Nanoarchitectonics of Sustainable Food Packaging: Materials, Methods, and Environmental Factors
Abstract
:1. Introduction
2. Nanoarchitectonics in the Context of Food Packaging
3. Foods That Are Packaged and Protected: Meat-, Fish-, Dairy-, Plant-, Bakery-, Gelled-, and Beverage-Based Products
3.1. Meat Protection—Meat Protection Can Be Specific for Different Types of Meat, Where Beef, Poultry, Pork, Lamb, and Processed Meat Can Be Distinguished
3.2. Fish and Seafood
3.3. Dairy Products
3.4. Plant-Based Products: Fruits, Vegetables, and Shell-Based Products
3.5. Other Products: Gelled, Cans, Bread and Patisserie, Beverages and Drinks, Etc.
3.6. Nanostructure of Food
4. What Environmental Threats to Protect Food Against
4.1. Oxygen
4.2. Bacteria
4.3. Light
4.4. Temperature
4.5. Mechanical Injury
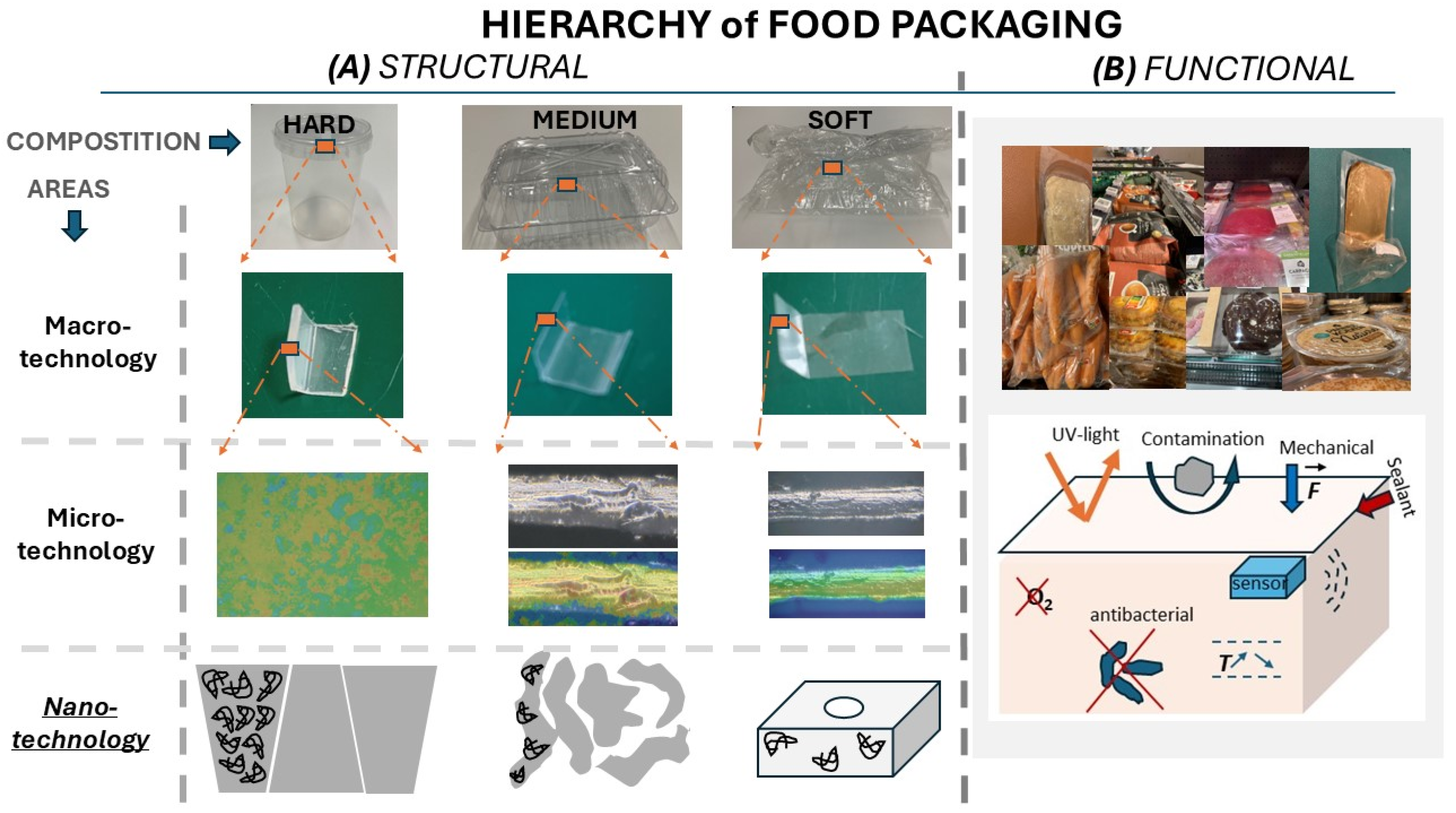
4.6. The Role of Nanoarchitectonics in Protection Against Environmental Factors
5. What Methods of Assembly, Structures, and Property Investigation Are Available
5.1. Active Packaging Materials and Molds
5.2. Barrier Permeations
5.3. Plasma Treatment
5.4. Sensors for Intelligent Packaging and Monitoring
5.5. Thermal Treatment
5.6. Light Treatment: Ultraviolet (UV), Visible, Pulsed, and Radiation
5.7. Assembly Methods and Structure: Microfluidics and Janus Structures
5.8. Enhancement of Mechanical Properties Needed for Transportation
5.9. Sealants
5.10. Analytical Techniques for Property Investigation and Characterization: Imaging, Spectroscopy, Analytics
5.11. Nanoarchitectonics and Assembly Methods and Their Application for Food Packaging
6. Materials in Packaging: Key Components of Developing Innovative, Sustainable/Recyclable, and Safe Packaging Approaches
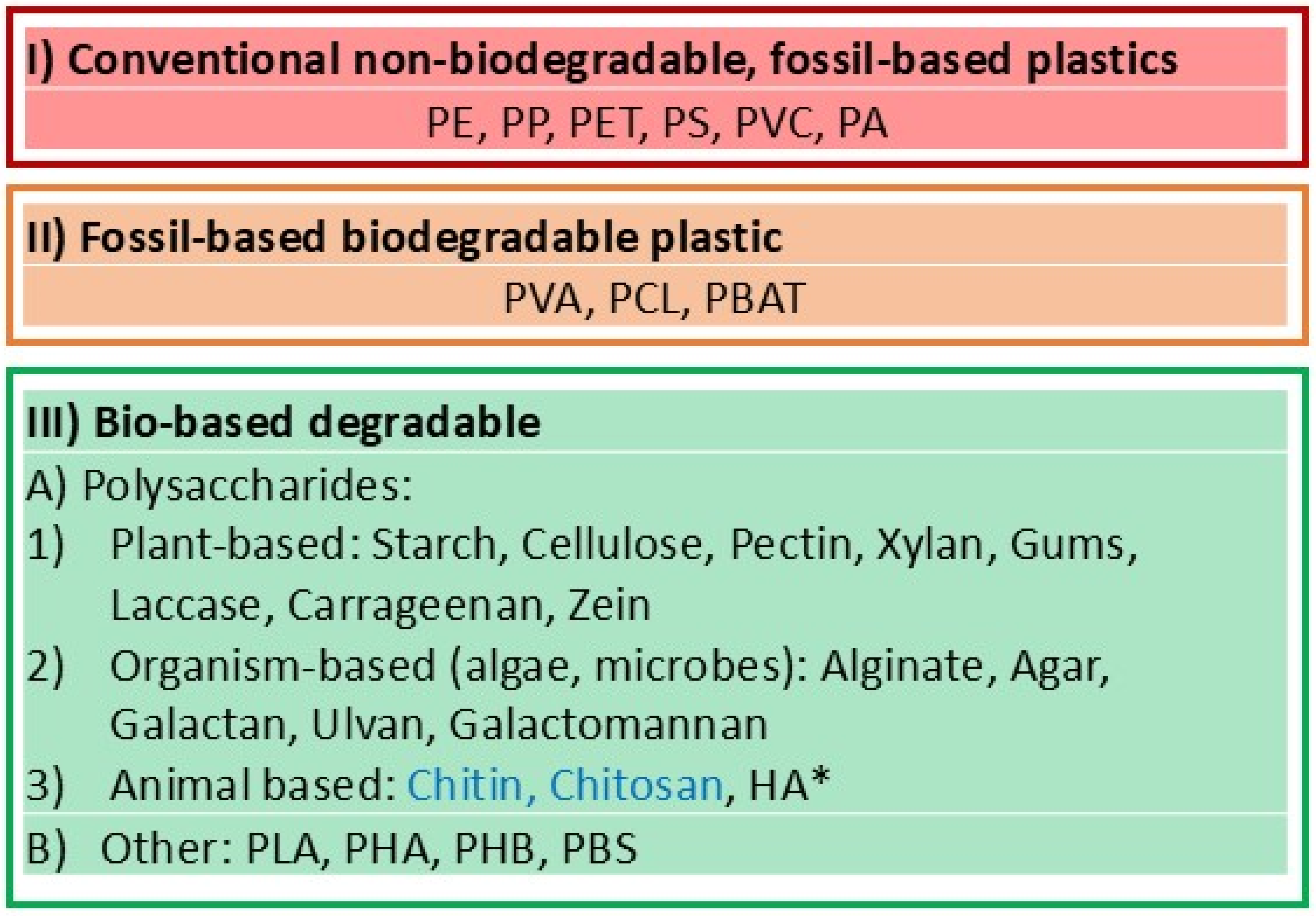
6.1. From Plastic-Based, to Bioplastic-Based, to Biodegradable Materials
- (I)
- Conventional polymers for plastic were some of the first.
- (II)
- Fossil-based biodegradable plastic.
- (III)
- Bio-based degradable.
- (A)
- Polysaccharides.
- (B)
- Other biodegradable polymers: PLA, PHA, PHB, PBS.
6.2. Edible Materials as a Developing Trend in Packaging
6.3. Hybrid/Composite Materials and Methods of Their Assembly

6.4. Nanoarchitectonics and the Role of Materials for Food Packaging
7. Administrative and Societal Factors: Safety, Ethics, Sustainability, and Marketing as Key Driving Forces in Food Packaging
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aono, M.; Ariga, K. The Way to Nanoarchitectonics and the Way of Nanoarchitectonics. Adv. Mater. 2016, 28, 989–992. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Y.; Parakhonskiy, B.; Skirtach, A.G. Nanoarchitectonics beyond perfect order—Not quite perfect but quite useful. Nanoscale 2022, 14, 15964–16002. [Google Scholar] [CrossRef]
- Ariga, K. Interface-Interactive Nanoarchitectonics: Solid and/or Liquid. Chemphyschem 2024, 25, e202400596. [Google Scholar] [CrossRef]
- Ariga, K. Molecular nanoarchitectonics: Unification of nanotechnology and molecular/materials science. Beilstein J. Nanotechnol. 2023, 14, 434–453. [Google Scholar] [CrossRef]
- Ariga, K. Materials Nanoarchitectonics for Advanced Devices. Materials 2024, 17, 5918. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: The method for everything in materials science. Bull. Chem. Soc. Jpn. 2024, 97, uoad001. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- WWF. The Hidden Cost of Plastic. Available online: https://www.wwfdrc.org/?36252/The-hidden-cost-of-plastic (accessed on 12 June 2024).
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Parakhonskiy, B.V.; Hoogenboom, R.; Skirtach, A.; De Neve, S. Labelling of micro- and nanoplastics for environmental studies: State-of-the-art and future challenges. J. Hazard. Mater. 2024, 462, 132785. [Google Scholar] [CrossRef]
- Wang, X.; Tian, S. Microplastics and Nanoplastics in Atheromas. N. Engl. J. Med. 2024, 390, 1727. [Google Scholar]
- Wang, Y.; Liu, K.; Zhang, M.; Xu, T.; Du, H.; Pang, B.; Si, C. Sustainable polysaccharide-based materials for intelligent packaging. Carbohydr. Polym. 2023, 313, 120851. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.C.; Sharma, R.; Debnath, S.; Sharma, M.; Inbaraj, B.S.; Dikkala, P.K.; Nayak, P.K.; Sridhar, K. Recent trends in polysaccharide-based biodegradable polymers for smart food packaging industry. Int. J. Biol. Macromol. 2023, 253, 127524. [Google Scholar] [CrossRef] [PubMed]
- Gamage, A.; Thiviya, P.; Liyanapathiranage, A.; Wasana, M.L.D.; Jayakodi, Y.; Bandara, A.; Manamperi, A.; Dassanayake, R.S.; Evon, P.; Merah, O.; et al. Polysaccharide-Based Bioplastics: Eco-Friendly and Sustainable Solutions for Packaging. J. Compos. Sci. 2024, 8, 413. [Google Scholar] [CrossRef]
- National Gattliemen’s Beef Association. Consumers Overwhelmingly Choose Beef over Alternatives. Available online: https://www.ncba.org/ (accessed on 5 January 2025).
- Clark, D.S.; Lentz, C.P.; Roth, L.A. Use of Carbon Monoxide for Extending Shelf-life of Prepackaged Fresh Beef. Can. Inst. Food Sci. Technol. J. 1976, 9, 114–117. [Google Scholar] [CrossRef]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat: Review. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Frandsen, M.; Rosenvold, K. Effect of ageing prior to freezing on colour stability of ovine longissimus muscle. Meat Sci. 2011, 88, 332–337. [Google Scholar] [CrossRef]
- Food and Drug Administration (U.S.). GRAS Notice GRN 000083. 2002. Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=83 (accessed on 23 February 2025).
- Gee, D.L.; Brown, W.D. Extension of shelf life in refrigerated ground beef stored under an atmosphere containing carbon dioxide and carbon monoxide. J. Agric. Food Chem. 1978, 26, 274–276. [Google Scholar] [CrossRef]
- Luño, M.; Beltrán, J.A.; Roncalés, P. Shelf-life extension and colour stabilisation of beef packaged in a low O2 atmosphere containing CO: Loin steaks and ground meat. Meat Sci. 1998, 48, 75–84. [Google Scholar] [CrossRef]
- Utrera, M.; Parra, V.; Estévez, M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014, 96, 812–820. [Google Scholar] [CrossRef]
- Senapati, M.; Sahu, P.P. Meat quality assessment using Au patch electrode Ag-SnO2/SiO2/Si MIS capacitive gas sensor at room temperature. Food Chem. 2020, 324, 126893. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, J.; Zhang, R.; Lin, J.; Zhou, M.; Qin, X.; Wang, K.; Zhou, Y.; Zhu, Q.; Jin, Y.; et al. Development of multi-cross-linking, rapid curing, and easy cleaning, edible hydrogels for meat preservation. Food Hydrocoll. 2024, 155, 110186. [Google Scholar] [CrossRef]
- Huang, K.; Wang, Y. Effect of surface charge on mechano-bactericidal activity of cellulose nanocrystals constructed chevaux-de-frise and meat preservation. Food Packag. Shelf Life 2024, 46, 101379. [Google Scholar] [CrossRef]
- Kuuliala, L.; Pippuri, T.; Hultman, J.; Auvinen, S.M.; Kolppo, K.; Nieminen, T.; Karp, M.; Björkroth, J.; Kuusipalo, J.; Jääskeläinen, E. Preparation and antimicrobial characterization of silver-containing packaging materials for meat. Food Packag. Shelf Life 2015, 6, 53–60. [Google Scholar] [CrossRef]
- Vossen, E.; Dewulf, L.; Van Royen, G.; Van Damme, I.; De Zutter, L.; Fraeye, I.; De Smet, S. Influence of aging time, temperature and relative humidity on the sensory quality of dry-aged Belgian Blue beef. Meat Sci. 2022, 183, 108659. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Pérez-Álvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- Sarantópoulos, C.I.G.L.; Alves, R.M.V.; Contreras, C.J.C.; Galvão, M.T.E.L.; Gomes, T.C. Use of a modified atmosphere masterpack for extending the shelf life of chicken cuts. Packag. Technol. Sci. 1998, 11, 217–229. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Sun, X.; Lu, Q.; Huang, M.; Zhou, G. Effect of normal and modified atmosphere packaging on shelf life of roast chicken meat. J. Food Saf. 2018, 38, e12493. [Google Scholar] [CrossRef]
- Criado, P.; Fraschini, C.; Salmieri, S.; Lacroix, M. Cellulose nanocrystals (CNCs) loaded alginate films against lipid oxidation of chicken breast. Food Res. Int. 2020, 132, 109110. [Google Scholar] [CrossRef]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Application of a LED-UV based light technology for decontamination of chicken breast fillets: Impact on microbiota and quality attributes. LWT 2021, 145, 111297. [Google Scholar] [CrossRef]
- Wei, N.; Pan, Z.; Ning, Y.; Liu, W.; Wen, X.; Yang, C.; Wang, L. Cassia Seed Gum Films Incorporated with Partridge Tea Extract as an Edible Antioxidant Food Packaging Film for Preservation of Chicken Jerky. Polymers 2024, 16, 1086. [Google Scholar] [CrossRef]
- Ordonez, J.A.; Ledward, D.A. Lipid and myoglobin oxidation in pork stored in oxygen- and carbon dioxide-enriched atmospheres. Meat Sci. 1977, 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, L.E.; Gibson, L.L.; Argnosa, G.C. The influence of controlled atmosphere and vacuum packaging upon chilled pork keeping quality. Meat Sci. 1995, 40, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Blickstad, E.; Molin, G. Carbon Dioxide as a Controller of the Spoilage Flora of Pork, with Special Reference to Temperature and Sodium Chloride. J. Food Prot. 1983, 46, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Cheah, P.; Ledward, D. Catalytic Mechanism of Lipid Oxidation following High Pressure Treatment in Pork Fat and Meat. J. Food Sci. 2006, 62, 1135–1139. [Google Scholar] [CrossRef]
- Huang, N.-Y.; Ho, C.-P.; McMillin, K.W. Retail Shelf-Life of Pork Dipped in Organic Acid before Modified Atmosphere or Vacuum Packaging. J. Food Sci. 2005, 70, m382–m387. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Yang, Y.; Wu, R.; Zhang, L.; Mu, X.; Wang, S. Fabrication of chitosan-based smart film by the O/W emulsion containing curcumin for monitoring pork freshness. J. Food Eng. 2024, 379, 112115. [Google Scholar] [CrossRef]
- Bernués, A.; Ripoll, G.; Panea, B. Consumer segmentation based on convenience orientation and attitudes towards quality attributes of lamb meat. Food Qual. Prefer. 2012, 26, 211–220. [Google Scholar] [CrossRef]
- Bueno, M.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. A model explaining and predicting lamb flavour from the aroma-active chemical compounds released upon grilling light lamb loins. Meat Sci. 2014, 98, 622–628. [Google Scholar] [CrossRef]
- Sañudo, C.; Nute, G.R.; Campo, M.M.; María, G.; Baker, A.; Sierra, I.; Enser, M.E.; Wood, J.D. Assessment of commercial lamb meat quality by British and Spanish taste panels. Meat Sci. 1998, 48, 91–100. [Google Scholar] [CrossRef]
- Bueno, M.; Campo, M.; Cacho, J.; Ferreira, V.; Escudero, A. Gas chromatographic–olfactometric characterisation of headspace and mouthspace key aroma compounds in fresh and frozen lamb meat. Food Chem. 2011, 129, 1909–1918. [Google Scholar] [CrossRef]
- Camo, J.; Beltrán, J.A.; Roncalés, P. Extension of the display life of lamb with an antioxidant active packaging. Meat Sci. 2008, 80, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Ye, H.; Wang, Z.; Wu, X.; Han, Y.; Xu, B. Changes in the microbial communities in vacuum-packaged smoked bacon during storage. Food Microbiol. 2019, 77, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Muela, E.; Monge, P.; Sañudo, C.; Campo, M.M.; Beltrán, J.A. Sensory quality of lamb following long-term frozen storage. Meat Sci. 2016, 114, 32–37. [Google Scholar] [CrossRef]
- Rubino, R.; Morand-Fehr, P.; Renieri, C.; Peraza, C.; Sarti, F.M. Typical products of the small ruminant sector and the factors affecting their quality. Small Rumin. Res. 1999, 34, 289–302. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Actio; FAO: Rome, Italy, 2024. [Google Scholar]
- Sivertsvik, M.; Rosnes, J.T.; Kleiberg, G.H. Effect of Modified Atmosphere Packaging and Superchilled Storage on the Microbial and Sensory Quality of Atlantic Salmon (Salmo salar) Fillets. J. Food Sci. 2003, 68, 1467–1472. [Google Scholar] [CrossRef]
- Monteiro, M.L.G.; Rosário, D.K.A.; de Carvalho, A.P.A.; Conte-Junior, C.A. Application of UV-C light to improve safety and overall quality of fish: A systematic review and meta-analysis. Trends Food Sci. Technol. 2021, 116, 279–289. [Google Scholar] [CrossRef]
- Karaçam, H.; Kutlu, S.; Köse, S. Effect of salt concentrations and temperature on the quality and shelf-life of brined anchovies. Int. J. Food Sci. Technol. 2002, 37, 19–28. [Google Scholar] [CrossRef]
- Hattula, T.; Elfving, K.; Mroueh, U.-M.; Luoma, T. Use of Liquid Smoke Flavouring as an Alternative to Traditional Flue Gas Smoking of Rainbow Trout Fillets (Oncorhynchus mykiss). LWT—Food Sci. Technol. 2001, 34, 521–525. [Google Scholar] [CrossRef]
- Niedziela, J.C.; MacRae, M.; Ogden, I.D.; Nesvadba, P. Control ofListeria monocytogenesin Salmon; Antimicrobial Effect of Salting, Smoking and Specific Smoke Compounds. LWT—Food Sci. Technol. 1998, 31, 155–161. [Google Scholar] [CrossRef]
- Trang Si, T.; Pham Thi Dan, P. Bioactive Compounds from By-Products of Shrimp Processing Industry in Vietnam. J. Food Drug Anal. 2012, 20, 194–197. [Google Scholar]
- Venugopal, V. Seafood Processing: Adding Value Through Quick Freezing, Retortable Packaging and Cook-Chilling, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Zhan, Z.; Feng, Y.; Zhao, J.; Qiao, M.; Jin, Q. Valorization of Seafood Waste for Food Packaging Development. Foods 2024, 13, 2122. [Google Scholar] [CrossRef] [PubMed]
- Cadwallader, D.C.; Gerard, P.D.; Drake, M.A. The role of packaging on the flavor of fluid milk. J. Dairy Sci. 2023, 106, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Rejeesh, C.R.; Anto, T. Packaging of milk and dairy products: Approaches to sustainable packaging. Mater. Today Proc. 2023, 72, 2946–2951. [Google Scholar] [CrossRef]
- Singh, N.; Mann, B.; Sharma, R.; Verma, A.; Panjagari, N.R.; Gandhi, K. Identification of polymer additives from multilayer milk packaging materials by liquid-solid extraction coupled with GC-MS. Food Packag. Shelf Life 2022, 34, 100975. [Google Scholar] [CrossRef]
- Renoldi, N.; Calligaris, S.; Nicoli, M.C.; Marino, M.; Rossi, A.; Innocente, N. Effect of the shifting from multi-layer systems towards recyclable mono-material packaging solutions on the shelf-life of portioned semi-hard cheese. Food Packag. Shelf Life 2024, 46, 101363. [Google Scholar] [CrossRef]
- Lafarga, T.; Colás-Medà, P.; Abadías, M.; Aguiló-Aguayo, I.; Bobo, G.; Viñas, I. Strategies to reduce microbial risk and improve quality of fresh and processed strawberries: A review. Innov. Food Sci. Emerg. Technol. 2019, 52, 197–212. [Google Scholar] [CrossRef]
- Lin, W.; Wang, J.; Wang, M.; Li, Z.; Ni, Y.; Wang, J. Recyclable bactericidal packaging films for emperor banana preservation. Food Chem. 2024, 438, 138002. [Google Scholar] [CrossRef]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Shelf life extension of strawberry juice by equivalent ultrasound, high pressure, and pulsed electric fields processes. Food Res. Int. 2021, 140, 110040. [Google Scholar] [CrossRef]
- Sheng, K.; Zheng, H.; Shui, S.; Yan, L.; Liu, C.; Zheng, L. Comparison of postharvest UV-B and UV-C treatments on table grape: Changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest. Biol. Technol. 2018, 138, 74–81. [Google Scholar] [CrossRef]
- Yu, K.; Yang, L.; Zhang, S.; Zhang, N.; Zhu, D.; He, Y.; Cao, X.; Liu, H. Tough, antibacterial, antioxidant, antifogging and washable chitosan/nanocellulose-based edible coatings for grape preservation. Food Chem. 2025, 468, 142513. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Raspberry fresh fruit quality as affected by pectin- and alginate-based edible coatings enriched with essential oils. Sci. Hortic. 2015, 194, 138–146. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, P.; Wu, Y.; Ouyang, J. Chitosan-hydroxypropyl methylcellulose and sodium alginate bilayer edible films for chestnut preservation. Food Chem. 2025, 466, 142254. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fogliano, V.; Heising, J.; Dekker, M. The effect of pore size on the diffusion of volatile antimicrobials is a key factor to preserve gelled foods. Food Chem. 2021, 351, 129316. [Google Scholar] [CrossRef]
- Canpack. Create Your Beverage Can. Available online: https://www.canpack.com/capabilities/beverage-cans/ (accessed on 30 January 2025).
- European Commission. Nanomaterials in Food. Available online: https://food.ec.europa.eu/food-safety/novel-food/nanomaterials_en (accessed on 23 February 2025).
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Robinson, J.; Binner, E. Understanding heat and mass transfer processes during microwave-assisted and conventional solvent extraction. Chem. Eng. Sci. 2021, 233, 116418. [Google Scholar] [CrossRef]
- Song, J.; Parakhonskiy, B.V.; Skirtach, A.G. Energy Transfer Influence on Superfast Calcium Carbonate Synthesis: Using Microwave Heating, Ultrasound Cavitation and Mechanical Stirring. J. Mater. Res. Technol. 2025, 35, 5600–5613. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Moreno-González, M.; Ottens, M. A Structured Approach to Recover Valuable Compounds from Agri-food Side Streams. Food Bioprocess Technol. 2021, 14, 1387–1406. [Google Scholar] [CrossRef]
- Verduijn, J.; Degroote, E.; Skirtach, A.G. Machine learning with label-free Raman microscopy to investigate ferroptosis in comparison with apoptosis and necroptosis. Commun. Biol. 2025, 8, 218. [Google Scholar] [CrossRef]
- Bratashov, D.N.; Masic, A.; Yashchenok, A.M.; Bedard, M.F.; Inozemtseva, O.A.; Gorin, D.A.; Basova, T.; Sievers, T.K.; Sukhorukov, G.B.; Winterhalter, M.; et al. Raman imaging and photodegradation study of phthalocyanine containing microcapsules and coated particles. J. Raman Spectrosc. 2011, 42, 1901–1907. [Google Scholar] [CrossRef]
- Huang, Y.; Spiegeleer, B.D.; Parakhonskiy, B.; Skirtach, A.G. Machine learning insights into CaCO3 phase transitions: Synthesis and phase prediction. Ceram. Int. 2024, 50, 23284–23295. [Google Scholar] [CrossRef]
- Verwee, E.; Vanleenhove, B.; Van den Wouwer, B.; Van de Walle, D.; Brijs, K.; Raes, K.; Damme, E.J.M.V.; Dewettinck, K.; Skirtach, A.G. Microscopic study of proteins, starch and cell walls in potato trimmings. LWT 2024, 209, 116798. [Google Scholar] [CrossRef]
- Verwee, E.; Chaerle, P.; Verduijn, J.; Mienis, E.; Sekulic, M.; De Keersmaecker, H.; Vyverman, W.; Foubert, I.; Skirtach, A.G.; Van Damme, E.J.M. Microalgal lipid bodies: Detection and comparative analysis using imaging flow cytometry, confocal laser scanning and Raman microscopy. Algal Res. 2024, 80, 103553. [Google Scholar] [CrossRef]
- De Witte, F.; Penagos, I.A.; Moens, K.; Skirtach, A.G.; Van Bockstaele, F.; Dewettinck, K. Multiscale assessment of the effect of a stearic-palmitic sucrose ester on the crystallization of anhydrous milk fat. Food Res. Int. 2024, 197, 115243. [Google Scholar] [CrossRef]
- Escudero, A.; Bueno-Aventin, E.; Ontanon, I.; Fernadez-Zurbano, P.; Ferreira, V. The role of polyphenols in oxygen consumption and in the accumulation of acetaldehyde and Strecker aldehydes during wine oxidation. Food Chem. 2025, 466, 142242. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J. Food Sci. 2011, 76, R164–R177. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Nieto, G.; Rosmini, M.R.; Munekata, P.E.S.; Sosa-Morales, M.E.; Lorenzo, J.M. Lipid oxidation of vegetable oils. In Food Lipids; Academic Press: Cambridge, MA, USA, 2022; pp. 127–152. [Google Scholar]
- García-Torres, R.; Ponagandla, N.R.; Rouseff, R.L.; Goodrich-Schneider, R.M.; Reyes-De-Corcuera, J.I. Effects of Dissolved Oxygen in Fruit Juices and Methods of Removal. Compr. Rev. Food Sci. Food Saf. 2009, 8, 409–423. [Google Scholar] [CrossRef]
- Coray, N.M.; Yildirim, S. Application of a Gallic Acid–Based Oxygen Scavenger Label for the Preservation of L-Ascorbic Acid in Orange Juice. Packag. Technol. Sci. 2024, 37, 917–923. [Google Scholar] [CrossRef]
- Alvarado, J.F.; Rozo, D.F.; Chaparro, L.M.; Medina, J.A.; Salcedo-Galan, F. Synthesis and Characterization of Reproducible Linseed Oil-Loaded Silica Nanoparticles with Potential Use as Oxygen Scavengers in Active Packaging. Nanomaterials 2022, 12, 3257. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Cao, S.; Tang, Y.; Wang, M.; Qu, C. Photodynamic bactericidal nanomaterials in food packaging: From principle to application. J. Food Sci. 2025, 90, e17606. [Google Scholar] [CrossRef]
- Rüegg, N.; Teixeira, S.R.; Beck, B.M.; Monnard, F.W.; Menard, R.; Yildirim, S. Application of antimicrobial packaging based on modified calcium carbonate and EOs for RTE meat products. Food Packag. Shelf Life 2022, 34, 100982. [Google Scholar] [CrossRef]
- Weldrick, P.J.; San, S.; Paunov, V.N. Advanced Alcalase-Coated Clindamycin-Loaded Carbopol Nanogels for Removal of Persistent Bacterial Biofilms. ACS Appl. Nano Mater. 2021, 4, 1187–1201. [Google Scholar] [CrossRef]
- Priyanka, S.; RS, A.B.; John, A. Biocompatible green technology principles for the fabrication of food packaging material with noteworthy mechanical and antimicrobial properties—A sustainable developmental goal towards the effective, safe food preservation strategy. Chemosphere 2023, 336, 139240. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A.; Pace, A. Photochemically Produced Singlet Oxygen: Applications and Perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Min, D.B.; Boff, J.M. Chemistry and Reaction of Singlet Oxygen in Foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Insinska-Rak, M.; Sikorski, M. Riboflavin interactions with oxygen-a survey from the photochemical perspective. Chemistry 2014, 20, 15280–15291. [Google Scholar] [CrossRef]
- Kim, N.; Choe, E. Singlet oxygen-related photooxidative stability and antioxidant changes of diacylglycerol-rich oil derived from mixture of olive and perilla oil. J. Food Sci. 2012, 77, C1185–C1191. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Khezerlou, A.; Tavassoli, M.; Abedini, A.H.; McClements, D.J. Development of sustainable UV-screening food packaging materials: A review of recent advances. Trends Food Sci. Technol. 2024, 145, 104366. [Google Scholar] [CrossRef]
- Khan, J.; Alam, S.; Begeno, T.A.; Du, Z. Anti-bacterial films developed by incorporating shikonin extracted from radix lithospermi and nano-ZnO into chitosan/polyvinyl alcohol for visual monitoring of shrimp freshness. Int. J. Biol. Macromol. 2024, 260, 129542. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, Y.; Sheng, W.; Yang, L.; Yang, H.; Tang, T.; Wang, C.; Tian, Y. Preparation and characterization of kappa-carrageenan/dextran films blended with nano-ZnO and anthocyanin for intelligent food packaging. Int. J. Biol. Macromol. 2024, 282, 137203. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Goksen, G.; Zhang, W.L. Rosemary essential oil: Chemical and biological properties, with emphasis on its delivery systems for food preservation. Food Control. 2023, 154, 110003. [Google Scholar] [CrossRef]
- Fernandes, B.C.N.; Paulo, B.B.; Guimaraes, M.C.; Sarantopoulos, C.; de Melo, N.R.; Prata, A.S. Prospection of the use of encapsulation in food packaging. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2309–2334. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Bahrami, S.; Sablani, S.S. Phase Change Materials in Food Packaging: A Review. Food Bioprocess Technol. 2024. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chanasut, U.; Kumpoun, W. 3—Enzymatic browning and its amelioration in fresh-cut tropical fruits. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Opara, U.L. Mechanical damage of fresh produce in postharvest transportation: Current status and future prospects. Trends Food Sci. Technol. 2022, 124, 195–207. [Google Scholar] [CrossRef]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent Developments in Smart Food Packaging Focused on Biobased and Biodegradable Polymers. Front. Sustain. Food S 2021, 5, 630393. [Google Scholar] [CrossRef]
- Akhrib, S.; Djellali, S.; Haddaoui, N.; Karimian, D.; Carraro, M. Biocomposites and Poly(lactic acid) in Active Packaging: A Review of Current Research and Future Directions. Polymers 2025, 17, 3. [Google Scholar] [CrossRef]
- Abbadessa, A.; Dogaris, I.; Farahani, S.; Reid, M.; Rautkoski, H.; Holopainen-Mantila, U.; Oinonen, P.; Henriksson, G. Layer-by-layer assembly of sustainable lignin-based coatings for food packaging applications. Prog. Org. Coat. 2023, 182, 107676. [Google Scholar] [CrossRef]
- Srinivasan, L.V.; Rana, S.S. A critical review of various synthesis methods of nanoparticles and their applications in biomedical, regenerative medicine, food packaging, and environment. Discov. Appl. Sci. 2024, 6, 371. [Google Scholar] [CrossRef]
- Weronika, S.; Paweł, J.; Tomasz Arkadiusz, T. Electrospraying and Electrospinning in Food Industry. In New Topics in Electrospraying; Weronika, S., Paweł, J., Tomasz Arkadiusz, T., Eds.; IntechOpen: Rijeka, Croatia, 2024; p. 1. [Google Scholar]
- Sultana, A.; Kathuria, A.; Gaikwad, K.K. Metal–organic frameworks for active food packaging. A review. Environ. Chem. Lett. 2022, 20, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Koreshkov, M.; Antreich, S.J.; Bismarck, A.; Fritz, I.; Reimhult, E.; Takatsuna, Y.; Zirbs, R. Sustainable food packaging using modified SiO nanofillers in biodegradable polymers. Mater. Chem. Front. 2024, 8, 2754–2763. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Kumar, Y.; Roy, S.; Sharma, A.; Yadav, D.K.; Kishore, A.; Kumar, N.; Meghwal, M. Edible Packaging: Mechanical Properties and Testing Methods. In Edible Food Packaging: Applications, Innovations and Sustainability; Poonia, A., Dhewa, T., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 331–352. [Google Scholar]
- Bahmid, N.A.; Suloi, A.N.F.; Engelen, A.; Anwar, M.; Hernawan. Antimicrobial Food Packaging—Interaction of Compounds and Bacterial Growth. Curr. Food Sci. Technol. Rep. 2024, 2, 121–131. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014, 140, 185–192. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Wang, L.; Goksen, G.; Shao, P. Multifunctional pectin films based on mussel-inspired modified 2D Ag nanosheets for long-lasting antibacterial and enhanced barrier properties. Food Hydrocoll. 2023, 137, 108331. [Google Scholar] [CrossRef]
- Li, N.; Jiang, D.; Zhou, Z.; Lu, Y.; Lei, Z.; Law, W.C.; Tang, C.Y. Development of carboxymethyl cellulose/starch films enriched with ZnO-NPs and anthocyanins for antimicrobial and pH-indicating food packaging. Int. J. Biol. Macromol. 2024, 282, 136814. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Q.; Wei, L. Active and smart biomass film with curcumin Pickering emulsion stabilized by chitosan-adsorbed laurate esterified starch for meat freshness monitoring. Int. J. Biol. Macromol. 2024, 275, 133331. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Amin, B.H.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Elbahnasawy, M.A. Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Causing Aspergillosis: Ultrastructure Study. J. Funct. Biomater. 2022, 13, 242. [Google Scholar] [CrossRef]
- Biswas, A.; Kar, U.; Jana, N.R. Cytotoxicity of ZnO nanoparticles under dark conditions oxygen vacancy dependent reactive oxygen species generation. Phys. Chem. Chem. Phys. 2022, 24, 13965–13975. [Google Scholar] [CrossRef]
- Turan, D.; Keukens, B.M.; Schifferstein, H.N.J. Food packaging technology considerations for designers: Attending to food, consumer, manufacturer, and environmental issues. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70058. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zhu, J.; Sun, P.; Yang, F.; Wu, H.; Li, W. Permeability of biodegradable film comprising biopolymers derived from marine origin for food packaging application: A review. Trends Food Sci. Technol. 2023, 136, 295–307. [Google Scholar] [CrossRef]
- Sun, C.; Li, G.; Wang, J.; Fang, Z.; Qin, F.; Chen, K.; Zhou, J.; Qiu, X. Transparent montmorillonite/cellulose nanofibril nanocomposite films: The influence of exfoliation degree and interfacial interaction. Cellulose 2022, 29, 7111–7124. [Google Scholar] [CrossRef]
- Freeland, B.; Ahad, I.U.; Foley, G.; Brabazon, D. Advanced Characterization Techniques for Nanostructures. In Micro and Nanomanufacturing Volume II; Jackson, M.J., Ahmed, W., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 53–89. [Google Scholar]
- Herrera-Rivera, M.D.; Torres-Arellanes, S.P.; Cortés-Martínez, C.I.; Navarro-Ibarra, D.C.; Hernández-Sánchez, L.; Solis-Pomar, F.; Pérez-Tijerina, E.; Román-Doval, R. Nanotechnology in food packaging materials: Role and application of nanoparticles. RSC Adv. 2024, 14, 21832–21858. [Google Scholar] [CrossRef]
- Nadeem, H.; Dehghani, M.; Miri, S.; Pazirofteh, M.; Garnier, G.; Batchelor, W. Highly hydrophobic and moisture barrier nanocellulose based films produced via spray deposition. Cellulose 2023, 30, 5157–5170. [Google Scholar] [CrossRef]
- Venezia, V.; Prieto, C.; Verrillo, M.; Grumi, M.; Silvestri, B.; Vitiello, G.; Luciani, G.; Lagaron, J.M. Electrospun films incorporating humic substances of application interest in sustainable active food packaging. Int. J. Biol. Macromol. 2024, 263, 130210. [Google Scholar] [CrossRef]
- Adibi, A.; Trinh, B.M.; Mekonnen, T.H. Recent progress in sustainable barrier paper coating for food packaging applications. Prog. Org. Coat. 2023, 181, 107566. [Google Scholar] [CrossRef]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold plasma in food processing: Design, mechanisms, and application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- He, M.; Yeo, C. Evaluation of Thermal Degradation of DLC Film Using a Novel Raman Spectroscopy Technique. Coatings 2018, 8, 143. [Google Scholar] [CrossRef]
- Alebachew, A.W.; Abdalkarim, S.Y.H.; Zhu, J.; Wu, S.; Zhang, Y.; Yu, H.Y.; Yunusov, K.E. Two-directions mechanical strength and high-barrier mechanisms of cellulose nanocrystal- based hybrids reinforced packaging with nacre-mimetic structure. Carbohydr. Polym. 2025, 348, 122910. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.J. Nanotechnology mediated intelligent and improved food packaging. Int. Nano Lett. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Vanderroost, M.; Ragaert, P.; Devlieghere, F.; De Meulenaer, B. Intelligent food packaging: The next generation. Trends Food Sci. Technol. 2014, 39, 47–62. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Pulsed light for food decontamination: A review. Trends Food Sci. Technol. 2007, 18, 464–473. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Demirci, A.; Irudayaraj, J.M. Inactivation of Staphylococcus aureus in Milk Using Flow-Through Pulsed UV-Light Treatment System. J. Food Sci. 2007, 72, M233–M239. [Google Scholar] [CrossRef]
- Cui, L.; Her, S.; Borst, G.R.; Bristow, R.G.; Jaffray, D.A.; Allen, C. Radiosensitization by gold nanoparticles: Will they ever make it to the clinic? Radiother. Oncol. 2017, 124, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Schroën, K.; de Ruiter, J.; Berton-Carabin, C.C. Microtechnological Tools to Achieve Sustainable Food Processes, Products, and Ingredients. Food Eng. Rev. 2020, 12, 101–120. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Middelberg, A.P.J. Microfluidic Mass-Transfer Control for the Simple Formation of Complex Multiple Emulsions. Angew. Chem. Int. Ed. 2009, 48, 7208–7211. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, S.; Sun, Y.; Fang, X.; Wu, L. Fabrication, properties and applications of Janus particles. Chem. Soc. Rev. 2012, 41, 4356–4378. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Parak, W.J.; Volodkin, D.; Skirtach, A.G. Hybrids of Polymeric Capsules, Lipids, and Nanoparticles: Thermodynamics and Temperature Rise at the Nanoscale and Emerging Applications. Langmuir 2019, 35, 8574–8583. [Google Scholar] [CrossRef]
- Yang, T.; Zan, S.; Li, B.; Li, L.; Zhang, X. Interfacial adsorption dynamics of solid lipid particles at oil/water interfaces through QCM-D technique. Food Hydrocoll. 2024, 148, 109431. [Google Scholar] [CrossRef]
- Kassab, Z.; Aziz, F.; Hannache, H.; Ben Youcef, H.; El Achaby, M. Improved mechanical properties of k-carrageenan-based nanocomposite films reinforced with cellulose nanocrystals. Int. J. Biol. Macromol. 2019, 123, 1248–1256. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of Mechanical Properties, Migration, and Potential Applications in Active Food Packaging Systems Containing Nanoclays and Nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced Mechanical Properties of Graphene-Based Poly(vinyl alcohol) Composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Liu, Y.; Yang, X.; Wang, X. Highly sensitive smart hydrogels with pH-tunable toughness via signaling cascade amplification. Giant 2023, 16, 100197. [Google Scholar] [CrossRef]
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.L.; Skirtach, A.G.; Mohan, M.K. Surface functionalization of chitosan as a coating material for orthopaedic applications: A comprehensive review. Carbohydr. Polym. 2021, 255, 117487. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Zhang, F.; Ding, X. An approach to evaluate the sealing performance of sealing structures based on multiscale contact analyses. J. Comput. Des. Eng. 2021, 8, 1433–1445. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Bastani, S.; Saeb, M.R.; Croutxé-Barghorn, C.; Allonas, X. High-performance water-based UV-curable soft systems with variable chain architecture for advanced coating applications. Prog. Org. Coat. 2019, 130, 99–113. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Song, T.; Liu, L.; Xu, F.; Pan, Y.-t.; Qian, M.; Li, D.; Yang, R. Multi-dimensional characterizations of washing durable ZnO/phosphazene-siloxane coated fabrics via ToF-SIMS and XPS. Polym. Test. 2022, 114, 107684. [Google Scholar] [CrossRef]
- Sameen, D.E.; Ahmed, S.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Zhang, Q.; Li, S.; Liu, Y. Electrospun nanofibers food packaging: Trends and applications in food systems. Crit. Rev. Food Sci. Nutr. 2022, 62, 6238–6251. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, Y.; Wang, C.; Liu, X.J.; El-Seedi, H.R.; Gomez, P.L.; Alzamora, S.M.; Zou, X.B.; Guo, Z.M. Enhanced composite Co-MOF-derived sodium carboxymethyl cellulose visual films for real-time and in situ monitoring fresh-cut apple freshness. Food Hydrocoll. 2024, 157, 6238–6251. [Google Scholar] [CrossRef]
- Eftekhari, K.; Parakhonskiy, B.V.; Grigoriev, D.; Skirtach, A.G. Advances in Nanoarchitectonics: A Review of “Static” and “Dynamic” Particle Assembly Methods. Materials 2024, 17, 1051. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, K.; Danglad-Flores, J.A.; Li, J.; Riegler, H.; Parakhonskiy, B.V.; Skirtach, A.G. Calcium carbonate particle synthesis in a confined and dynamically thinning layer on a spin-coater—In situ deposition for cell adhesion. Mater. Chem. Phys. 2023, 310, 128462. [Google Scholar] [CrossRef]
- Eftekhari, K.; Van der Meeren, L.; Depla, D.; Parakhonskiy, B.; Skirtach, A.G. PM2.5 and PM10 adsorption onto filters and surfaces functionalized with calcium carbonate particle assembly. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132617. [Google Scholar] [CrossRef]
- Ariga, K. Layered nanoarchitectonics for condensed hard matter, soft matter, and living matter. J. Phys. Condens. Matter 2025, 37, 053001. [Google Scholar] [CrossRef] [PubMed]
- Gutfreund, P.; Higy, C.; Fragneto, G.; Tschopp, M.; Felix, O.; Decher, G. Molecular conformation of polyelectrolytes inside Layer-by-Layer assembled films. Nat. Commun. 2023, 14, 4076. [Google Scholar] [CrossRef] [PubMed]
- Proshin, P.I.; Abdurashitov, A.S.; Sindeeva, O.A.; Ivanova, A.A.; Sukhorukov, G.B. Additive Manufacturing of Drug-Eluting Multilayer Biodegradable Films. Polymers 2022, 14, 4318. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Li, J.; Parakhonskiy, B.V.; Skirtach, A.G. A decade of developing applications exploiting the properties of polyelectrolyte multilayer capsules. Chem. Commun. 2022, 59, 807–835. [Google Scholar] [CrossRef]
- Gulin-Sarfraz, T.; Grøvlen, M.S.; Rosqvist, E.; Pettersen, M.K.; Peltonen, J.; Sarfraz, J. Optimized multilayer coating using layer-by-layer assembly method for excellent oxygen barrier of poly(lactic acid) based film. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131155. [Google Scholar] [CrossRef]
- del Hoyo-Gallego, S.; Pérez-Álvarez, L.; Gómez-Galván, F.; Lizundia, E.; Kuritka, I.; Sedlarik, V.; Laza, J.M.; Vila-Vilela, J.L. Construction of antibacterial poly(ethylene terephthalate) films via layer by layer assembly of chitosan and hyaluronic acid. Carbohydr. Polym. 2016, 143, 35–43. [Google Scholar] [CrossRef]
- Sun, J.; Ren, R.; Yao, L.; Li, J.; Tong, L.; Yuan, J.; Wang, D. Effect of Combined Chitosan and Hyperbranched Poly-L-Lysine Based Coating on Prolonging the Shelf Life of Oyster Mushroom (Pleurotus ostreatus). Foods 2024, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Dong, H.; Wu, W.; Yang, X.; He, Q. Functional biopolymers for food packaging: Formation mechanism and performance improvement of chitosan-based composites. Food Biosci. 2023, 54, 102927. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- European Bioplastics e.V. What Are Bioplastics? Available online: https://www.european-bioplastics.org/bioplastics/ (accessed on 9 January 2025).
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khan, S.; Mehdizadeh, M.; Bahmid, N.A.; Adli, D.N.; Walker, T.R.; Perestrelo, R.; Câmara, J.S. Phytochemicals and bioactive constituents in food packaging—A systematic review. Heliyon 2023, 9, e21196. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Dziadek, M.; Schietse, J.; Boone, M.; Declercq, H.A.; Coenye, T.; Vanhoorne, V.; Vervaet, C.; Balcaen, L.; Buchweitz, M.; et al. Pectin-bioactive glass self-gelling, injectable composites with high antibacterial activity. Carbohydr. Polym. 2019, 205, 427–436. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Zhang, Z.; Ye, C.; Chen, Y.; Wei, P.; Wang, Y.; Xia, Y. Thermoplastic starch plasticized by polymeric ionic liquid. Eur. Polym. J. 2021, 148, 110367. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Huang, X.; Ge, X.; Zhou, L.; Wang, Y. Eugenol embedded zein and poly(lactic acid) film as active food packaging: Formation, characterization, and antimicrobial effects. Food Chem. 2022, 384, 132482. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef]
- Alam, J.; Alhoshan, M.; Shukla, A.K.; Aldalbahi, A.; Ali, F.A.A. k-Carrageenan—A versatile biopolymer for the preparation of a hydrophilic PVDF composite membrane. Eur. Polym. J. 2019, 120, 109219. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Zhu, F.; Li, G. Alginate-based active and intelligent packaging: Preparation, properties, and applications. Int. J. Biol. Macromol. 2024, 279, 135441. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Chawla, R.; Santhosh, R.; Thakur, R.; Sarkar, P.; Zhang, W. Agar-based edible films and food packaging application: A comprehensive review. Trends Food Sci. Technol. 2023, 141, 104198. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Westlake, J.R.; Laabei, M.; Jiang, Y.; Yew, W.C.; Smith, D.L.; Burrows, A.D.; Xie, M. Vanillin Cross-Linked Chitosan Film with Controlled Release of Green Tea Polyphenols for Active Food Packaging. ACS Food Sci. Technol. 2023, 3, 1680–1693. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Priyadarshi, R.; Zhang, W.; Khezerlou, A.; Rhim, J.-W. Innovative application of laccase enzyme in food packaging. Trends Food Sci. Technol. 2024, 151, 104623. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of volatile compounds and their sensory impact in a biopolymer based on polylactic acid (PLA) and polyester. Food Chem. 2019, 294, 171–178. [Google Scholar] [CrossRef]
- Malinowski, R.; Rytlewski, P.; Barczewski, M.; Krasinskyi, V.; Dulebová, Ľ.; Kaczor, D. Polylactide films modified with glass microspheres—Morphology and properties. Food Packag. Shelf Life 2024, 46, 101356. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly(lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. LWT 2021, 135, 110034. [Google Scholar] [CrossRef]
- Zamir, S.S.; Frouzanmehr, M.R.; Nagalakshmaiah, M.; Ajji, A.; Robert, M.; Elkoun, S. Chemical compatibility of lactic acid-grafted starch nanocrystals (SNCs) with polylactic acid (PLA). Polym. Bull. 2019, 76, 3481–3499. [Google Scholar] [CrossRef]
- Bulota, M.; Budtova, T. PLA/algae composites: Morphology and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2015, 73, 109–115. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinho, F.; Sanches Silva, A.; Andrade, M.; Machado, A.V.; Castilho, M.C.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active polylactic acid film incorporated with green tea extract: Development, characterization and effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Iversen, L.J.; Rovina, K.; Vonnie, J.M.; Matanjun, P.; Erna, K.H.; ‘Aqilah, N.M.; Felicia, W.X.; Funk, A.A. The Emergence of Edible and Food-Application Coatings for Food Packaging: A Review. Molecules 2022, 27, 5604. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials-The Place of Inorganics-in-Organics in it, Their Composition and Applications. Front. Chem. 2019, 7, 179. [Google Scholar] [CrossRef]
- Song, J.; Vikulina, A.S.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of hybrid materials. Part-II: The place of organics-on-inorganics in it, their composition and applications. Front. Chem. 2023, 11, 1078840. [Google Scholar] [CrossRef]
- Ariga, K. Composite Nanoarchitectonics Towards Method for Everything in Materials Science. J. Inorg. Organomet. Polym. Mater. 2024, 34, 2926–2947. [Google Scholar] [CrossRef]
- Malherbi, N.M.; Schmitz, A.C.; Grando, R.C.; Bilck, A.P.; Yamashita, F.; Tormen, L.; Fakhouri, F.M.; Velasco, J.I.; Bertan, L.C. Corn starch and gelatin-based films added with guabiroba pulp for application in food packaging. Food Packag. Shelf Life 2019, 19, 140–146. [Google Scholar] [CrossRef]
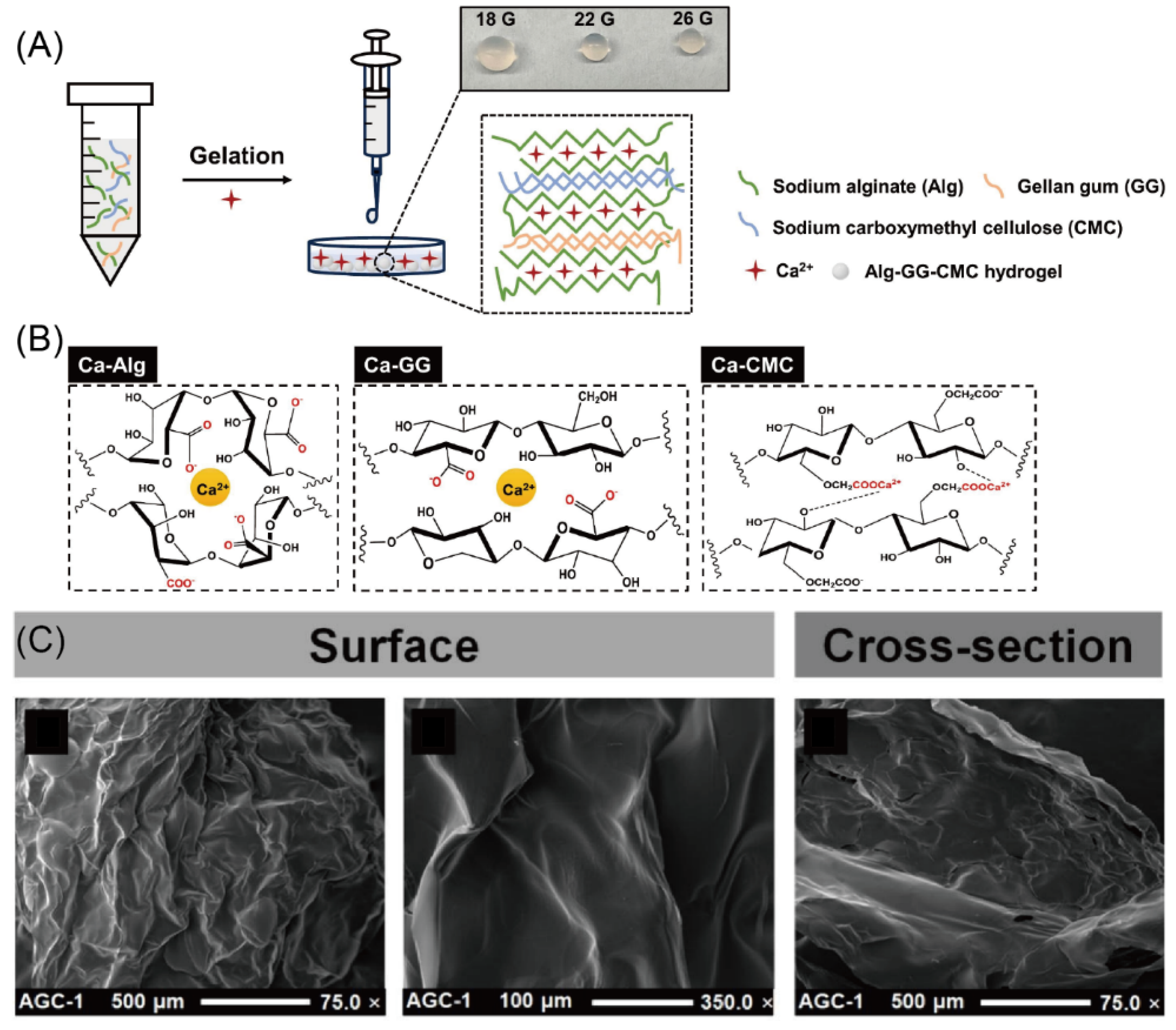
- Cao, L.; Lewille, B.; Dewettinck, K.; Willaert, R.G.; Tan, M.; Skirtach, A.G.; Parakhonskiy, B.V. Emulsion-filled bulk gels based on alginate and gellan gum: The fabrication, characterization, curcumin delivery, and antioxidative properties. Chem. Eng. J. 2024, 501, 157649. [Google Scholar] [CrossRef]
- Ghosh, S.; Su, Y.H.; Yang, C.J.; Lai, J.Y. Design of Highly Adhesive Urchin-Like Gold Nanostructures for Effective Topical Drug Administration and Symptomatic Relief of Corneal Dryness. Small Struct. 2024, 6, 2400484. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S. Development of poly (vinyl alcohol)/nano-clay/Ipomoea cairica anthocyanin based halochromic smart films for freshness monitoring of lamb meat. Food Biosci. 2024, 60, 104441. [Google Scholar] [CrossRef]
- Kuswandi, B. Environmental friendly food nano-packaging. Environ. Chem. Lett. 2017, 15, 205–221. [Google Scholar] [CrossRef]
- Koshy, R.R.; Reghunadhan, A.; Mary, S.K.; Sadanandan, S.; Jose, S.; Thomas, S.; Pothen, L.A. AgNP anchored carbon dots and chitin nanowhisker embedded soy protein isolate films with freshness preservation for active packaging. Food Packag. Shelf Life 2022, 33, 100876. [Google Scholar] [CrossRef]
- Thang, T.H.; Nguyen, T.A. Polymer nanocomposites for food-packaging applications. In Smart Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 333–354. [Google Scholar]
- Zdyrko, B.; Klep, V.; Li, X.; Kang, Q.; Minko, S.; Wen, X.; Luzinov, I. Polymer brushes as active nanolayers for tunable bacteria adhesion. Mater. Sci. Eng. C 2009, 29, 680–684. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, Y.Q.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.J. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules 2019, 20, 4171–4179. [Google Scholar] [CrossRef]
- Cao, L.; Van de Walle, D.; Hirmz, H.; Wynendaele, E.; Dewettinck, K.; Parakhonskiy, B.V.; Skirtach, A.G. Food-based biomaterials: pH-responsive alginate/gellan gum/carboxymethyl cellulose hydrogel beads for lactoferrin delivery. Biomater. Adv. 2024, 165, 213999. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Tymetska, S.; Shymborska, Y.; Raczkowska, J.; Awsiuk, K.; Skirtach, A.G.; Korolko, S.; Chebotar, A.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive properties of pH-sensitive poly(methacrylic acid)-grafted brush coatings with controlled wettability for cell culture. J. Mater. Chem. B 2025. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Goyanes, S.; Garrigós, M.C.; Jiménez, A. Antioxidant water-resistant fish gelatin nanofibers: A comparative analysis of fructose and citric acid crosslinking and investigation of chlorogenic acid release kinetics. Food Hydrocoll. 2024, 150, 109696. [Google Scholar] [CrossRef]
- Fenko, A.; Lotterman, H.; Galetzka, M. What’s in a name? The effects of sound symbolism and package shape on consumer responses to food products. Food Qual. Prefer. 2016, 51, 100–108. [Google Scholar] [CrossRef]
- Skaczkowski, G.; Durkin, S.; Kashima, Y.; Wakefield, M. The effect of packaging, branding and labeling on the experience of unhealthy food and drink: A review. Appetite 2016, 99, 219–234. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 11 January 2025).
- European Commission. Food Safety. Available online: https://food.ec.europa.eu/index_en (accessed on 20 January 2025).
- FoodSafety.gov. Your Gateway to Food Safety Information. Available online: https://www.foodsafety.gov/ (accessed on 25 January 2025).
- U.S. Food & Drug Administration. Safe Food Handling. Available online: https://www.fda.gov/food/buy-store-serve-safe-food/safe-food-handling (accessed on 28 January 2025).
- Sciensano. Food Consumption and Food Safety. Available online: https://www.sciensano.be/en/health-topics/food-consumption-and-food-safety (accessed on 26 January 2025).
- Food Standards Agency. Food-Safety. Available online: https://www.food.gov.uk/food-safety (accessed on 24 January 2025).
- EuChinaSafe. Delivering an Effective, Resilient and Sustainable EU-China Food Safety Partnership. Available online: http://www.euchinasafe.eu/index.html (accessed on 28 January 2025).
- International Atomic Energy Agency, Food Safety Asia (FSA). Available online: https://www.iaea.org/services/networks/fsa (accessed on 19 January 2025).
- Stokstad, E. Nitrogen crisis threatens Dutch environment—And economy. Science 2019, 366, 1180–1181. [Google Scholar] [CrossRef]
- FAO. Blue Transformation—Roadmap 2022–2030: A Vision for FAO’s Work on Aquatic Food Systems; FAO: Rome, Italy, 2022. [Google Scholar]
- FAO. Strategic Framework 2022-31; FAO: Rome, Italy, 2021. [Google Scholar]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Hoppe, M.; de Voogt, P.; Franz, R. Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates—A review. Trends Food Sci. Technol. 2016, 50, 118–130. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Rosenmai, A.K.; Vinggaard, A.M.; Nerín, C. Migration studies and toxicity evaluation of cyclic polyesters oligomers from food packaging adhesives. Food Chem. 2020, 311, 125918. [Google Scholar] [CrossRef]
- NEN. Welfare Quality, Assessment Protocol for Cattle; NEN: Delft, The Netherlands, 2009. [Google Scholar]
- María, G.A.; Villarroel, M.; Chacón, G.; Gebresenbet, G. Scoring system for evaluating the stress to cattle of commercial loading and unloading. Vet. Rec. 2004, 154, 818–821. [Google Scholar] [CrossRef]
- Grandin, T. Objective scoring of animal handling and stunning practices at slaughter plants. J. Am. Vet. Med. Assoc. 1998, 212, 36–39. [Google Scholar] [CrossRef]
- Simon, G.E.; Hoar, B.R.; Tucker, C.B. Assessing cow-calf welfare. Part 1: Benchmarking beef cow health and behavior, handling; and management, facilities, and producer perspectives. J. Anim. Sci. 2016, 94, 3476–3487. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Auditing animal welfare at slaughter plants. Meat Sci. 2010, 86, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.D.; Nicholson, K.L.; Frenzel, L.L.; Maddock, R.J.; Delmore, R.J., Jr.; Lawrence, T.E.; Henning, W.R.; Pringle, T.D.; Johnson, D.D.; Paschal, J.C.; et al. Survey of transportation procedures, management practices, and health assessment related to quality, quantity, and value for market beef and dairy cows and bulls. J. Anim. Sci. 2013, 91, 5026–5036. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Maintenance of good animal welfare standards in beef slaughter plants by use of auditing programs. J. Am. Vet. Med. Assoc. 2005, 226, 370–373. [Google Scholar] [CrossRef]
- Troy, D.; Kerry, J. Consumer perception and the role of science in the meat industry. Meat Sci. 2010, 86, 214–226. [Google Scholar] [CrossRef]

| Dairy Product | Packaging Material |
|---|---|
| Liquid Milk | Polyethylene pouches (LDPE or LLDPE), paper board cartons (Tetra Pak or Tetra Brik), glass bottles, PET bottles. |
| Milk Powder | Flexible laminates (PET/BOPP/Al foil), tin container. |
| Ice Cream | Polypropylene containers, PET Laminates, paper board carton. |
| Butter | Parchment paper, wax-coated paper, cellophane, aluminum foil laminates, lacquered tin cans. |
| Cheese | Aluminum foil/paper laminates, cellophane/paper combinations, tinplate container. |
| Ghee | Polypropylene bottles, glass bottles, HDPE film pouches, tinplate container, polyethylene film pouches, LDPE/HDPE films. |
| Traditional Sweets | Aluminum foil, cellophane, HDPE films, LDPE films, PE laminate, tinplate container. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Skirtach, A.G. Nanoarchitectonics of Sustainable Food Packaging: Materials, Methods, and Environmental Factors. Materials 2025, 18, 1167. https://doi.org/10.3390/ma18051167
Yang T, Skirtach AG. Nanoarchitectonics of Sustainable Food Packaging: Materials, Methods, and Environmental Factors. Materials. 2025; 18(5):1167. https://doi.org/10.3390/ma18051167
Chicago/Turabian StyleYang, Tangyu, and Andre G. Skirtach. 2025. "Nanoarchitectonics of Sustainable Food Packaging: Materials, Methods, and Environmental Factors" Materials 18, no. 5: 1167. https://doi.org/10.3390/ma18051167
APA StyleYang, T., & Skirtach, A. G. (2025). Nanoarchitectonics of Sustainable Food Packaging: Materials, Methods, and Environmental Factors. Materials, 18(5), 1167. https://doi.org/10.3390/ma18051167









