Study on the Influence of Nickel Plating on the Structure and Properties of Aluminum/Steel Bimetallic Bonding
Abstract
1. Introduction
2. Experimental Procedures
2.1. Experimental Materials
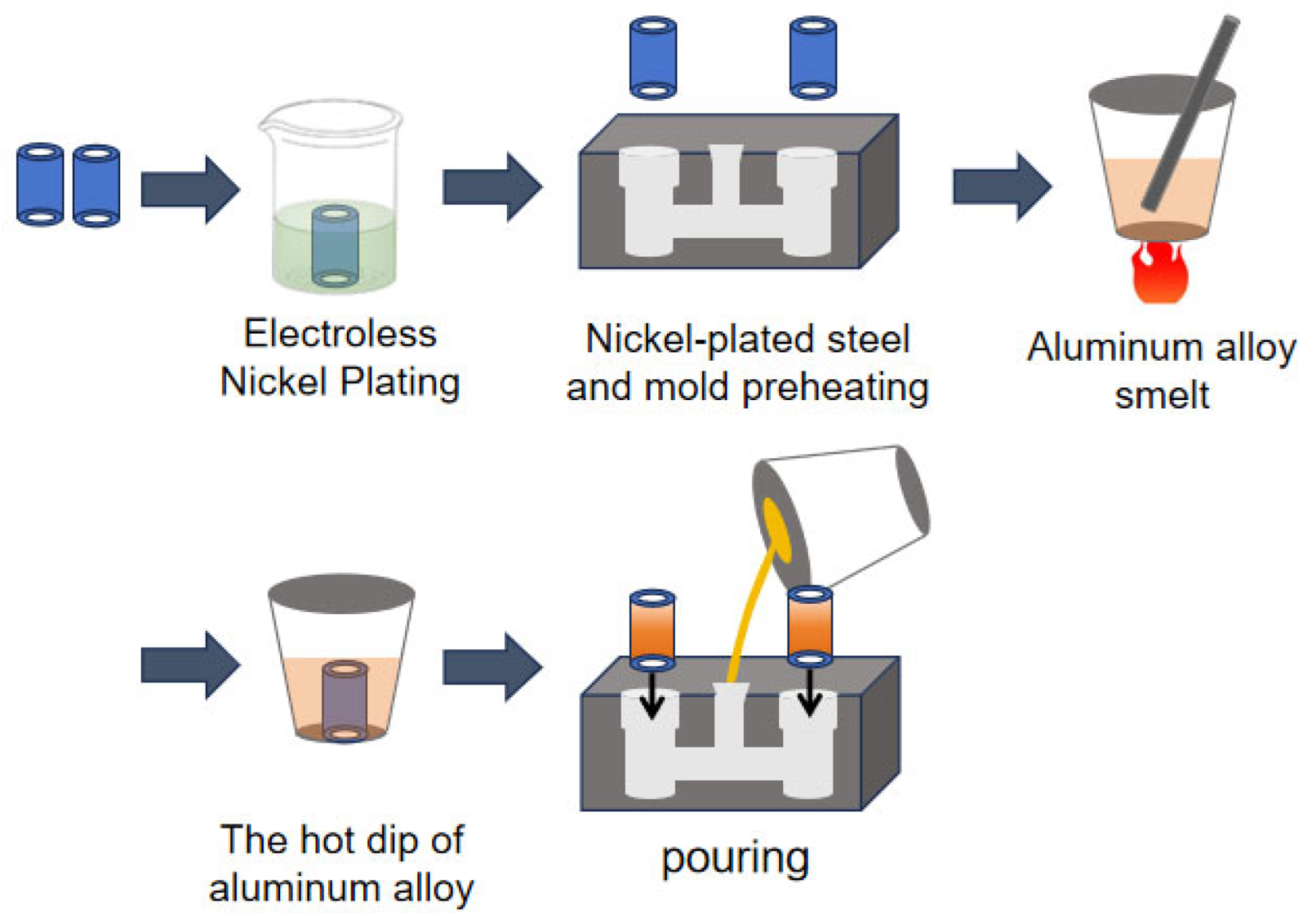
2.2. Preparation of Al/Steel Bimetal with Ni Interlayer
2.3. Microstructural Characterizations
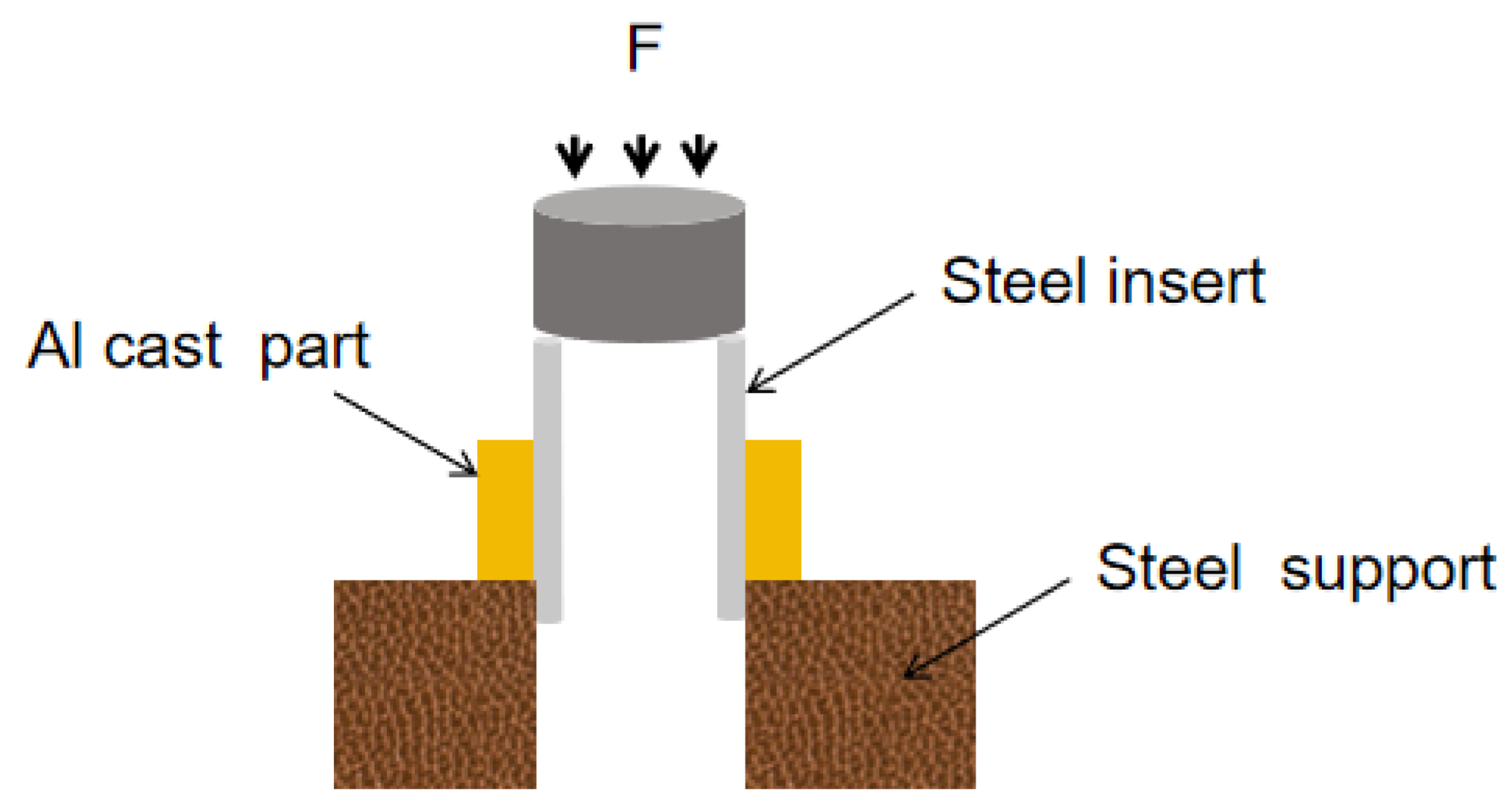
2.4. Mechanical Properties
3. Results and Discussion
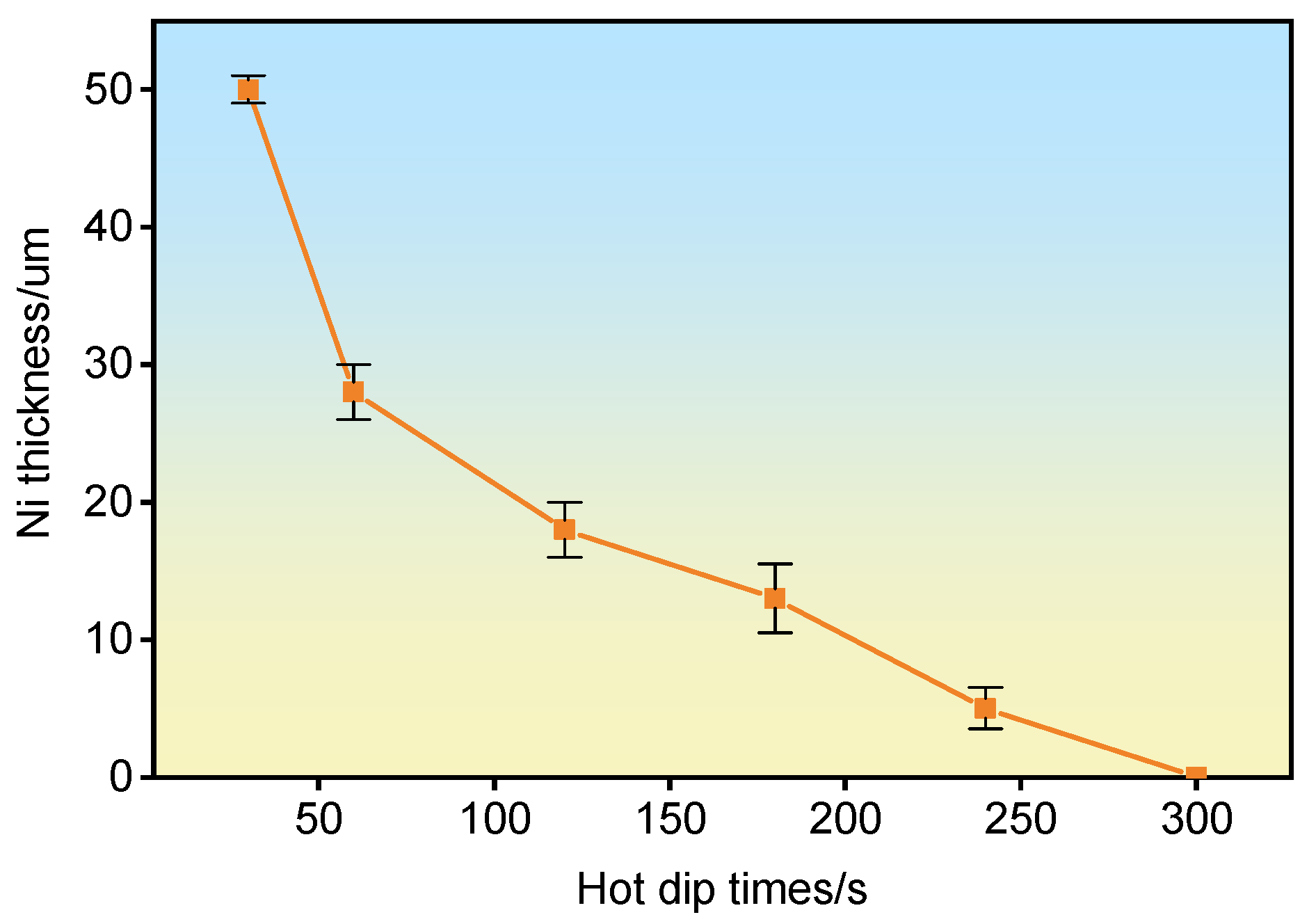
3.1. Nickel-Plated Coating
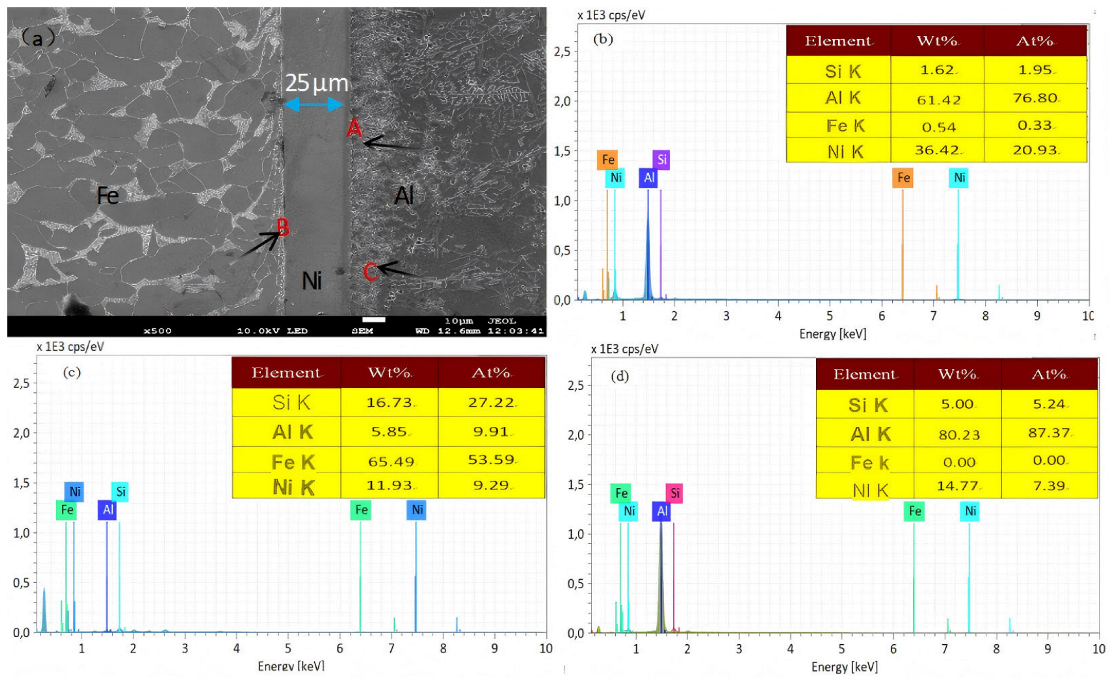
3.2. Microstructure
3.3. Shear Strength
4. Discussion
4.1. Interface Formation Mechanism
4.2. Relationship Between Microstructure and Mechanical Properties
5. Conclusions
- When the thickness of the nickel-plated layer is 50 µm, the hot-dip post-casting process is used to obtain the interface diffusion layer of nickel-plated steel/Al bimetallic. The total thickness of the diffusion layer composed of τ1-Al2Fe3Si3 and FeAl3 on the steel side increases first and then decreases. When the hot dip time is 240 s, the thickness reaches the maximum of 8 µm. The diffusion layer composed of τ5–Al8Fe2Si and Fe2Al5 on the aluminum side decreases first and then increases, and the minimum of 6 µm is reached at 240 s. This confirms hot-dipping time as a critical lever for interfacial architecture control;
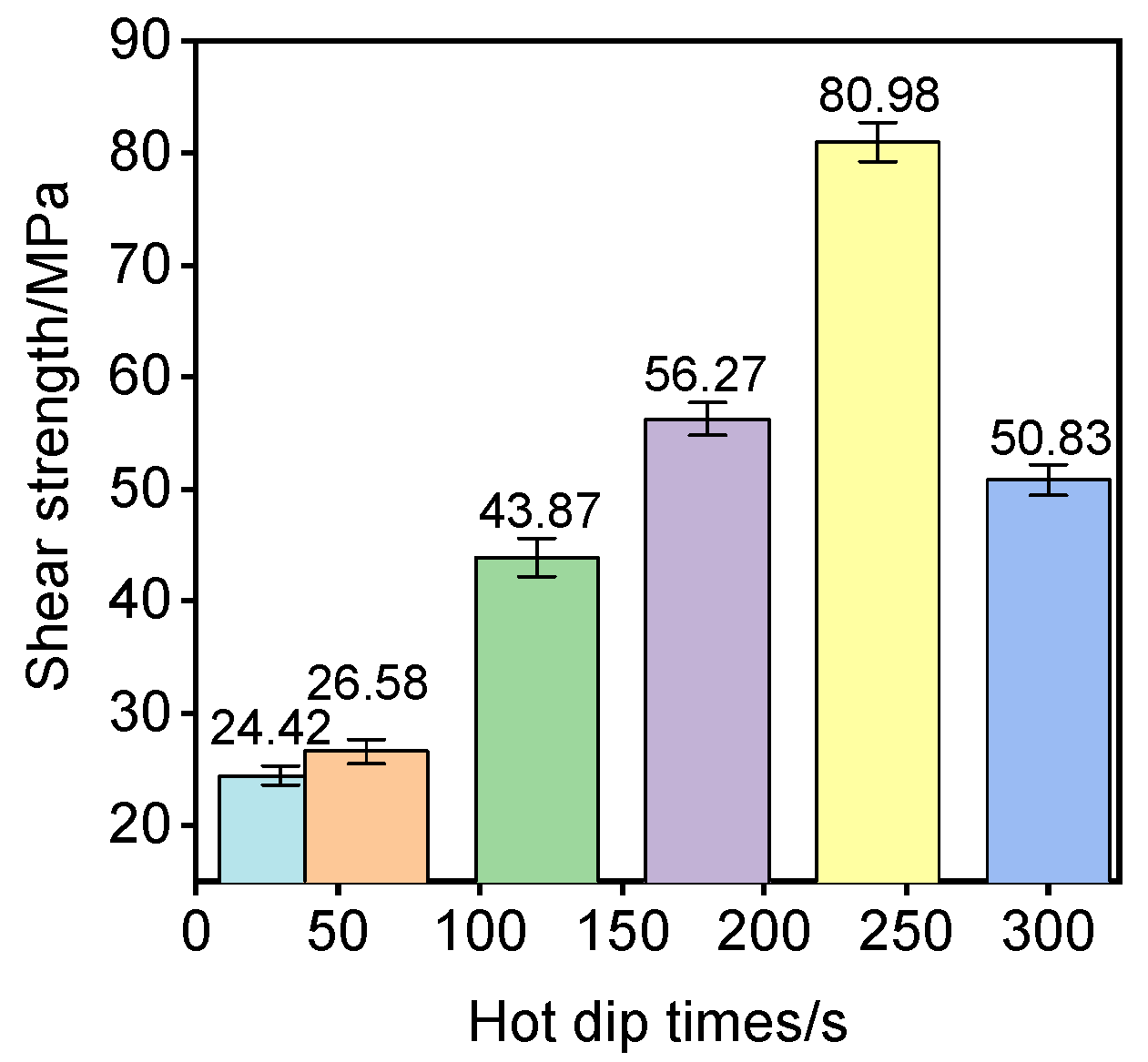
- Peak interfacial shear strength of 80.98 MPa was achieved at 240 s, representing a 75% enhancement over non-Ni-interlayered systems. Strength degradation beyond 240 s correlated with excessive brittle IMC formation and interfacial stress concentration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Zhao, W.; Qu, S.; Zhang, Y. Microstructures and mechanical properties of AZ91D/0Cr19Ni9 bimetal composite prepared by liquid-solid compound casting. Trans. Nonferrous Met. Soc. China 2019, 29, 51–58. [Google Scholar] [CrossRef]
- Rao, H.C.; Varghese, J.; Suresh, A. Dual metal core multilayer boards (MLBS)—A better option for high speed spacecraft electronic packaging applications. Circuit World 2006, 32, 30–38. [Google Scholar]
- Xu, Q.; Li, X.; Sari, H.M.; Li, W.; Liu, W.; Hao, Y.; Qin, J.; Cao, B.; Xiao, W.; Xu, Y.; et al. Surface engineering of LiNi0.8Mn0.1Co0.1O2 towards boosting lithium storage: Bimetallic oxides versus monometallic oxides. Nano Energy 2020, 77, 105034. [Google Scholar] [CrossRef]
- Oloyede, O.; Bigg, T.D.; Cochrane, R.F.; Mullis, A.M. Microstructure evolution and mechanical properties of drop-tube processed, rapidly solidified grey cast iron. Mater. Sci. Eng. 2016, 654, 143–150. [Google Scholar] [CrossRef]
- Guler, K.A.; Kisasoz, A.; Karaaslan, A. Investigation of Al/Steel bimetal composite fabrication by vacuum assisted solid mould investment casting. Acta Phys. Pol. A 2014, 126, 1327–1330. [Google Scholar] [CrossRef]
- Mao, F.; Zhang, P.; Wei, S.; Chen, C.; Zhang, G.; Xiong, M.; Wang, T.; Guo, J.; Wang, C. Interface Microstructure and Mechanical Properties of Al/Steel Bimetallic Composites Fabricated by Liquid-Solid Casting with Rare Earth Eu Additions. Materials 2022, 15, 6507. [Google Scholar] [CrossRef]
- Lombardi, A.; D’Elia, F.; Ravindran, C.; MacKay, R. Replication of engine block cylinder bridge microstructure and mechanical properties with lab scale 319 Al alloy billet castings. Mater. Charact. 2014, 137, 87125. [Google Scholar] [CrossRef]
- Yang, J.; Oliveira, J.; Li, Y.; Tan, C.; Gao, C.; Zhao, Y.; Yu, Z. Laser techniques for dissimilar joining of aluminum alloys to steels: A critical review. Mater. Process Technol. 2022, 301, 117443. [Google Scholar] [CrossRef]
- Yin, F.-C.; Zhao, M.-X.; Liu, Y.-X.; Han, W.; Li, Z. Effect of Si on growth kinetics of intermetallic compounds during reaction between solid iron and molten aluminum. Trans. Nonferrous Met. Soc. China 2013, 23, 556–561. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, G.; Yu, H.; Lv, W.; Wen, K.; Xu, H. Controlling interfacial composition and improvement in bonding strength of compound casted Al/steel bimetal via Cr interlayer. J. Mater. Res. Technol. 2023, 23, 4385–4395. [Google Scholar] [CrossRef]
- Liu, B.; Yang, Q.; Wang, Y. Intereaction and intermetallic phase formation between aluminum and stainless steel. Results Phys. 2019, 12, 514–524. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Liu, X.; Liu, F. Effects of hot-dip galvanizing and aluminizing on interfacial microstructures and mechanical properties of aluminum/iron bimetallic composites. J. Alloys Compd. 2016, 688, 742–751. [Google Scholar] [CrossRef]
- Tavakoli, A.M.; Nami, B.; Malekan, M.; Khoubrou, I. Influences of Coating Type on Microstructure and Strength of Aluminum–Steel Bimetal Composite Interface. Int. J. Met. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajihara, M. Kinetics of isothermal reactive diffusion between solid Fe and liquid Al. J. Mater. Sci. 2010, 45, 5676–5684. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, S.; Han, R.; Tang, M.; Zhao, C.; Zhang, H. Microstructure and mechanical properties of aluminum/steel bimetal using compound casting with electroless nickel plating. Mater. Res. Express 2021, 8, 016517. [Google Scholar] [CrossRef]
- Ma, J.; Wang, T.; Chen, C.; Xiong, M.; Xiao, L.; Wei, S.; Mao, F.; Zhang, C. Effect of High Entropy Alloy Coating on Microstructure and Mechanical Properties of Al/Fe Liquid-Solid Bimetal Composite. Mater. Guide 2023, 37, 159–166. [Google Scholar]
- Liu, S.; Xu, H.; Zhang, B.; Zhang, G.; Bai, L.; Song, H.; Zhang, D.; Chang, C.; Yu, H.; Yang, C. The Effect of the Cu Interlayer on the Interfacial Microstructure and Mechanical Properties of Al/Fe Bimetal by Compound Casting. Materials 2023, 16, 15. [Google Scholar] [CrossRef]
- Gerald, O.J.; Wenge, L.; Tao, Z.Y.; Long, L.C.; Qiang, L. Influence of plasma spraying current on the microstructural characteristics and tribological behaviour of plasma sprayed Cr2O3 coating. Boletín Soc. Española Cerámica Y Vidr. 2020, 60, 338–346. [Google Scholar] [CrossRef]
- Hu, X.P.; Zhao, Y.; Wang, Q.; Zhang, X.Z.; Li, R.X.; Zhang, B.R. Effect of pouring and cooling temperatures on microstructures and mechanical properties of as-cast and T6 treated A356 alloy. China Foundry 2019, 16, 380–385. [Google Scholar] [CrossRef]
- Durkin, B. Passing Customer Specs for High-Phosphorus Electroless Nickel Deposits. Prod. Finish. 2018, 83, 64–65. [Google Scholar]
- Ingersoll rand oil-free compressors continue their breath of fresh air. Munic. Eng. Aust. 2013, 40, 26.
- Walnsch, A.; Kriegel, M.J.; Fabrichnaya, O.; Leineweber, A. Thermodynamic assessment and experimental investigation of the systems Al–Fe–Mn and Al–Fe–Mn–Ni. Calphad 2019, 66, 101621. [Google Scholar] [CrossRef]
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminµm alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Van Alboom, A.; Lemmens, B.; Breitbach, B.; De Grave, E.; Cottenier, S.; Verbeken, K. Multi-method identification and characterization of the intermetallic surface layers of hot-dip Al-coated steel: FeAl3 or Fe4Al13 and Fe2Al5 or Fe2Al5+x. Surf. Coat. Technol. 2017, 324, 419–428. [Google Scholar] [CrossRef]
- Azimaee, H.; Sarfaraz, M.; Mirjalili, M.; Aminian, K. Effect of silicon and manganese on the kinetics and morphology of the intermetallic layer growth during hot-dip aluminizing. Surf. Coat. Technol. 2018, 357, 483–496. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, Q.; Lu, W.; Sun, S.; Xia, M.; Li, J. In situ observation on the formation of intermetallics compounds at the interface of liquid Al/solid Ni. Scr. Mater. 2016, 130, 214–218. [Google Scholar] [CrossRef]
- Nam, D.B.; Hoang TN, O.; Hoang, N.V. Al-Fe-Ni Metallic Glasses via Mechanical Alloying and Its Consolidation. Appl. Sci. 2022, 12, 10561. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Yu, Y.; Fan, Z. Improving mechanical properties of AZ91D magnesium/A356 aluminum bimetal prepared by compound casting via a high velocity oxygen fuel sprayed Ni coating. J. Magnes. Alloys 2022, 10, 1075–1085. [Google Scholar] [CrossRef]
- Xu, G.; Luo, A.A.; Chen, Y.; Sachdev, A.K. Interfacial phenomena in magnesium/aluminum bi-metallic castings. Mater. Sci. Eng. A 2014, 595, 154–158. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, L.; Xu, R. Effect of interlayer composition on the microstructure and strength of diffusion bonded Mg/Al joint. Mater. Des. 2009, 30, 4548–4551. [Google Scholar] [CrossRef]
- Janusz, C.; Stanisław, L. Kinetics of Corrosion Processes of Alloy on the Intermetallic Phase Matrix FeAl in High Temperature in Air Atmosphere. Solid. State Phenom. 2013, 212, 137–140. [Google Scholar]




| C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|
| 0.2 | 0.3 | 0.5 | ≤0.045 | ≤0.045 | Bal. |
| Si | Fe | Mn | Mg | Ti | Al |
|---|---|---|---|---|---|
| 7.5 | ≤0.20 | ≤0.10 | 0.45 | 0.1 | Bal. |
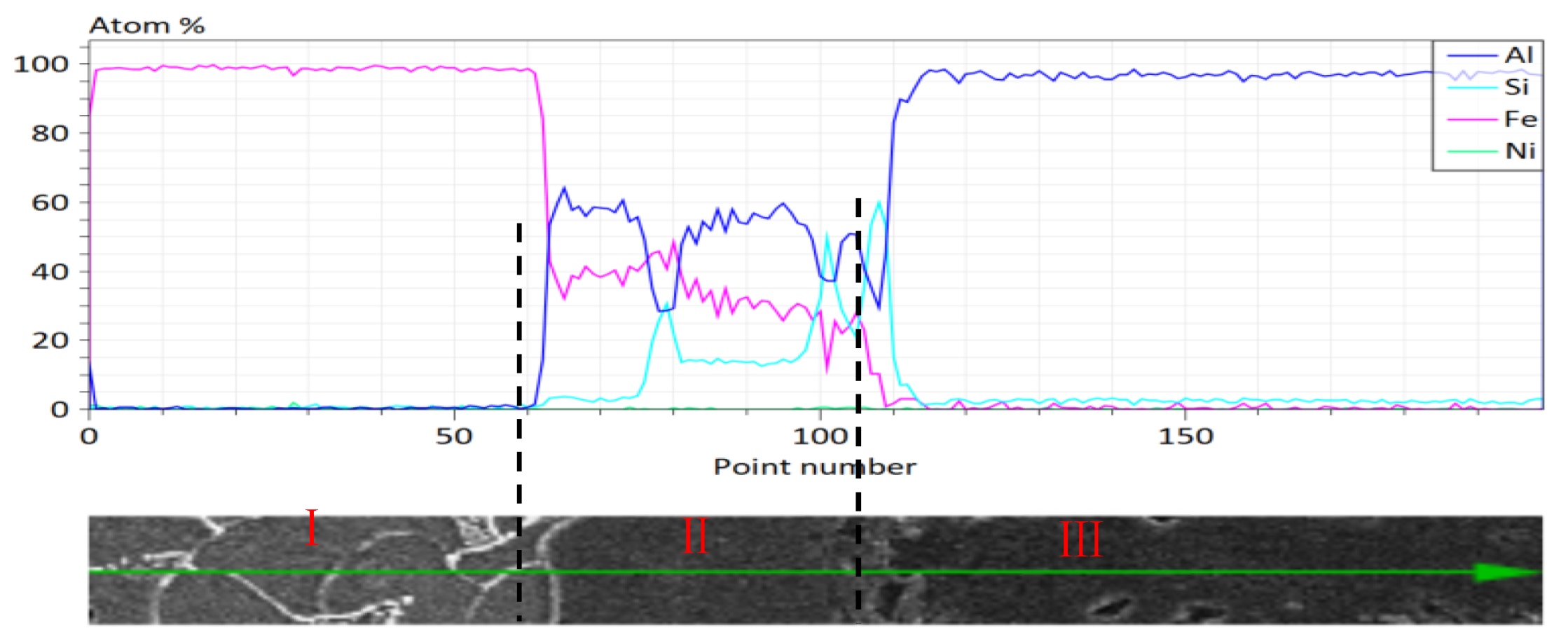
| Region | Element Contents (at.%) | |||
|---|---|---|---|---|
| Al | Fe | Si | Ni | |
| A | 76.80 | 0.33 | 1.95 | 20.93 |
| B | 9.91 | 53.59 | 27.22 | 9.29 |
| C | 87.37 | 0.00 | 5.24 | 7.39 |
| Region | Element Content (at.%) | |||
|---|---|---|---|---|
| Al | Fe | Si | Ni | |
| A | 46.95 | 40.83 | 12.21 | 0.00 |
| B | 57.09 | 29.49 | 13.41 | 0.00 |
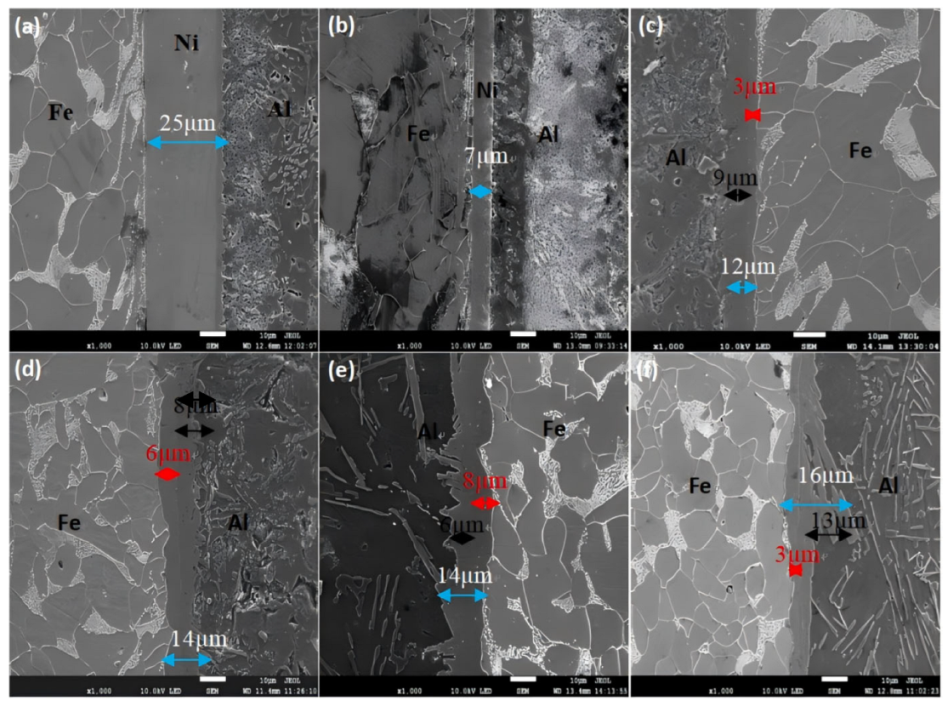
| Hot Dip Time (s) | τ1-Al2Fe3Si3 and FeAl3 Thickness (μm) | τ5-Al8Fe2Si and Fe2Al5 Thickness (μm) | Ni Layer Thickness (μm) |
|---|---|---|---|
| 30 | 0 | 0 | 25 |
| 60 | 0 | 0 | 7 |
| 120 | 3 | 9 | 0 |
| 180 | 6 | 8 | 0 |
| 240 | 8 | 6 | 0 |
| 300 | 3 | 13 | 0 |
| Sample | 1# | 2# | 3# | 4# | 5# | 6# |
|---|---|---|---|---|---|---|
| Hot dip time (s) | 30 | 60 | 120 | 180 | 240 | 300 |
| Shear strength (MPa) | 24.42 | 26.58 | 43.87 | 56.27 | 80.98 | 50.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, G.; Wang, M.; Xu, H. Study on the Influence of Nickel Plating on the Structure and Properties of Aluminum/Steel Bimetallic Bonding. Materials 2025, 18, 1898. https://doi.org/10.3390/ma18091898
Zhang Y, Zhang G, Wang M, Xu H. Study on the Influence of Nickel Plating on the Structure and Properties of Aluminum/Steel Bimetallic Bonding. Materials. 2025; 18(9):1898. https://doi.org/10.3390/ma18091898
Chicago/Turabian StyleZhang, Yufei, Guowei Zhang, Mingjie Wang, and Hong Xu. 2025. "Study on the Influence of Nickel Plating on the Structure and Properties of Aluminum/Steel Bimetallic Bonding" Materials 18, no. 9: 1898. https://doi.org/10.3390/ma18091898
APA StyleZhang, Y., Zhang, G., Wang, M., & Xu, H. (2025). Study on the Influence of Nickel Plating on the Structure and Properties of Aluminum/Steel Bimetallic Bonding. Materials, 18(9), 1898. https://doi.org/10.3390/ma18091898





