Investigation of the Etching Resistance of Yttrium Oxyfluoride Coating Deposited via Atmospheric Plasma Spraying Against Cl2/O2 Plasma
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
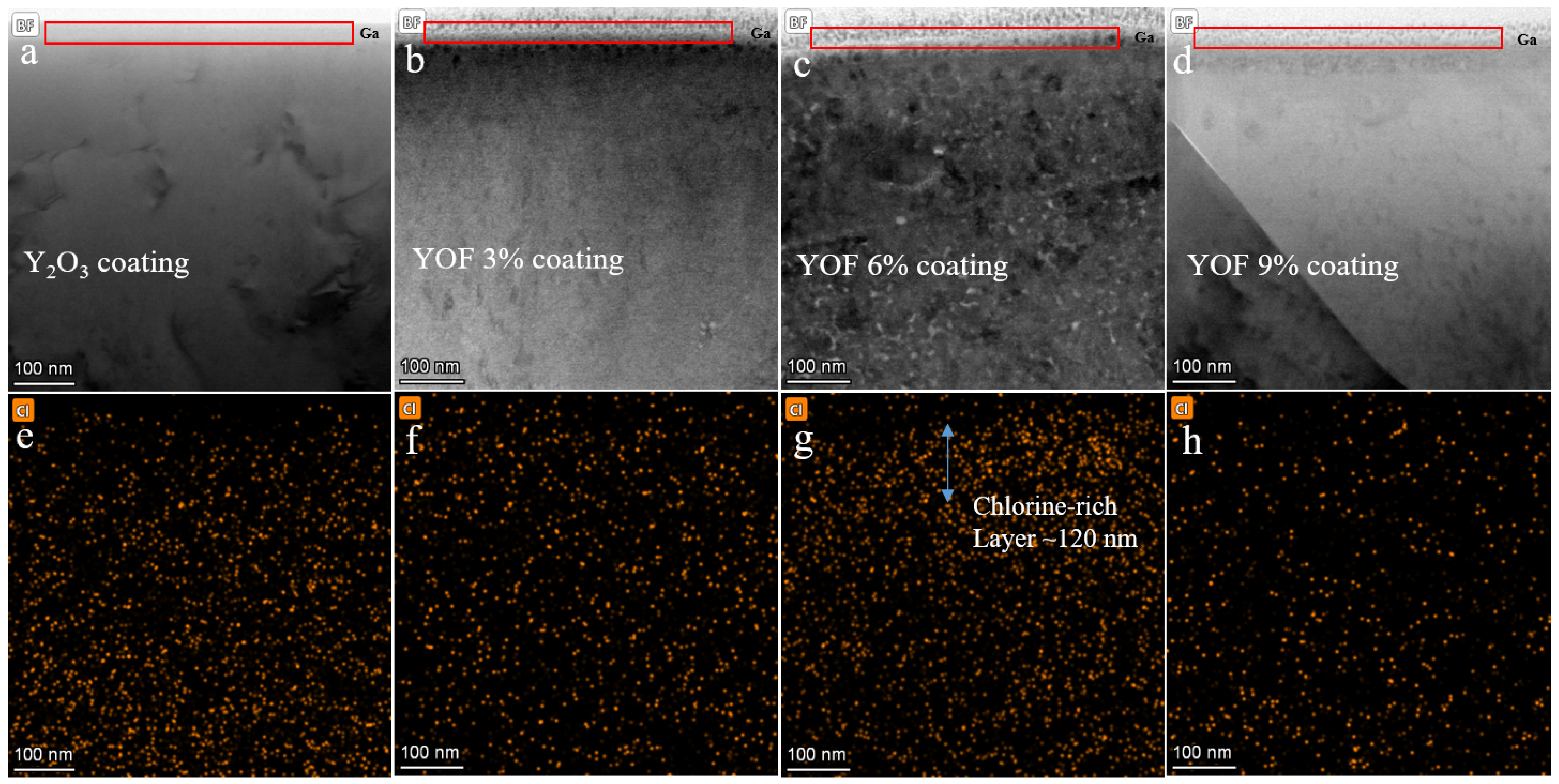
3. Discussion and Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryu, J.E.; Park, S.; Park, Y.; Ryu, S.W.; Hwang, K.; Jang, H.W. Technological breakthroughs in chip fabrication, transfer, and color conversion for high-performance micro-LED displays. Adv. Mater. 2023, 35, 2204947. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, X.; Wu, W.; Li, F.; Wang, R.; Lu, H.; Pan, C. Controlled on-chip fabrication of large-scale perovskite single crystal arrays for high-performance laser and photodetector integration. Light. Sci. Appl. 2023, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, J.; Zhang, Y.; Qu, Y.; Jia, B.; Chen, Z.; Moss, D.J. Fabrication technologies for the on-chip integration of 2D materials. Small Methods 2022, 6, 2101435. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, M.; Wu, Z.; Gao, Y.; Xia, Y.; Yang, P.; Jiang, Z. Advances in high-performance MEMS pressure sensors: Design, fabrication, and packaging. Microsystems Nanoeng. 2023, 9, 156. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Shu, H.; Zhang, Z.; Yi, S.; Bai, B.; Zou, W. Optical coherent dot-product chip for sophisticated deep learning regression. Light. Sci. Appl. 2021, 10, 221. [Google Scholar] [CrossRef]
- Economou, D.J. Pulsed plasma etching for semiconductor manufacturing. J. Phys. Appl. Phys. 2014, 47, 303001. [Google Scholar] [CrossRef]
- Cardinaud, C.; Peignon, M.C.; Tessier, P.Y. Plasma etching: Principles, mechanisms, application to micro-and nano-technologies. Appl. Surf. Sci. 2000, 164, 72–83. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, Y.; Park, H.; Lee, C. The importance of intimate inter-crystallite bonding for the plasma erosion resistance of vacuum kinetic sprayed Y2O3 coating. Surf. Coatings Technol. 2019, 374, 493–499. [Google Scholar] [CrossRef]
- Tezani, L.L.; Pessoa, R.S.; Maciel, H.S.; Petraconi, G. Chemistry studies of SF6/CF4, SF6/O2 and CF4/O2 gas phase during hollow cathode reactive ion etching plasma. Vacuum 2014, 106, 64–68. [Google Scholar] [CrossRef]
- Coburn, J.W.; Winters, H.F. Plasma etching—A discussion of mechanisms. J. Vac. Sci. Technol. 1979, 16, 391–403. [Google Scholar] [CrossRef]
- Lee, C.G.; Kanarik, K.J.; Gottscho, R.A. The grand challenges of plasma etching: A manufacturing perspective. J. Phys. Appl. Phys. 2014, 47, 273001. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Lee, J.; Hwang, H.W.; Jeong, G.; Kim, K.Y.; Jeon, H. Improvement of yttrium oxyfluoride coating with modified precursor solution for laser-induced hydrothermal synthesis. Coatings 2022, 12, 740. [Google Scholar] [CrossRef]
- So, J.; Kim, M.; Kwon, H.; Maeng, S.; Choi, E.; Chung, C.W.; Yun, J.Y. Investigation of contamination particles generation and surface chemical reactions on Al2O3, Y2O3, and YF3 coatings in F-based plasma. Appl. Surf. Sci. 2023, 629, 157367. [Google Scholar] [CrossRef]
- Lim, K.Y.; Kim, Y.W.; Kim, K.J. Mechanical properties of electrically conductive silicon carbide ceramics. Ceram. Int. 2014, 40, 10577–10582. [Google Scholar] [CrossRef]
- Zou, B.; Khan, Z.S.; Gu, L.; Fan, X.; Huang, W.; Wang, Y.; Cao, X. Microstructure, oxidation protection and failure mechanism of Yb2SiO5/LaMgAl11O19 coating deposited on C/SiC composites by atmospheric plasma spraying. Corros. Sci. 2012, 62, 192–200. [Google Scholar] [CrossRef]
- Kreethi, R.; Hwang, Y.J.; Lee, H.Y.; Park, J.H.; Lee, K.A. Surface analysis of yttrium oxyfluoride deposited via air plasma spraying for Erosion resistance against NF3 plasma. J. Korean Ceram. Soc. 2024, 61, 63–70. [Google Scholar] [CrossRef]
- Kreethi, R.; Hwang, Y.J.; Lee, H.Y.; Park, J.H.; Lee, K.A. Stability and plasma etching behavior of yttrium-based coatings by air plasma spray process. Surf. Coatings Technol. 2023, 454, 129182. [Google Scholar] [CrossRef]
- Ku, C.M.; Cheng, S. Factor design for the oxide etching process to reduce edge particle contamination in capacitively coupled plasma etching equipment. Appl. Sci. 2022, 12, 5684. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, C.; Chen, Y.; Wang, Y. Phase composition, structural, and plasma erosion properties of ceramic coating prepared by suspension plasma spraying. Int. J. Appl. Ceram. Technol. 2018, 15, 1388–1396. [Google Scholar] [CrossRef]
- Miwa, K.; Takada, N.; Sasaki, K. Fluorination mechanisms of Al2O3 and Y2O3 surfaces irradiated by high-density CF4/O2 and SF6/O2 plasmas. J. Vac. Sci. Technol. 2009, 27, 831–835. [Google Scholar] [CrossRef]
- Kim, D.M.; Lee, S.H.; Alexander, W.B.; Kim, K.B.; Oh, Y.S.; Lee, S.M. X-ray photoelectron spectroscopy study on the interaction of yttrium–aluminum oxide with fluorine-based plasma. J. Am. Ceram. Soc. 2011, 94, 3455–3459. [Google Scholar] [CrossRef]
- Qin, X.; Zhou, G.; Yang, H.; Wong, J.I.; Zhang, J.; Luo, D.; Tang, D. Fabrication and plasma resistance properties of transparent YAG ceramics. Ceram. Int. 2012, 38, 2529–2535. [Google Scholar] [CrossRef]
- Lim, K.S.; Jang, T.S.; Jeong, J.H.; Hong, S.H.; Jin, J. Effect of Interlayer on Flatness and Adhesion of Aerosol-Deposited Yttrium Oxide Coating. Materials 2024, 17, 3533. [Google Scholar] [CrossRef]
- Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication, characterization, and fluorine-plasma exposure behavior of dense yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2017, 56, 06HC02. [Google Scholar] [CrossRef]
- Shiba, Y.; Teramoto, A.; Goto, T.; Kishi, Y.; Shirai, Y.; Sugawa, S. Stable yttrium oxyfluoride used in plasma process chamber. J. Vac. Sci. Technol. 2017, 35, 2. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Han, H.N.; Kim, W.; Hwang, N.M. Yttrium oxyfluoride coatings deposited by suspension plasma spraying using coaxial feeding. Coatings 2020, 10, 481. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, W.; Hwang, N.M. Plasma etching behavior of YOF coating deposited by suspension plasma spraying in inductively coupled CHF3/Ar plasma. Coatings 2020, 10, 1023. [Google Scholar] [CrossRef]
- Lin, T.K.; Wang, W.K.; Huang, S.Y.; Tasi, C.T.; Wuu, D.S. Comparison of erosion behavior and particle contamination in mass-production CF4/O2 plasma chambers using Y2O3 and YF3 protective coatings. Nanomaterials 2017, 7, 183. [Google Scholar] [CrossRef]
- Ma, T.; List, T.; Donnelly, V.M. Comparisons of NF3 plasma-cleaned Y2O3, YOF, and YF3 chamber coatings during silicon etching in Cl2 plasmas. J. Vac. Sci. Technol. 2018, 36, 3. [Google Scholar] [CrossRef]
- Tahara, R.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication of dense yttrium oxyfluoride ceramics by hot pressing and their mechanical, thermal, and electrical properties. Jpn. J. Appl. Phys. 2018, 57, 06JF04. [Google Scholar] [CrossRef]
- Miyashita, K.; Yoshida, K.; Yano, T.; Matsukura, K.; Kishi, Y. Corrosion behavior of yttrium oxyfluoride ceramics in HCl, HNO3 and HF solutions at room temperature. Jpn. J. Appl. Phys. 2020, 59, SJJB02. [Google Scholar] [CrossRef]
- Wang, W.K.; Lin, Y.X.; Xu, Y.J. Structural and fluorine plasma etching behavior of sputter-deposition yttrium fluoride film. Nanomaterials 2018, 8, 936. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Wuu, D.S.; Huang, S.Y.; Wang, W.K. Preparation and characterization of sprayed-yttrium oxyfluoride corrosion protective coating for plasma process chambers. Coatings 2018, 8, 373. [Google Scholar] [CrossRef]
- Ma, T.; List, T.; Donnelly, V.M. Y2O3 wall interactions in Cl2 etching and NF3 cleaning plasmas. J. Vac. Sci. Technol. 2017, 35, 3. [Google Scholar] [CrossRef]

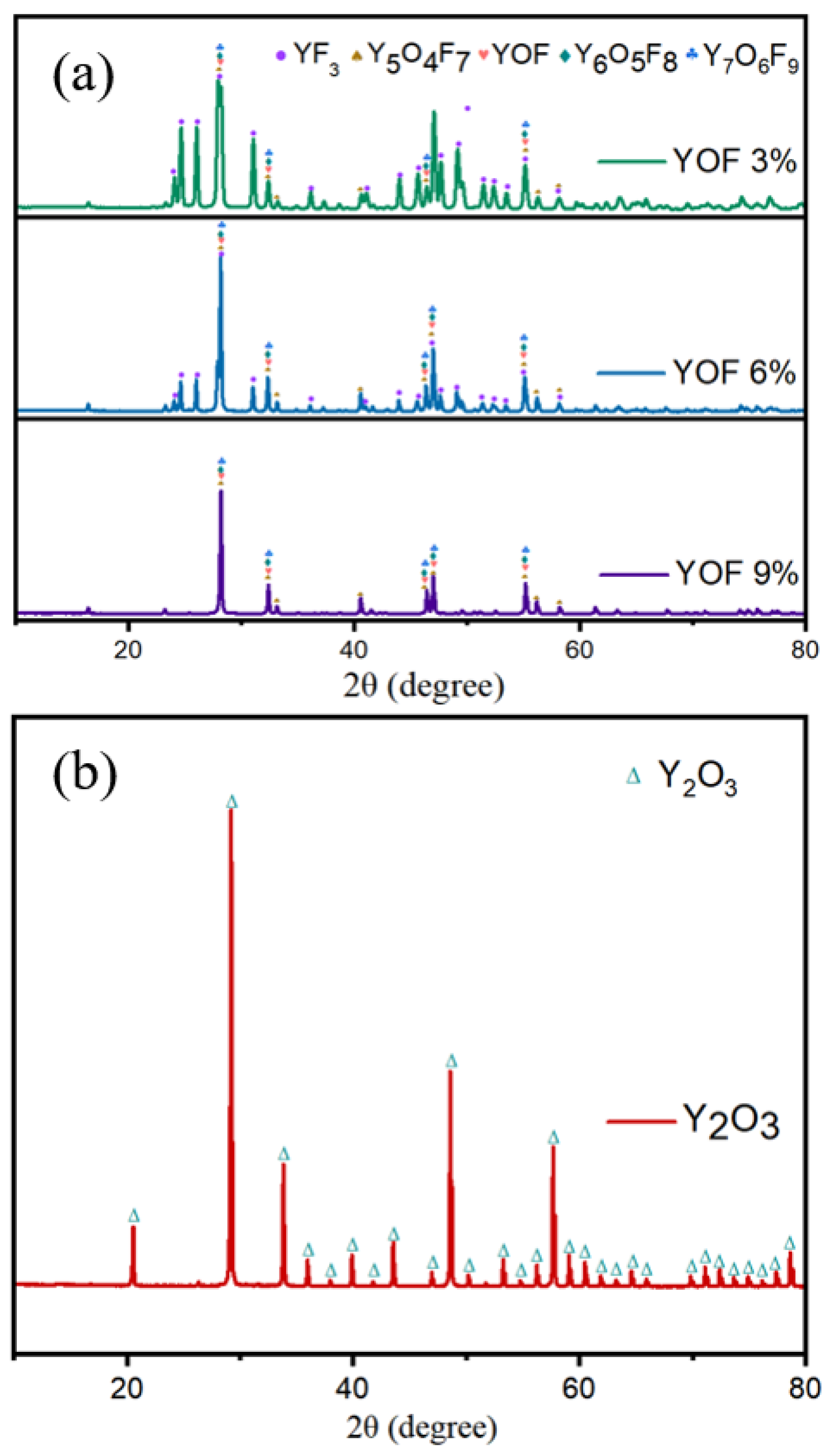
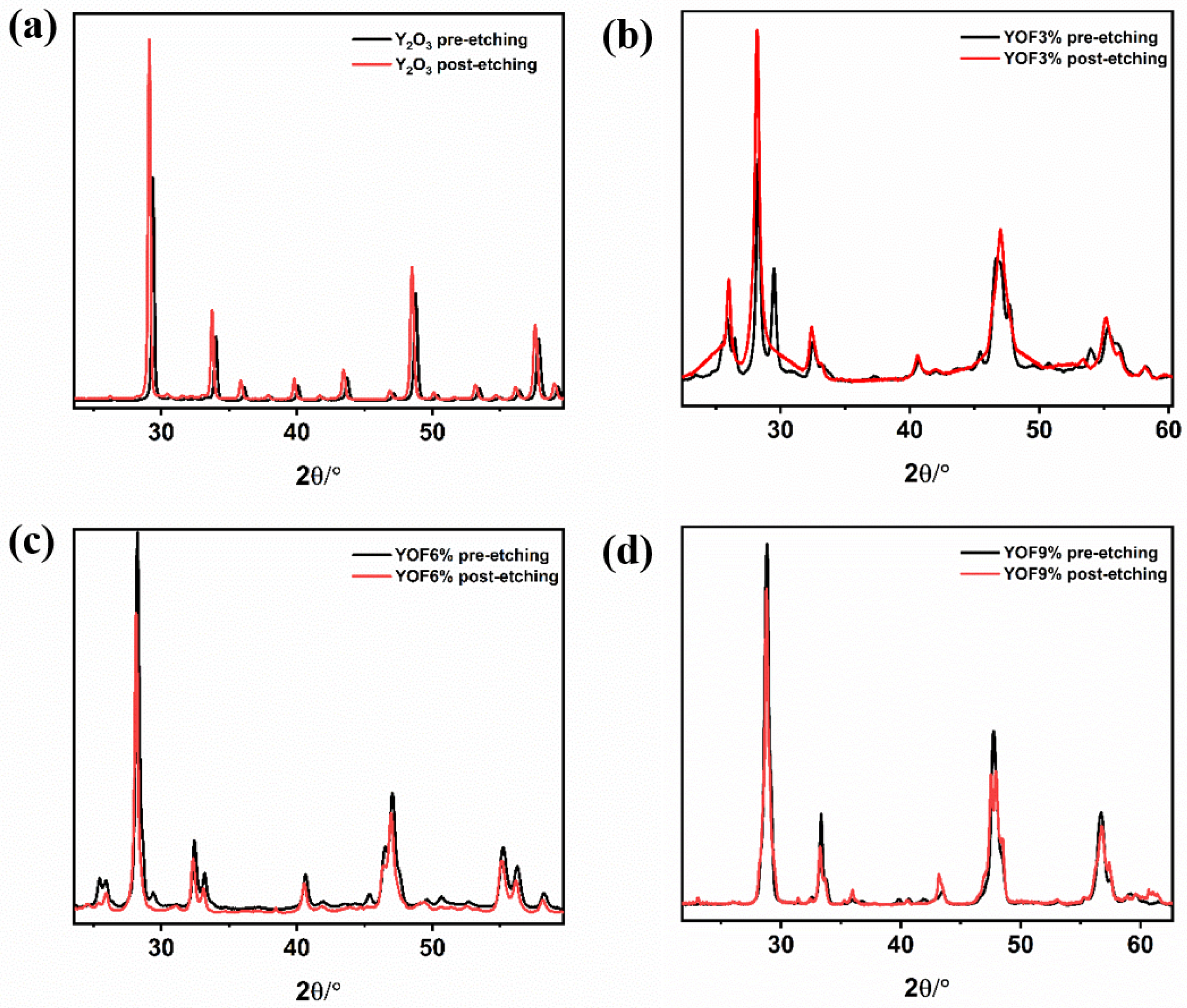


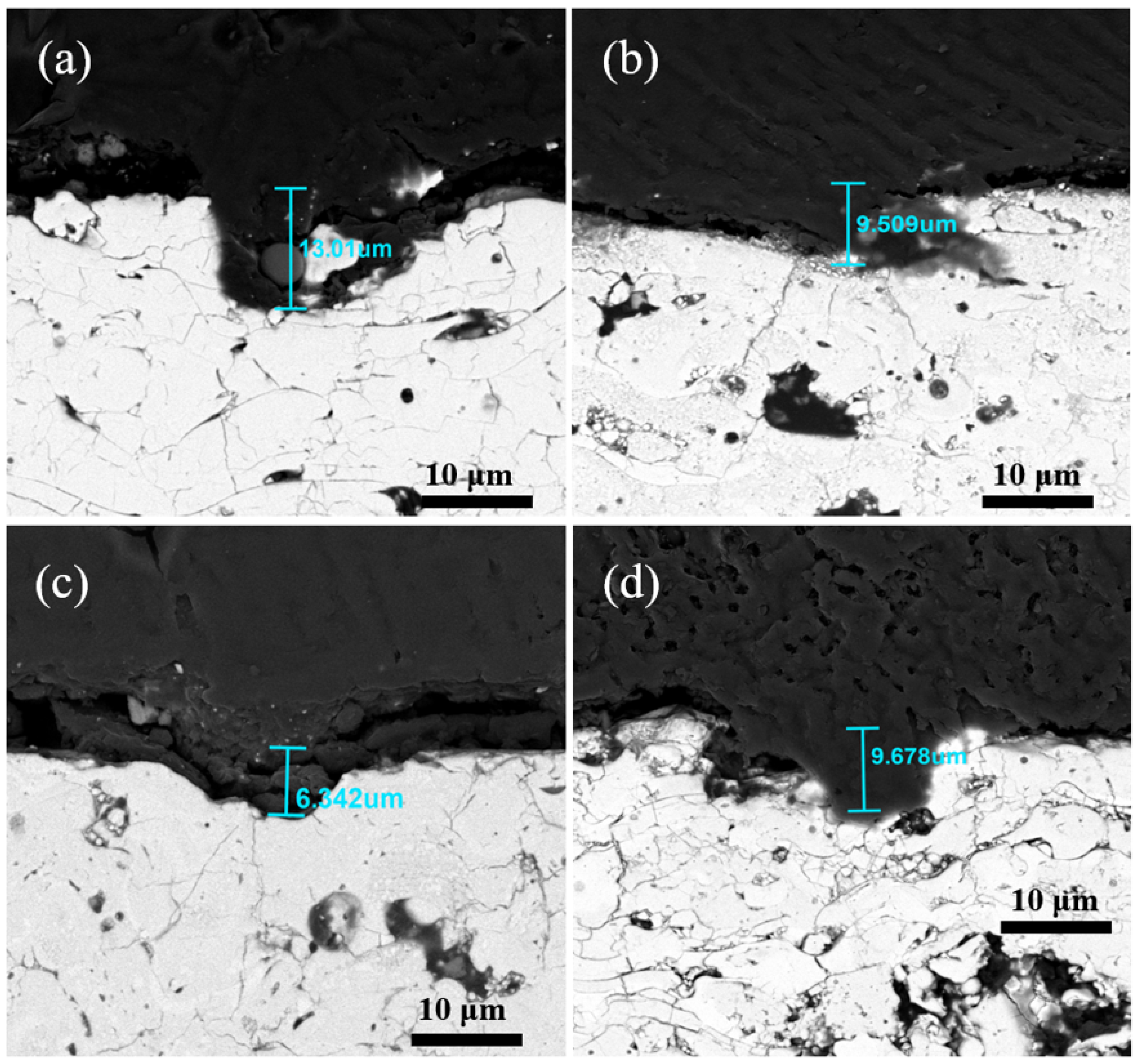
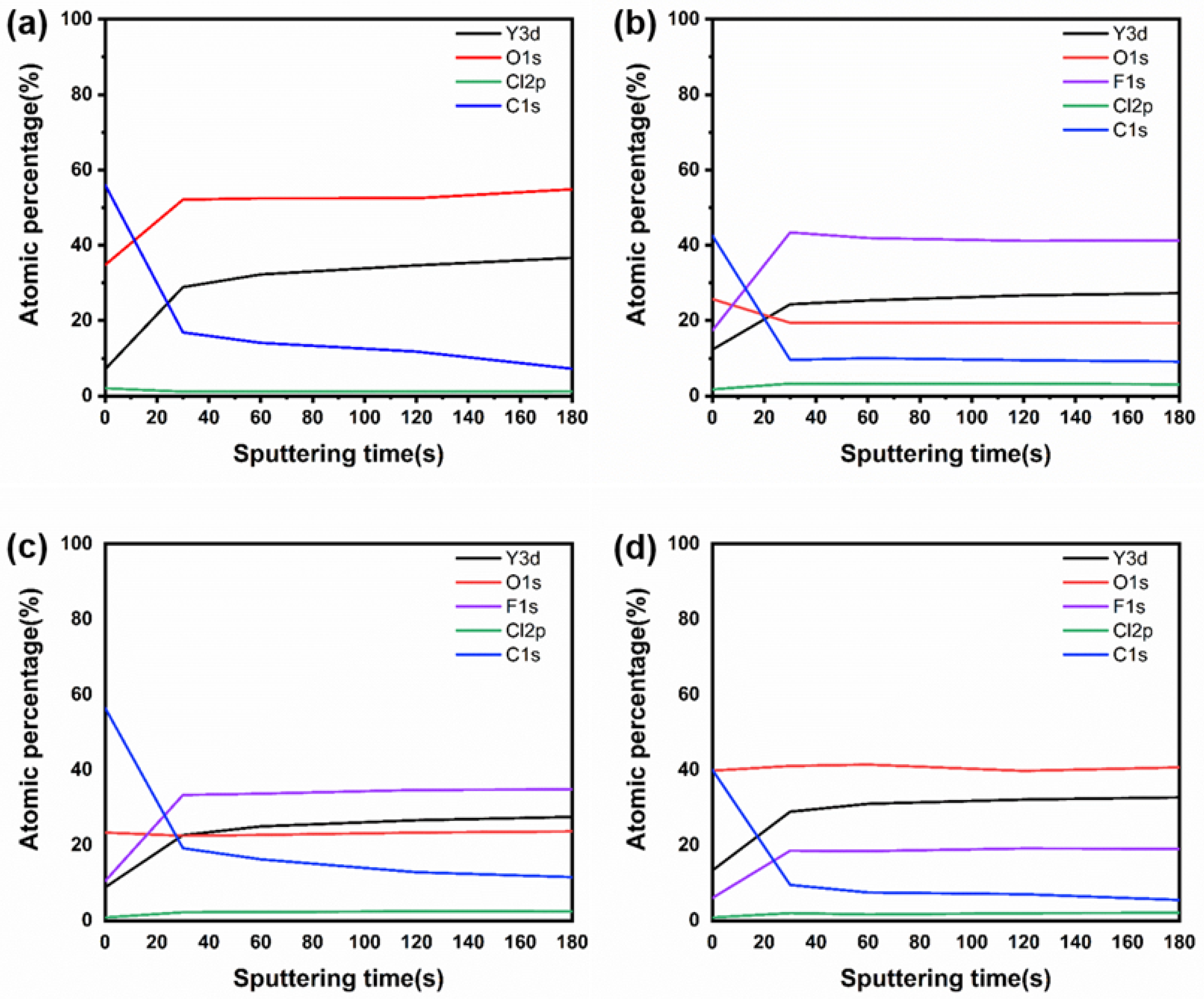
| Parameter | Y2O3 | YOF 3% | YOF 6% | YOF 9% |
|---|---|---|---|---|
| Median particle size (D50, µm) | 34.0 | 27.1 | 26.6 | 27.7 |
| Angle of repose (∘) | 33.5 | 33.7 | 31.4 | 30.6 |
| Y2O3 | YOF 3% | YOF 6% | YOF 9% | |
|---|---|---|---|---|
| Pre-etching (g) | 24.19 | 25.33 | 25.38 | 25.32 |
| Post-etching (g) | 23.74 | 24.54 | 25.11 | 24.59 |
| Mass variation (g) | −0.45 | −0.79 | −0.27 | −0.73 |
| Decrement (g/cm2) | 0.046 | 0.127 | 0.018 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Lv, Y.; Ang, K.; Wang, B.; Jiang, X.; Wang, Y.; Xu, J.; Meng, H.; Chen, H.; Shi, Y.; et al. Investigation of the Etching Resistance of Yttrium Oxyfluoride Coating Deposited via Atmospheric Plasma Spraying Against Cl2/O2 Plasma. Materials 2025, 18, 1903. https://doi.org/10.3390/ma18091903
Tang Z, Lv Y, Ang K, Wang B, Jiang X, Wang Y, Xu J, Meng H, Chen H, Shi Y, et al. Investigation of the Etching Resistance of Yttrium Oxyfluoride Coating Deposited via Atmospheric Plasma Spraying Against Cl2/O2 Plasma. Materials. 2025; 18(9):1903. https://doi.org/10.3390/ma18091903
Chicago/Turabian StyleTang, Zaifeng, Yukun Lv, Kaiqu Ang, Bing Wang, Xiaojun Jiang, Yuwei Wang, Jin Xu, Hua Meng, Hongli Chen, Ying Shi, and et al. 2025. "Investigation of the Etching Resistance of Yttrium Oxyfluoride Coating Deposited via Atmospheric Plasma Spraying Against Cl2/O2 Plasma" Materials 18, no. 9: 1903. https://doi.org/10.3390/ma18091903
APA StyleTang, Z., Lv, Y., Ang, K., Wang, B., Jiang, X., Wang, Y., Xu, J., Meng, H., Chen, H., Shi, Y., & Wang, L. (2025). Investigation of the Etching Resistance of Yttrium Oxyfluoride Coating Deposited via Atmospheric Plasma Spraying Against Cl2/O2 Plasma. Materials, 18(9), 1903. https://doi.org/10.3390/ma18091903





