Kinetic Study and Simulation of Titanium Carbide-Supported, Platinum-Doped Tetrahedral Amorphous Carbon Electrodes for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Mathematical Modelling
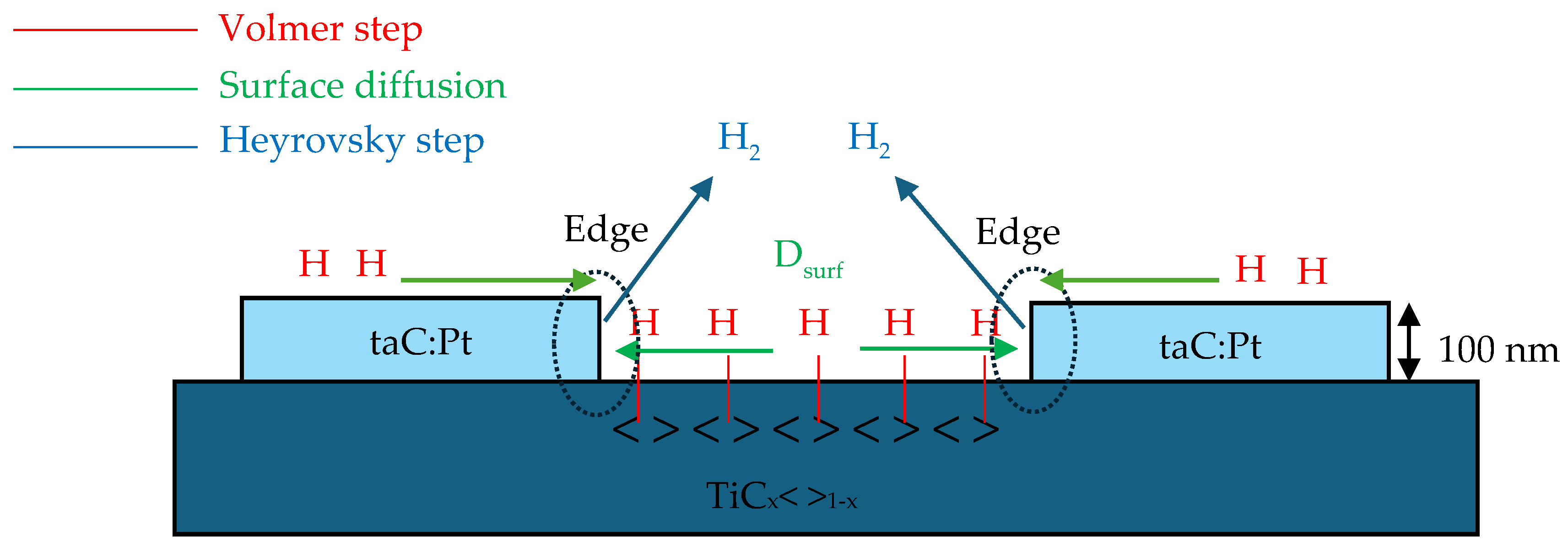
3. Volmer–Heyrovsky–Tafel Mechanism
- (1)
- 0.5 M of H2SO4 of high acidic concentration, hence cH+ = 1000 mol/m3.
- (2)
- The maximum surface concentration of the material, = 1 × 10−5 mol/m2 [38].
- (3)
4. Time-Dependent Properties
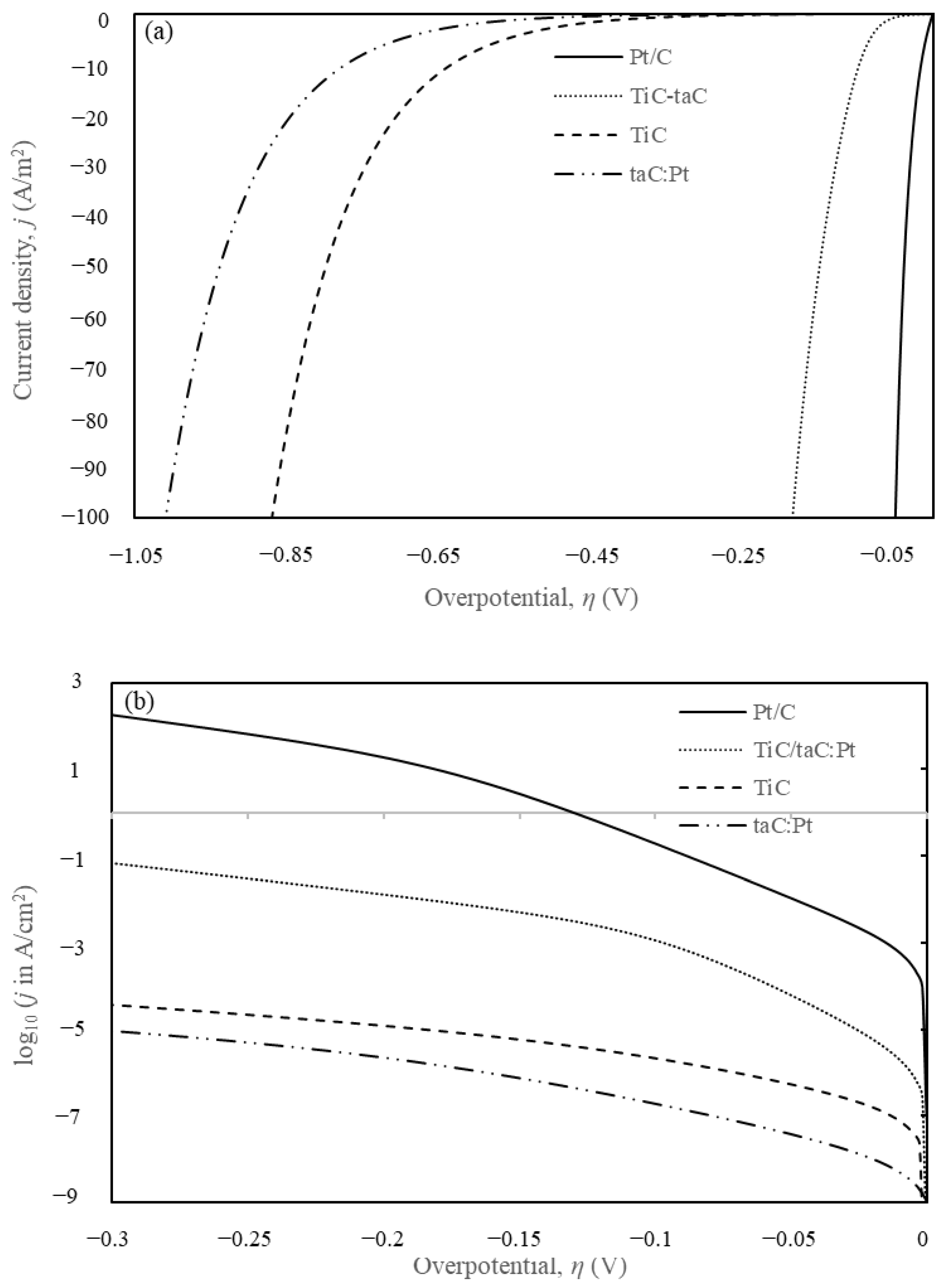
5. Results and Discussion
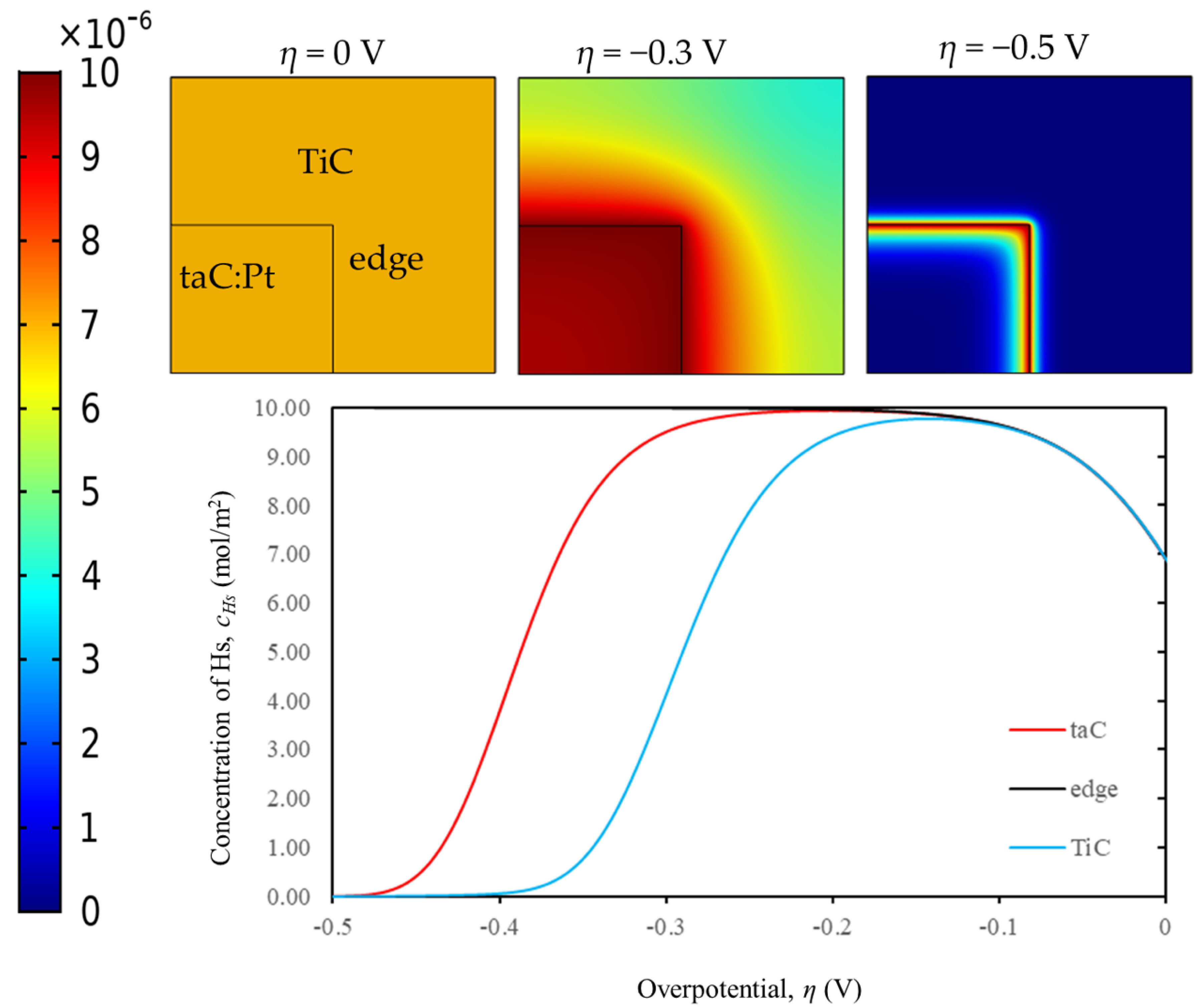
5.1. Finite Element Method (FEM) Simulation
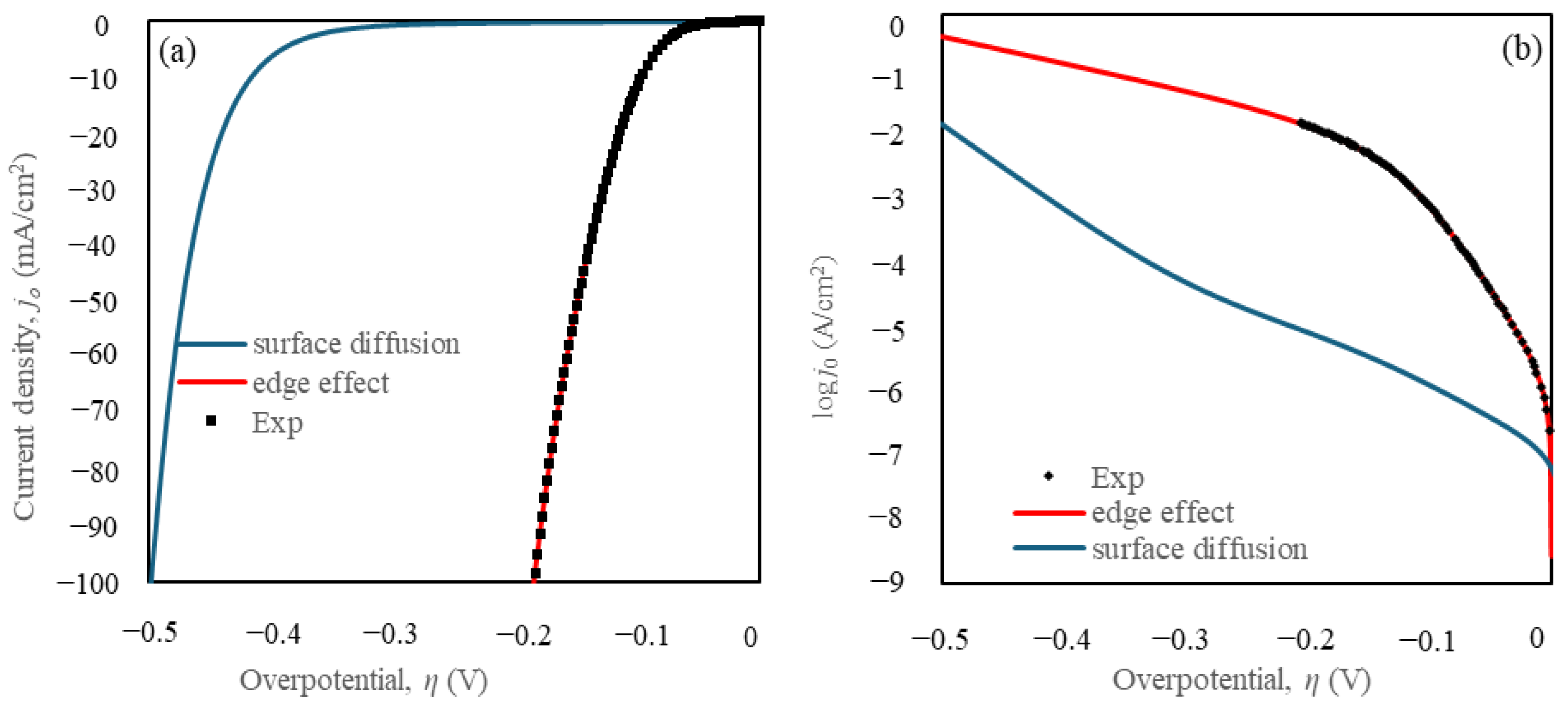
5.2. Effect of Surface Diffusion and the Edge Effect Between TiC Substrate and taC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Secher, N.M.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is there anything better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, J.; Li, J.; Li, Y.; Jin, X.; Zhu, Y.; Liu, Y.; Li, Z.; El-Harairy, A.; Xiao, C.; et al. Artificial heterointerfaces achieve delicate reaction kinetics towards hydrogen evolution and hydrazine oxidation catalysis. Angew. Chem. 2021, 133, 6049–6058. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Sun, Z. Novel 2D transition-metal carbides: Ultrahigh performance electrocatalysts for overall water splitting and oxygen reduction. Adv. Funct. Mater. 2020, 30, 2000570. [Google Scholar] [CrossRef]
- Chen, P.; Ye, J.; Wang, H.; Ouyang, L.; Zhu, M. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting. J. Alloys Compd. 2021, 883, 160833. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, Q.; Zhu, X.; Wei, Y.; Wang, J. Synergetic Effect and Phase Engineering by Formation of Ti3C2Tx Modified 2H/1T-MoSe2 Composites for Enhanced HER. Materials 2023, 16, 6991. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic water splitting hydrogen production via environmental benign carbon based nanomaterials. Int. J. Hydrogen Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Ahmed, K.; Hameed, S.; Patchigolla, K.; Dawood, N.; Ghouri, Z.K. Carbon-based electrocatalysts for hydrogen evolution reaction. Energy Convers. Manag. X 2025, 26, 100892. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Zocche, N.; Lorenzi, R.; Ferrara, C.; Poli, F.; Soavi, F.; Santoro, C. Valorization of the inedible pistachio shells into nanoscale transition metal and nitrogen codoped carbon-based electrocatalysts for hydrogen evolution reaction and oxygen reduction reaction. Mater. Renew. Sustain. Energy 2022, 11, 131–141. [Google Scholar] [CrossRef]
- Ferri, T.; Gozzi, D.; Latini, A. Hydrogen evolution reaction (HER) at thin film and bulk TiC electrodes. Int. J. Hydrogen Energy 2007, 32, 4692–4701. [Google Scholar] [CrossRef]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Song, M.; Chen, D.; Yang, Y.; Xiang, M.; Zhu, Q.; Zhao, H.; Ward, L.; Chen, X. Crystal Facet Engineering of Single-Crystalline TiC Nanocubes for Improved Hydrogen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2008028. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Ye, Y.; Lee, S.; Park, J.; Lee, H.; Lee, J.; Han, J.W. Rational design of TiC-supported single-atom electrocatalysts for hydrogen evolution and selective oxygen reduction reactions. ACS Energy Lett. 2018, 4, 126–132. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Z.; Liao, B.; Wu, X.; Zhang, X. Comparative study on effects of Ni ion implantation on amorphous carbon (aC) coating and tetrahedral amorphous carbon (ta-C) coating. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 467, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Chen, H.; Wang, M. Nanoindentation-induced deformation behaviors of tetrahedral amorphous carbon film deposited by cathodic vacuum arc with different substrate bias voltages. Appl. Surf. Sci. 2022, 576, 151741. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kim, J.-I.; Lee, W.; Kim, J. Tribological properties of multilayer tetrahedral amorphous carbon coatings deposited by filtered cathodic vacuum arc deposition. Friction 2021, 9, 1292–1302. [Google Scholar] [CrossRef]
- Indra, A.; Menezes, P.W.; Sahraie, N.R.; Bergmann, A.; Das, C.; Tallarida, M.; Schmeißer, D.; Strasser, P.; Driess, M. Unification of catalytic water oxidation and oxygen reduction reactions: Amorphous beat crystalline cobalt iron oxides. J. Am. Chem. Soc. 2014, 136, 17530–17536. [Google Scholar] [CrossRef]
- Liu, J.; Nai, J.; You, T.; An, P.; Zhang, J.; Ma, G.; Niu, X.; Liang, C.; Yang, S.; Guo, L. The flexibility of an amorphous cobalt hydroxide nanomaterial promotes the electrocatalysis of oxygen evolution reaction. Small 2018, 14, 1703514. [Google Scholar] [CrossRef]
- Laurila, T.; Caro, M.A. Special features of the electrochemistry of undoped tetrahedral amorphous carbon (ta-c) thin films. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 856–862. [Google Scholar]
- Huang, R.Q.; Liao, W.P.; Yan, M.X.; Liu, S.; Li, Y.M.; Kang, X.W. P-doped Ru-pt alloy catalyst toward high performance alkaline hydrogen evolution reaction. J. Electrochem. 2023, 29, 3. [Google Scholar]
- Chen, W.; Zhu, X.; Wei, W.; Chen, H.; Dong, T.; Wang, R.; Liu, M.; Ostrikov, K.; Peng, P.; Zang, S. Neighboring platinum atomic sites activate platinum–cobalt nanoclusters as high-performance ORR/OER/HER electrocatalysts. Small 2023, 19, 2304294. [Google Scholar] [CrossRef]
- Maheskumar, V.; Min, A.; Kumar, A.; Senthil, R.A.; Moon, C.J.; Choi, M.Y. Accelerating the Hydrogen Evolution Kinetics with a Pulsed Laser–Synthesized Platinum Nanocluster–Decorated Nitrogen-Doped Carbon Electrocatalyst for Alkaline Seawater Electrolysis. Small 2024, 20, 2403314. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Rajbongshi, B.M.; Ramani, V.; Verma, A. Titanium carbide: An emerging electrocatalyst for fuel cell and electrolyser. Int. J. Hydrogen Energy 2021, 46, 12801–12821. [Google Scholar] [CrossRef]
- Farid, W.; Li, H.; Wang, Z.; Cui, H.; Kong, C.; Yu, H. Integrating Experimental and Computational Analyses for Mechanical Characterization of Titanium Carbide/Aluminum Metal Matrix Composites. Materials 2024, 17, 2093. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Garcia, E.M.; Hebenstreit, J.; Khalifa, Y.; Soltero, C.R.; Rakos, J.; Kaulfuss, F.; A Rusinek, C. Electrochemical Performance of Boron-and Nitrogen-Doped Tetrahedral Amorphous Carbon. Electrochim. Acta 2024, 502, 144655. [Google Scholar] [CrossRef]
- Rao, L.; Liu, H.; Hu, T.; Shao, W.; Shi, Z.; Xing, X.; Zhou, Y.; Yang, Q. Relationship between bonding characteristic and thermal property of amorphous carbon structure: Ab initio molecular dynamics study. Diam. Relat. Mater. 2021, 111, 108211. [Google Scholar] [CrossRef]
- Yang, T.T.; Tan, T.L.; Saidi, W.A. High activity toward the hydrogen evolution reaction on the edges of MoS2-supported platinum nanoclusters using cluster expansion and electrochemical modeling. Chem. Mater. 2020, 32, 1315–1321. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Tan, Q.; Wang, J.; Liu, J.; Wan, H.; Miao, L.; Jiang, J. Simultaneous interfacial chemistry and inner Helmholtz plane regulation for superior alkaline hydrogen evolution. Energy Environ. Sci. 2020, 13, 3007–3013. [Google Scholar] [CrossRef]
- Xiao, D.; Ruan, Q.; Bao, D.L.; Luo, Y.; Huang, C.; Tang, S.; Shen, J.; Cheng, C.; Chu, P.K. Effects of ion energy and density on the plasma etching-induced surface area, edge electrical field, and multivacancies in MoSe2 nanosheets for enhancement of the hydrogen evolution reaction. Small 2020, 16, 2001470. [Google Scholar] [CrossRef]
- Chu, Y.-M.; Nazir, U.; Sohail, M.; Selim, M.M.; Lee, J.-R. Enhancement in thermal energy and solute particles using hybrid nanoparticles by engaging activation energy and chemical reaction over a parabolic surface via finite element approach. Fractal Fract. 2021, 5, 119. [Google Scholar] [CrossRef]
- Lao, M.; Li, P.; Jiang, Y.; Pan, H.; Dou, S.X.; Sun, W. From fundamentals and theories to heterostructured electrocatalyst design: An in-depth understanding of alkaline hydrogen evolution reaction. Nano Energy 2022, 98, 107231. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef] [PubMed]
- Kahyarian, A.; Brown, B.; Nesic, S. Mechanism of the hydrogen evolution reaction in mildly acidic environments on gold. J. Electrochem. Soc. 2017, 164, H365. [Google Scholar] [CrossRef]
- Kichigin, V.I.; Shein, A.B. Kinetics and mechanism of hydrogen evolution reaction on cobalt silicides in alkaline solutions. Electrochim. Acta 2015, 164, 260–266. [Google Scholar] [CrossRef]
- Kichigin, V.I.; Shein, A.B. The kinetics of hydrogen evolution reaction accompanied by hydrogen absorption reaction with consideration of subsurface hydrogen as an adsorbed species: Polarization curve. J. Electroanal. Chem. 2020, 873, 114427. [Google Scholar] [CrossRef]
- Vilekar, S.A.; Fishtik, I.; Datta, R. Kinetics of the hydrogen electrode reaction. J. Electrochem. Soc. 2010, 157, B1040. [Google Scholar] [CrossRef]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry; World Scientific: Singapore, 2018. [Google Scholar]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2022. [Google Scholar]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Kemppainen, E.; Halme, J.; Hansen, O.; Seger, B.; Lund, P.D. Two-phase model of hydrogen transport to optimize nanoparticle catalyst loading for hydrogen evolution reaction. Int. J. Hydrogen Energy 2016, 41, 7568–7581. [Google Scholar] [CrossRef]
- Glandut, N.; Malec, A.D.; Mirkin, M.V.; Majda, M. Electrochemical studies of the lateral diffusion of TEMPO in the aqueous liquid/vapor interfacial region. J. Phys. Chem. B 2006, 110, 6101–6109. [Google Scholar] [CrossRef]
- Glandut, N.; Monson, C.F.; Majda, M. Electrochemistry of TEMPO in the aqueous liquid/vapor interfacial region: Measurements of the lateral mobility and kinetics of surface partitioning. Langmuir 2006, 22, 10697–10704. [Google Scholar] [CrossRef]
- Zubair, M.; Hassan, M.M.U.; Mehran, M.T.; Baig, M.M.; Hussain, S.; Shahzad, F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: A review. Int. J. Hydrogen Energy 2022, 47, 2794–2818. [Google Scholar] [CrossRef]
- Glandut, N.; Orlianges, J.C.; Bidron, G. Hydrogen evolution reaction at titanium carbide-supported, platinum-doped tetrahedral amorphous carbon array electrodes. In Proceedings of the EMN Ceramics Meeting, Orlando, FL, USA, 26–29 January 2015. [Google Scholar]
- Liu, Y.; Wang, Q.; Zhang, J.; Ding, J.; Cheng, Y.; Wang, T.; Li, J.; Hu, F.; Yang, H.B.; Liu, B. Recent advances in carbon-supported noble-metal electrocatalysts for hydrogen evolution reaction: Syntheses, structures, and properties. Adv. Energy Mater. 2022, 12, 2200928. [Google Scholar] [CrossRef]
- Protopopova, V.; Iyer, A.; Wester, N.; Kondrateva, A.; Sainio, S.; Palomäki, T.; Laurila, T.; Mishin, M.; Koskinen, J. Ultrathin undoped tetrahedral amorphous carbon films: The role of the underlying titanium layer on the electronic structure. Diam. Relat. Mater. 2015, 57, 43–52. [Google Scholar] [CrossRef]
- Zhu, Q.; Qu, Y.; Liu, D.; Ng, K.W.; Pan, H. Two-dimensional layered materials: High-efficient electrocatalysts for hydrogen evolution reaction. ACS Appl. Nano Mater. 2020, 3, 6270–6296. [Google Scholar] [CrossRef]
- Jian, C.; Cai, Q.; Hong, W.; Li, J.; Liu, W. Edge-riched MoSe2/MoO2 hybrid electrocatalyst for efficient hydrogen evolution reaction. Small 2018, 14, 1703798. [Google Scholar] [CrossRef]
- Ramji, H.R. Parametric study and simulation of Titanium Carbide (TiC) supported, Platinum doped tetrahedral Carbon (taC) electrodes for Hydrogen Evolution Reactions (HER). Ph.D. Dissertation, Université de Limoges, Limoges, France, Université de Malaisie Sarawak, Kota Samarahan, Malaysia, 2023. [Google Scholar]
- Campos-Roldán, C.; González-Huerta, R.; Alonso-Vante, N. The oxophilic and electronic effects on anchored platinum nanoparticles on sp2 carbon sites: The hydrogen evolution and oxidation reactions in alkaline medium. Electrochim. Acta 2018, 283, 1829–1834. [Google Scholar] [CrossRef]
- Wang, K.; Yu, J.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J. Loading of Single Atoms of Iron, Cobalt, or Nickel to Enhance the Electrocatalytic Hydrogen Evolution Reaction of Two-Dimensional Titanium Carbide. Int. J. Mol. Sci. 2024, 25, 4034. [Google Scholar] [CrossRef]
- Manikandan, A.; Ilango, P.R.; Chen, C.W.; Wang, Y.C.; Shih, Y.C.; Lee, L.; Wang, Z.M.; Ko, H.; Chueh, Y.L. A superior dye adsorbent towards the hydrogen evolution reaction combining active sites and phase-engineering of (1T/2H) MoS 2/α-MoO 3 hybrid heterostructured nanoflowers. J. Mater. Chem. A 2018, 6, 15320–15329. [Google Scholar] [CrossRef]
- Tan, Y.; Feng, J.; Dong, H.; Liu, L.; Zhao, S.; Lai, F.; Liu, T.; Bai, Y.; Parkin, I.P.; He, G. The edge effects boosting hydrogen evolution performance of platinum/transition bimetallic phosphide hybrid electrocatalysts. Adv. Funct. Mater. 2023, 33, 2209967. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Vasileff, A.; Jiao, Y.; Chen, S.; Song, L.; Zheng, B.; Zheng, Y.; Qiao, S.-Z. Strain effect in bimetallic electrocatalysts in the hydrogen evolution reaction. ACS Energy Lett. 2018, 3, 1198–1204. [Google Scholar] [CrossRef]
- Viswanathan, S.; Reddy, M.M.; Mohan, L.; Bera, P.; Barshilia, H.C.; Anandan, C. Corrosion and wear properties of Ti/tetrahedral amorphous carbon multilayered coating. J. Bio Tribo Corros. 2017, 3, 39. [Google Scholar] [CrossRef]




| Name | Value | Description |
|---|---|---|
| n | 1 | No of electron |
| R | 8.314 J/(mol·K) | Universal gas constant |
| T | 298.15 K | Temperature |
| F | 96,485 C/mol | Faraday’s constant |
| H2 | 1 mol/m3 | Hydrogen concentration (cH2) |
| vb | 1 × 10−8 V/s | Potential scan rate (vb) |
| Einit | 0.5 | Initial potential (Einit) |
| cstar | 1000 mol/m3 | Initial concentration for H+ (cH+) |
| Gmax | 1 × 10−5 mol/m2 | Maximum concentration of Hs () |
| tend | 2 × 108 | Time stop (tend) |
| tstep | 2 × 105 | Time step (tstep) |
| Erev | −0.5 V | Reverse potential (Erev) |
| Electrode | TiC | taC |
|---|---|---|
| kv (m3/(mol·s)) | 6.1 × 10−7 | 4.8 × 10−8 |
| k-v (1/s) | 8 × 10−3 | 4.8 × 10−5 |
| kh ((m3/(mol·s)) | 1.09 × 10−5 | 2.8 × 10−6 |
| k-h ((m3/(mol·s)) | 1.09 × 10−3 | 2.8 × 10−3 |
| kt (m2/(mol·s)) | 1 × 102 | 0 |
| k-t (m5/(mol2·s)) | 1 × 103 | 0 |
| βv | 0.77 | 0.82 |
| βh | 0.25 | 0.25 |
| Area (m2) | 7.5 × 10−9 | 2.5 × 10−9 |
| Electrode | TiC | taC:Pt | Edge | Pt/C |
|---|---|---|---|---|
| kv (m3/(mol·s) | 6.1 × 10−6 | 4.8 × 10−8 | 9 × 101 | 3 |
| k-v (1/s) | 8.0 × 10−2 | 4.8 × 10−5 | 9 × 101 | 3 × 107 |
| kh (m3/(mol·s) | 1.09 × 10−5 | 2.8 × 10−6 | 4 | 3 × 101 |
| k-h (m3/(mol·s) | 1.09 × 10−3 | 2.8 × 10−3 | 4 × 106 | 3 |
| kt (m2/(mol·s) | 1 × 102 | 0 | 0 | 0 |
| k-t (m5/(mol2·s) | 1 × 103 | 0 | 0 | 0 |
| βv | 0.77 | 0.82 | 0.9 | 0.5 |
| βh | 0.25 | 0.25 | 0.25 | 0.5 |
| Area (m2) | 7.5 × 10−9 | 2.5 × 10−9 | 1 × 10−11 |
| Materials | Tafel Slope (mV/dec) | Overpotentials at 10 mA/cm2, η10 (mV) | Exchange Current Density, jo (mA/cm2) | Ref. |
|---|---|---|---|---|
| taC:Pt | 60~104 @ −10~−200 mV | −1009 | 1.26 × 10−8 | This work |
| TiC | 54~114 @ −10~−200 mV | −871 | 2.00 × 10−7 | This work |
| TiC/taC:Pt | 20~40 @ −10~−100 mV | −185 | 1.00 × 10−6 | This work |
| TiC-NiSA | 70.3 | −149.8 | - | [51] |
| TiC-CoSA | 69.0 | −128.6 | - | [51] |
| TiC-FeSA | 61.1 | −123.4 | - | [51] |
| Pt/C | 15~40 @ −10~−100 mV | −50 | 2.00 × 10−3 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramji, H.R.; Glandut, N.; Orlianges, J.-C.; Absi, J.; Lim, S.F. Kinetic Study and Simulation of Titanium Carbide-Supported, Platinum-Doped Tetrahedral Amorphous Carbon Electrodes for Hydrogen Evolution Reaction. Materials 2025, 18, 1916. https://doi.org/10.3390/ma18091916
Ramji HR, Glandut N, Orlianges J-C, Absi J, Lim SF. Kinetic Study and Simulation of Titanium Carbide-Supported, Platinum-Doped Tetrahedral Amorphous Carbon Electrodes for Hydrogen Evolution Reaction. Materials. 2025; 18(9):1916. https://doi.org/10.3390/ma18091916
Chicago/Turabian StyleRamji, Harunal Rejan, Nicolas Glandut, Jean-Christophe Orlianges, Joseph Absi, and Soh Fong Lim. 2025. "Kinetic Study and Simulation of Titanium Carbide-Supported, Platinum-Doped Tetrahedral Amorphous Carbon Electrodes for Hydrogen Evolution Reaction" Materials 18, no. 9: 1916. https://doi.org/10.3390/ma18091916
APA StyleRamji, H. R., Glandut, N., Orlianges, J.-C., Absi, J., & Lim, S. F. (2025). Kinetic Study and Simulation of Titanium Carbide-Supported, Platinum-Doped Tetrahedral Amorphous Carbon Electrodes for Hydrogen Evolution Reaction. Materials, 18(9), 1916. https://doi.org/10.3390/ma18091916







