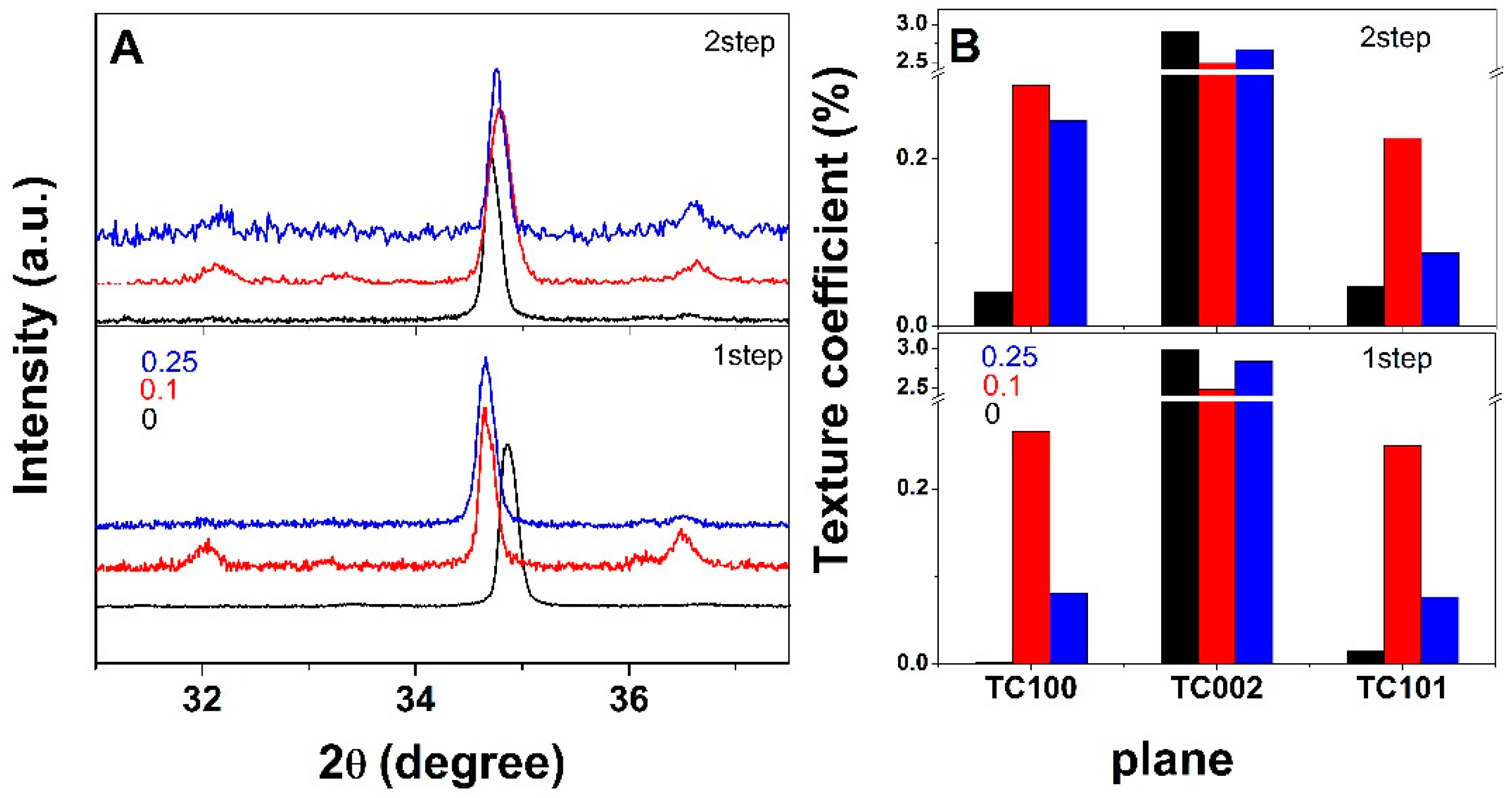
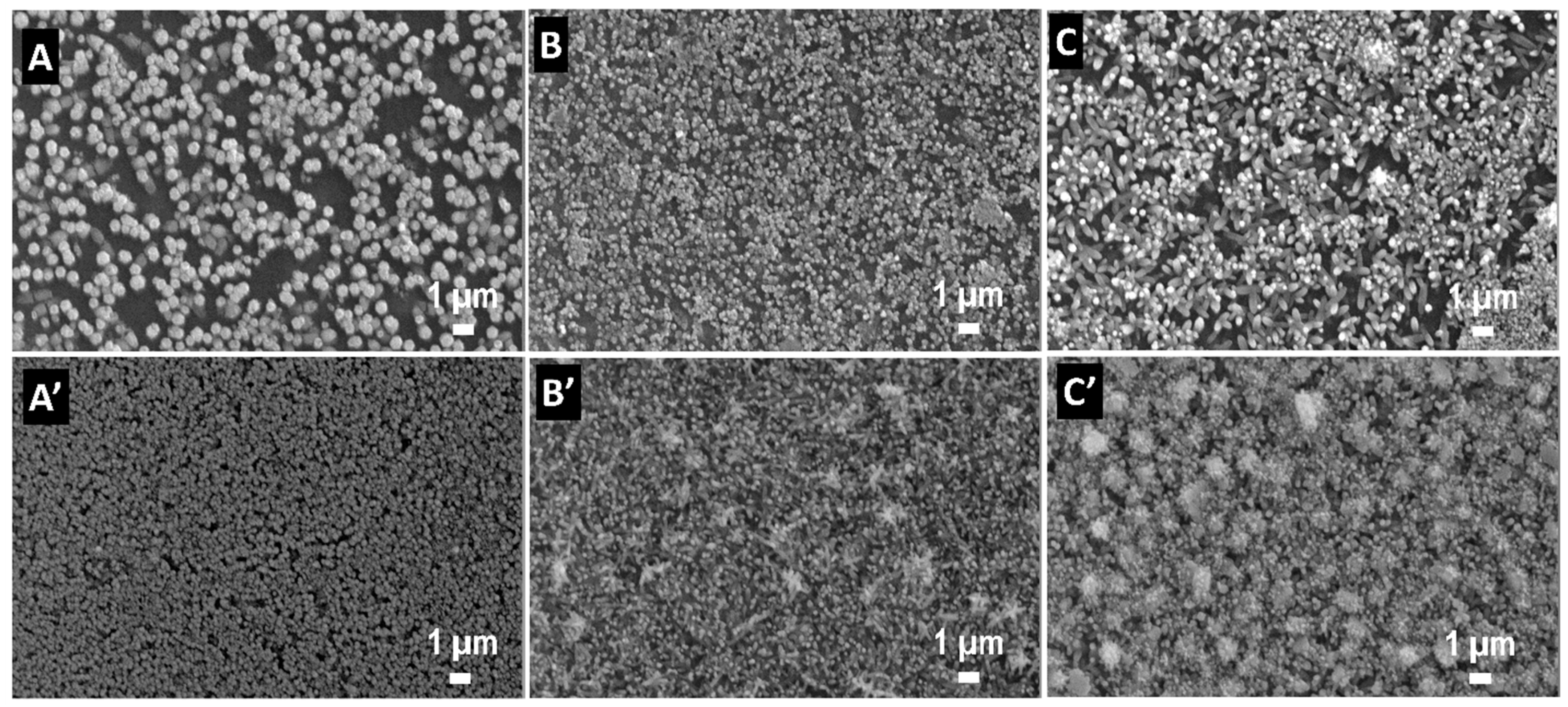
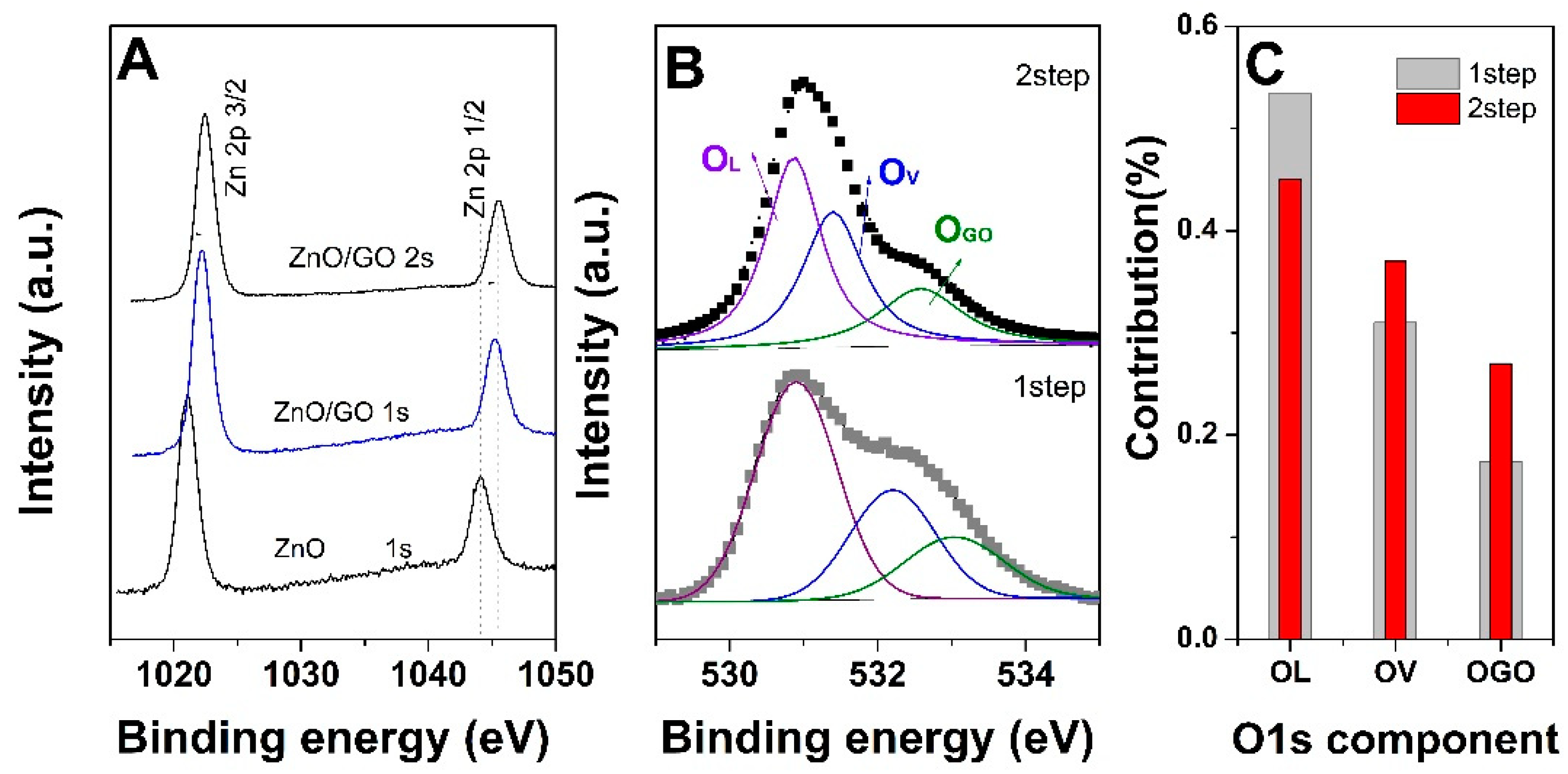
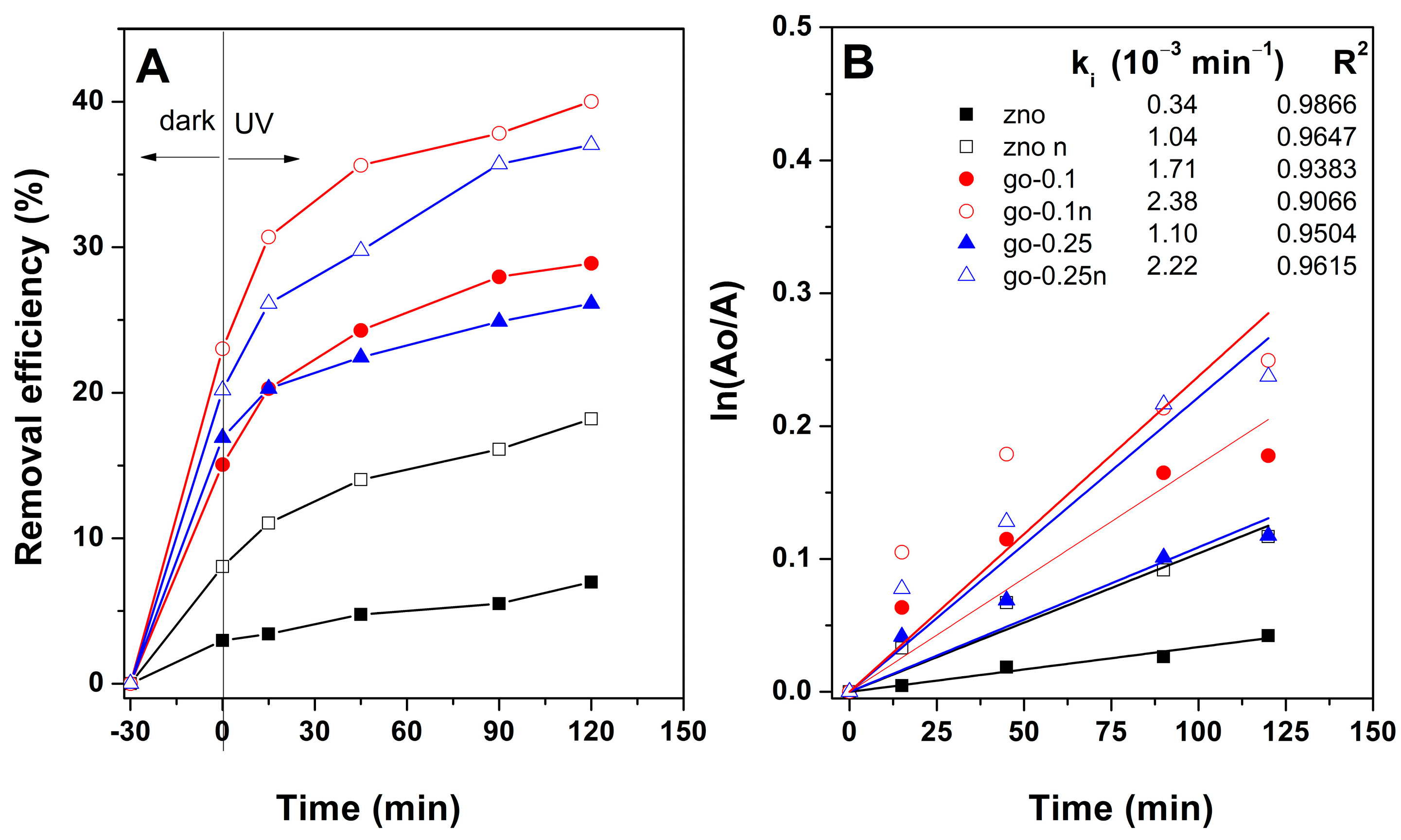
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guaraldo, T.T.; Vakili, R.; Wenk, J.; Mattia, D. Highly efficient ZnO photocatalytic foam reactors for micropollutant degradation. Chem. Eng. J. 2023, 455, 140784. [Google Scholar] [CrossRef]
- Khan, F.; Khan, M.S.; Kamal, S.; Arshad, M.; Ahmad, S.I.; Nami, S.A.A. Recent advances in graphene oxide and reduced graphene oxide based nanocomposites for the photodegradation of dyes. J. Mater. Chem. C 2020, 8, 15940–15955. [Google Scholar] [CrossRef]
- Hendrix, Y.; Rauwel, E.; Nagpal, K.; Haddad, R.; Estephan, E.; Boissière, C.; Rauwel, P. Revealing the Dependency of Dye Adsorption and Photocatalytic Activity of ZnO Nanoparticles on Their Morphology and Defect States. Nanomaterials 2023, 13, 1998. [Google Scholar] [CrossRef]
- Kumar, N.; Mittal, H.; Reddy, L.; Nair, P.; Ngila, J.C.; Parashar, V. Morphogenesis of ZnO nanostructures: Role of acetate (COOH−) and nitrate (NO3−) ligand donors from zinc salt precursors in synthesis and morphology dependent photocatalytic properties. RSC Adv. 2015, 5, 38801–38809. [Google Scholar] [CrossRef]
- Nouasria, F.Z.; Selloum, D.; Henni, A.; Tingry, S.; Hrbac, J. Improvement of the photocatalytic performance of ZnO thin films in the UV and sunlight range by Cu doping and additional coupling with Cu2O. Ceram. Int. 2022, 48, 13283–13294. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.Q.; Weng, B.; Xu, Y.J. Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891–16903. [Google Scholar] [CrossRef]
- Joshi, B.N.; Yoon, H.; Na, S.H.; Choi, J.Y.; Yoon, S.S. Enhanced photocatalytic performance of graphene-ZnO nanoplatelet composite thin films prepared by electrostatic spray deposition. Ceram. Int. 2014, 40, 3647–3654. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Sai, H.; Li, Z.; Li, F. Formation of uniform reduced graphene oxide films on modified PET substrates using drop-casting method. Particuology 2014, 17, 66–73. [Google Scholar] [CrossRef]
- Cembrero, J.; Pruna, A.; Pullini, D.; Busquets-Mataix, D. Effect of combined chemical and electrochemical reduction of graphene oxide on morphology and structure of electrodeposited ZnO. Ceram. Int. 2014, 40, 10351–10357. [Google Scholar] [CrossRef]
- Wu, S.; Yin, Z.; He, Q.; Huang, X.; Zhou, X.; Zhang, H. Electrochemical Deposition of Semiconductor Oxides on Reduced Graphene Oxide-Based Flexible, Transparent, and Conductive Electrodes. J. Phys. Chem. C 2010, 114, 11816–11821. [Google Scholar] [CrossRef]
- Pruna, A.; Shao, Q.; Kamruzzaman, M.; Zapien, J.A.; Ruotolo, A. Optimized properties of ZnO nanorod arrays grown on graphene oxide seed layer by combined chemical and electrochemical approach. Ceram. Int. 2016, 42, 17192–17201. [Google Scholar] [CrossRef]
- Ashikyn, N.; Abdul, H.; Hashim, M.; Hashim, A.M. Synthesis of Zinc Oxide Nanostructures on Graphene/Glass Substrate via Electrochemical Deposition: Effects of Potassium Chloride and Hexamethylenetetramine as Supporting Reagents. Nano-Micro Lett. 2015, 7, 317–324. [Google Scholar] [CrossRef]
- Aziz, N.S.A.; Mahmood, M.R.; Yasui, K.; Hashim, A.M. Seed/catalyst-free vertical growth of high-density electrodeposited zinc oxide nanostructures on a single-layer graphene. Nanoscale Res. Lett. 2014, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Kulis-Kapuscinska, A.; Kwoka, M.; Borysiewicz, M.A.; Wojciechowski, T.; Licciardello, N.; Sgarzi, M.; Cuniberti, G. Photocatalytic degradation of methylene blue at nanostructured ZnO thin films. Nanotechnology 2023, 34, 155702. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Precise Tuning of Surface Composition and Electron-Transfer Properties of Graphene Oxide Films through Electroreduction. Chem. A Eur. J. 2013, 19, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Henni, A.; Harfouche, N.; Karar, A.; Zerrouki, D.; Perrin, F.X.; Rosei, F. Synthesis of graphene–ZnO nanocomposites by a one-step electrochemical deposition for efficient photocatalytic degradation of organic pollutant. Solid State Sci. 2019, 98, 106039. [Google Scholar] [CrossRef]
- Tamvakos, A.; Calestani, D.; Tamvakos, D.; Mosca, R.; Pullini, D.; Pruna, A. Effect of grain-size on the ethanol vapor sensing properties of room-temperature sputtered ZnO thin films. Microchim. Acta 2015, 182, 1991–1999. [Google Scholar] [CrossRef]
- Gomez-Alvarez, M.A.; Morales, C.; Méndez, J.; del Campo, A.; Urbanos, F.J.; Díaz, A.; Reséndiz, L.; Flege, J.I.; Granados, D.; Soriano, L. A Comparative Study of the ZnO Growth on Graphene and Graphene Oxide: The Role of the Initial Oxidation State of Carbon. C 2020, 6, 41. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, F.; Zhang, M.; Guo, M. Effects of preparing conditions on controllable one-step electrodeposition of ZnO nanotube arrays. Electrochim. Acta 2014, 132, 370–376. [Google Scholar] [CrossRef]
- Pruna, A.; Wu, Z.; Zapien, J.A.; Li, Y.Y.; Ruotolo, A. Enhanced photocatalytic performance of ZnO nanostructures by electrochemical hybridization with graphene oxide. Appl. Surf. Sci. 2018, 441, 936–944. [Google Scholar] [CrossRef]
- Pauporté, T.; Rathouský, J. Electrodeposited Mesoporous ZnO Thin Films as Efficient Photocatalysts for the Degradation of Dye Pollutants. J. Phys. Chem. C 2007, 111, 7639–7644. [Google Scholar] [CrossRef]
- Sreejesh, M.; Dhanush, S.; Rossignol, F.; Nagaraja, H.S. Microwave assisted synthesis of rGO/ZnO composites for non-enzymatic glucose sensing and supercapacitor applications. Ceram. Int. 2017, 43, 4895–4903. [Google Scholar] [CrossRef]
- Hadadian, M.; Goharshadi, E.K.; Fard, M.M.; Ahmadzadeh, H. Synergistic effect of graphene nanosheets and zinc oxide nanoparticles for effective adsorption of Ni (II) ions from aqueous solutions. Appl. Phys. A Mater. Sci. Process. 2018, 124, 239. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Razavi, N.; Es’haghi, Z. Curcumin loaded magnetic graphene oxide solid-phase extraction for the determination of parabens in toothpaste and mouthwash coupled with high performance liquid chromatography. Microchem. J. 2019, 148, 616–625. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Wang, T.; Lu, M.; Wang, T. In-situ fabrication of reduced graphene oxide (rGO)/ZnO heterostructure: Surface functional groups induced electrical properties. Electrochim. Acta 2016, 196, 558–564. [Google Scholar] [CrossRef]
- del Rocío López Guemez, A.; Cordero García, A.; Cervantes López, J.L.; Pérez Vidal, H.; Díaz Flores, L.L. Evaluation of the photocatalytic activity of ZnO nanorods and nanoflowers grown from seed layers deposited by spin coating. Bol. La Soc. Esp. Ceram. Vidr. 2023, 63, 72–84. [Google Scholar] [CrossRef]
- Sigircik, G.; Erken, O.; Tuken, T.; Gumus, C.; Ozkendir, O.M.; Ufuktepe, Y. Electrosynthesis of ZnO nanorods and nanotowers: Morphology and X-ray Absorption Near Edge Spectroscopy studies. Appl. Surf. Sci. 2015, 340, 1–8. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Song, X.; Wei, Y.; Battaglia, V.S. Facile hydrothermal fabrication of ZnO–graphene hybrid anode materials with excellent lithium storage properties. Sustain. Energy Fuels 2017, 1, 767–779. [Google Scholar] [CrossRef]
- Narasimman, S.; Balakrishnan, L.; Alex, Z.C. Fiber optic magnetic field sensor using Co doped ZnO nanorods as cladding. RSC Adv. 2018, 8, 18243–18251. [Google Scholar] [CrossRef]
- Mishra, D.K.; Mohapatra, J.; Sharma, M.K.; Chattarjee, R.; Singh, S.K.; Varma, S.; Behera, S.N.; Nayak, S.K.; Entel, P. Carbon doped ZnO: Synthesis, characterization and interpretation. J. Magn. Magn. Mater. 2013, 329, 146–152. [Google Scholar] [CrossRef]
- Mirikaram, N.; Pérez-molina, Á.; Morales-torres, S.; Salemi, A.; Maldonado-hódar, F.J.; Pastrana-martínez, L.M. Photocatalytic Perfomance of ZnO-Graphene Oxide Composites towards the Degradation of Vanillic Acid under Solar Radiation and Visible-LED. Nanomaterials 2021, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Sáaedi, A.; Yousefi, R.; Jamali-Sheini, F.; Zak, A.K.; Cheraghizade, M.; Mahmoudian, M.R.; Baghchesara, M.A.; Dezaki, A.S. XPS studies and photocurrent applications of alkali-metals-doped ZnO nanoparticles under visible illumination conditions. Phys. Low-Dimens. Syst. Nanostruct. 2016, 79, 113–118. [Google Scholar] [CrossRef]
- Madhan Kumar, A.; Gasem, Z.M. In situ electrochemical synthesis of polyaniline/f-MWCNT nanocomposite coatings on mild steel for corrosion protection in 3.5% NaCl solution. Prog. Org. Coat. 2015, 78, 387–394. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Ong, W.J.; Voon, S.Y.; Tan, L.L.; Goh, B.T.; Yong, S.T.; Chai, S.P. Enhanced daylight-induced photocatalytic activity of solvent exfoliated graphene (SEG)/ZnO hybrid nanocomposites toward degradation of Reactive Black 5. Ind. Eng. Chem. Res. 2014, 53, 17333–17344. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Yang, F.; Zhang, T.; Chang, Y.; Liu, F. Seed-mediated synthesis and the photo-degradation activity of ZnO-graphene hybrids excluding the influence of dye adsorption. Appl. Surf. Sci. 2013, 283, 654–659. [Google Scholar] [CrossRef]
- Chen, J.; Xiong, Y.; Duan, M.; Li, X.; Li, J.; Fang, S.; Qin, S.; Zhang, R. Insight into the Synergistic Effect of Adsorption-Photocatalysis for the Removal of Organic Dye Pollutants by Cr-Doped ZnO. Langmuir 2020, 36, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Ndabakuranye, J.P.; Nadarajah, A.; Niyitanga, T.; Prawer, S.; Ahnood, A. Photodegradation kinetics for bilirubin sensing: New solutions for old problems. Biosens. Bioelectron. X 2022, 12, 100272. [Google Scholar] [CrossRef]
- Kenanakis, G.; Giannakoudakis, Z.; Vernardou, D.; Savvakis, C.; Katsarakis, N. Photocatalytic degradation of stearic acid by ZnO thin films and nanostructures deposited by different chemical routes. Catal. Today 2010, 151, 34–38. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Jiu, H.; Qi, G.; Huang, Y. ZnO-reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2. Ceram. Int. 2015, 41, 6256–6262. [Google Scholar] [CrossRef]
- Gupta, B.K.; Grover, V.; Gupta, G.; Shanker, V. Highly efficient luminescence from hybrid structures of ZnO/multi-walled carbon nanotubesfor high performance display applications. Nanotechnology 2010, 21, 475701. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.; Cui, P.; Bak, S.; Lee, H.; Lee, S.M.; Lee, H. Facile preparation of an n-type reduced graphene oxide field effect transistor at room temperature. Chem. Commun. 2014, 50, 1224–1226. [Google Scholar] [CrossRef]
- Atta, D.; Wahab, H.A.; Ibrahim, M.A.; Battisha, I.K. Photocatalytic degradation of methylene blue dye by ZnO nanoparticle thin films, using Sol–gel technique and UV laser irradiation. Sci. Rep. 2024, 14, 26961. [Google Scholar] [CrossRef]
- Cataño, F.A.; Gomez, H.; Dalchiele, E.A.; Marotti, R.E. Morphological and Structural Control of Electrodeposited ZnO Thin Films and Its Influence on the Photocatalytic Degradation of Methyl Orange Dye. Int. J. Electrochem. Sci. 2014, 9, 534–548. [Google Scholar] [CrossRef]
- Chen, S.; Song, X.; Song, X.; Zhang, Y. Novel electrochemical synthesis of N-doped ZnO–rGO films for the photoelectrocatalytic degradation of antibiotics. Opt. Mater. 2024, 157, 116308. [Google Scholar] [CrossRef]
- Melia, L.F.; Gallegos, M.V.; Juncal, L.; Meyer, M.; Ibañez, F.J.; Damonte, L.C. Enhanced photocatalytic performance by ZnO/Graphene heterojunction grown on Ni foam for methylene blue removal. Charact. Appl. Nanomater. 2024, 7, 5756. [Google Scholar] [CrossRef]
- Lu, H.; Sha, S.; Li, T.; Wen, Q.; Yang, S.; Wu, J.; Wang, K.; Sheng, Z.; Ma, J. One-step electrodeposition of ZnO/graphene composites with enhanced capability for photocatalytic degradation of organic dyes. Front. Chem. 2022, 10, 1061129. [Google Scholar] [CrossRef]

| Material, Exposed Surface | Electrodeposition Conditions | Electrolyte, Concentration | Analyte, Concentration | Irradiation (Source, Irradiance, Exposure Duration) | k, 10−3 min−1 | η, % | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO 6.25 cm2 | Sol–gel | MB 14.8 µM | 120 W, 24 h | 0.37 | 42 | [44] | |
| ZnO 1 cm2 | magnetron sputtering | - | MB 1 µM | 8 W UV lamp, 1.86 mW/cm2, 540 min | 1 | 64 | [14] |
| ZnO 4 cm2 | −0.8 V | 10 mM Zn(NO3)2 | MB 62 µM | 1000 W Xe/Hg lamp, 4 h | 55 | [45] | |
| ZnO–rGO 4 cm2 | −1.1 V, 1 min/ −1 V, 45 min | 50 mM Zn(NO3)2 + 0.1 M KCl + 5 mg/L GO | Ofloxacin 5 mg/L | 4 W Xe lamp 0.4 mW/cm2, 180 min | 2.24 | 35 | [46] |
| ZnO/G on Ni foam | −0.8 V, 60 min | 10 mM Zn(NO3)2 + 0.1 M KCl + 0.1 M KNO3 | MB 4 µM | UV, 8 W, 165 min | 57 | [47] | |
| ZnO-GO 1 cm2 | −1.3 V, 0.5 min/ −1.05 V, 10 min | 10 mM Zn(NO3)2, 0.1 mg/L GO | MB, 1 µM | 4 W, UV lamp, 0.4 mW/cm2, 90 min | 2.38 | 40 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruna, A.; Poliac, I.; Busquets-Mataix, D.; Ruotolo, A. Effect of Electrodeposition Conditions on Adsorption and Photocatalytic Properties of ZnO. Materials 2025, 18, 497. https://doi.org/10.3390/ma18030497
Pruna A, Poliac I, Busquets-Mataix D, Ruotolo A. Effect of Electrodeposition Conditions on Adsorption and Photocatalytic Properties of ZnO. Materials. 2025; 18(3):497. https://doi.org/10.3390/ma18030497
Chicago/Turabian StylePruna, Alina, Iulian Poliac, David Busquets-Mataix, and Antonio Ruotolo. 2025. "Effect of Electrodeposition Conditions on Adsorption and Photocatalytic Properties of ZnO" Materials 18, no. 3: 497. https://doi.org/10.3390/ma18030497
APA StylePruna, A., Poliac, I., Busquets-Mataix, D., & Ruotolo, A. (2025). Effect of Electrodeposition Conditions on Adsorption and Photocatalytic Properties of ZnO. Materials, 18(3), 497. https://doi.org/10.3390/ma18030497








