Optically Stimulated Luminescence Silicone Foils for 2D Dose Mapping in Proton Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of the 2D OSL Dosimeter
2.2. The 2nd Generation Optical Detection System
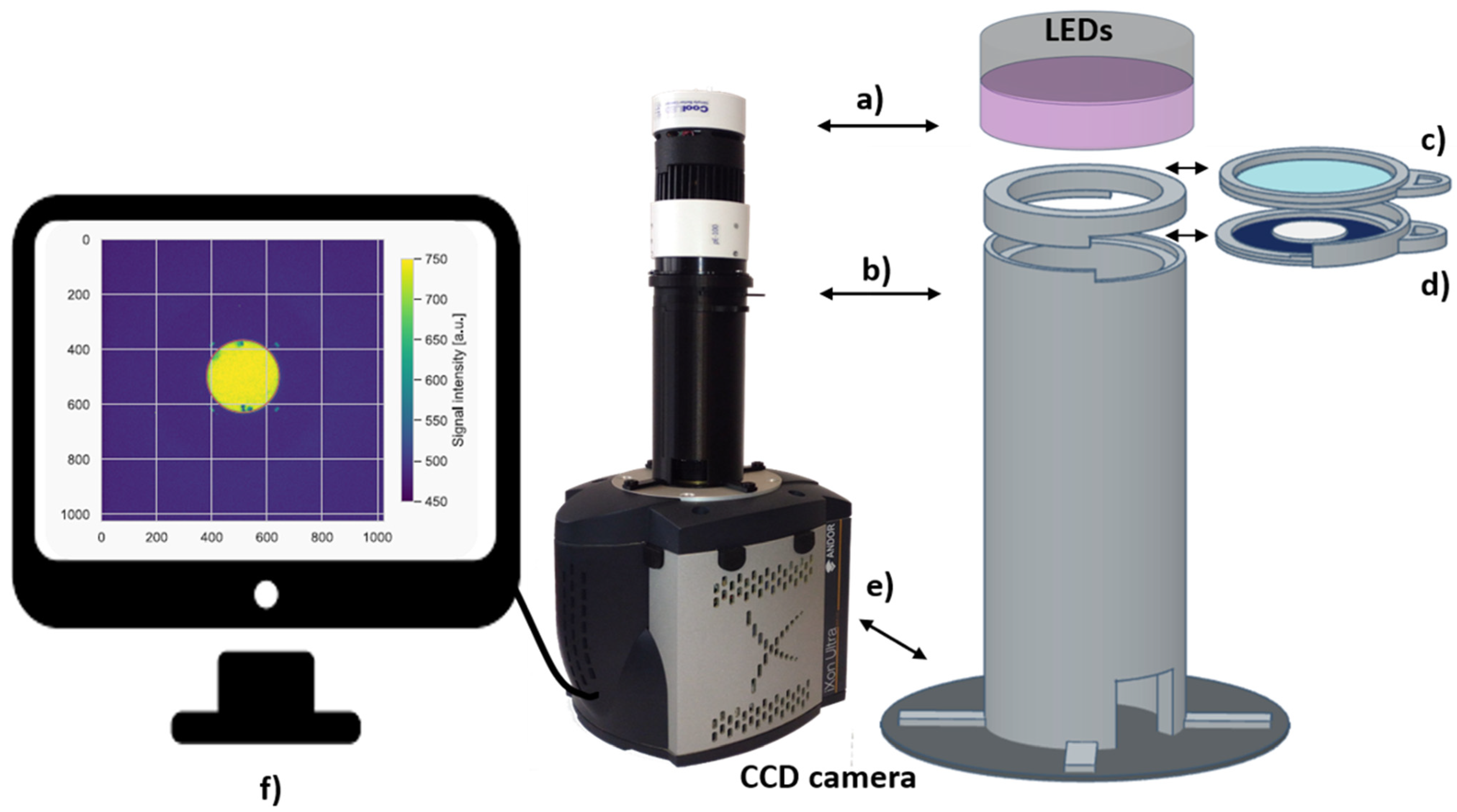

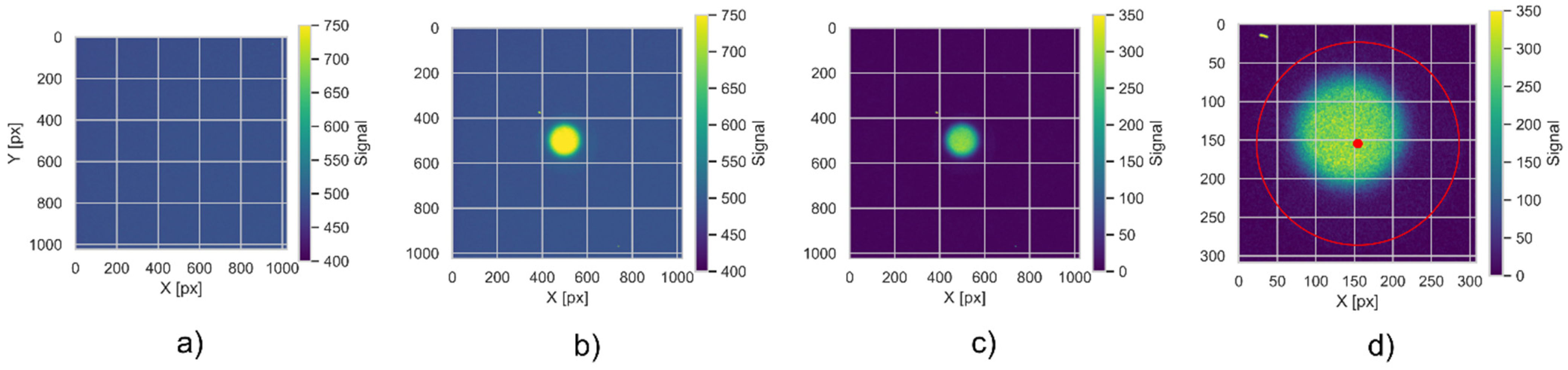
2.3. Image Acquisition and Data Analysis
- circular shape of signal on the detector (due to collimator shape);
- 2D signal shape related to the readout system and light propagation in the tube;
- noise levels typical of the filters used.
- The raw TIFF files are being read into a 2D NumPy array (from an open-source numerical Python library) with signal encoded as unsigned 16-bit integers, using the imread function from the matplotlib Python library. These arrays are stored in convenient data structures (Python data classes), enumerated by a unique detector id.
- The background signal is subtracted pixel-by-pixel from the raw data. All negative values are set to zero in the background-subtracted array. The background subtraction is performed separately for Co60 calibration data and for data from samples irradiated in proton beams.
- The live view data is used to perform detector position based on the method described in [36]. We apply the Hough method of circle detection, which is parametrized to locate a single circle of radius corresponding to expected detector dimensions [37]. The boolean mask is automatically created, based on LV signal levels within 3 standard deviations of all the samples contained within a circle of 100-pixel radius, located inside the expected detector area.
- In current experimental settings, the detector is visible as a circle with an area of 10% of the image. Therefore, after background subtraction, the images are centered on the detector’s central point and cropped so that the image frame is 20% larger than the detector radius. Further processing is done on the cropped image.
- In the irradiation where the 10 mm collimator is used, we also locate uniformly irradiated areas on the detector. This allows for a spatial alignment as the gravity center of an irradiated part does not necessarily correspond to the detector’s geometrical center. This is performed using the centre of mass function from a script.ndimage Python module for image processing.
- To account for various sensitivities in different areas of the foils, each of the foil samples was rotated. After rotation, the characteristic point, visible in the live image, was positioned in the top part of the image. Such an operation is necessary as the foils are being cut in a circular shape, making exact angular positioning difficult in the readout system. The rotation is performed automatically using custom code, which detects characteristic points and then rotates the image using the ‘rotate’ function from the Scipy image package [38].
- The detector sensitivity correction is obtained from reference radiation in the form of Individual Response Images (IRI, as described in [25]). Finally, the signal images are divided by IRI images to correct for the detector sensitivity spatial distribution.
- The last step of the data analysis is to translate the detector signal into the radiation dose. The detector signal is multiplied by the detector efficiency (as in [36]) and a scaling factor from the 60Co reference radiation.
2.4. Proton Beam Delivery and Reference Dosimetry
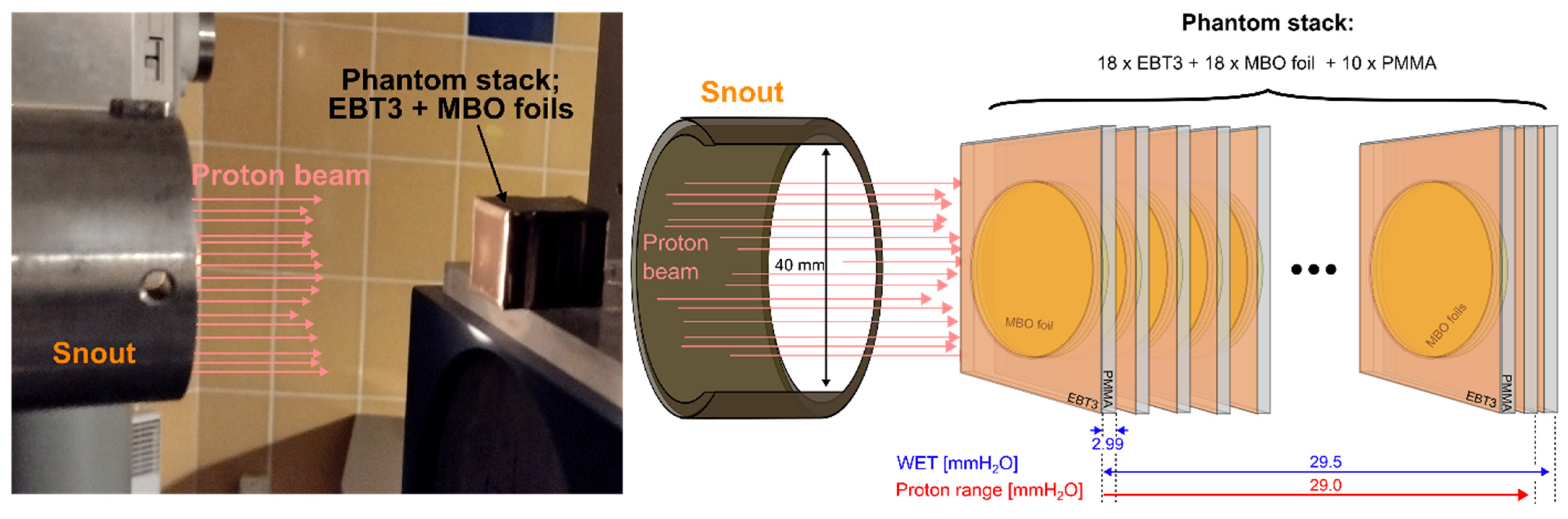
2.5. Experimental Phantom Used for Proton Irradiation
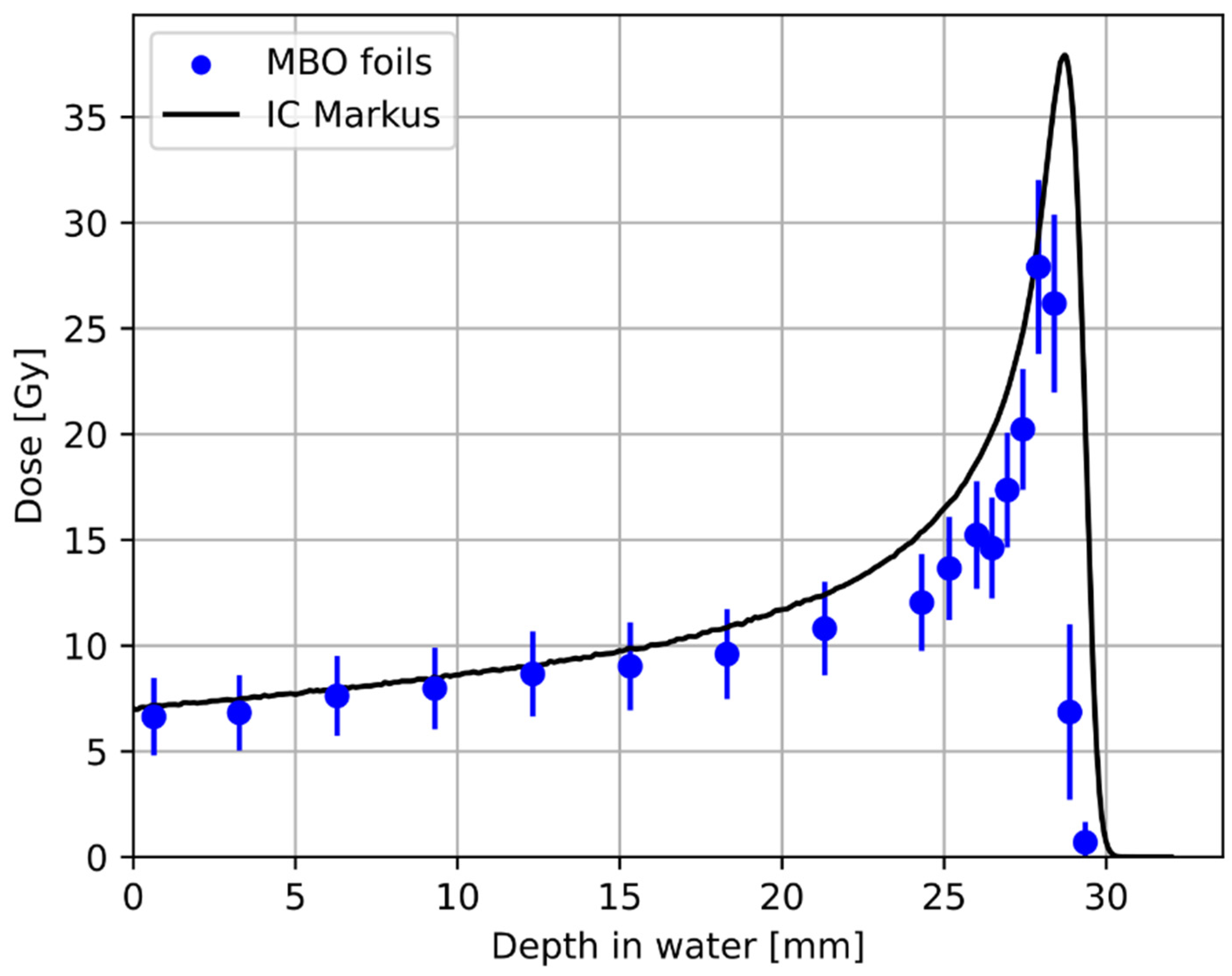
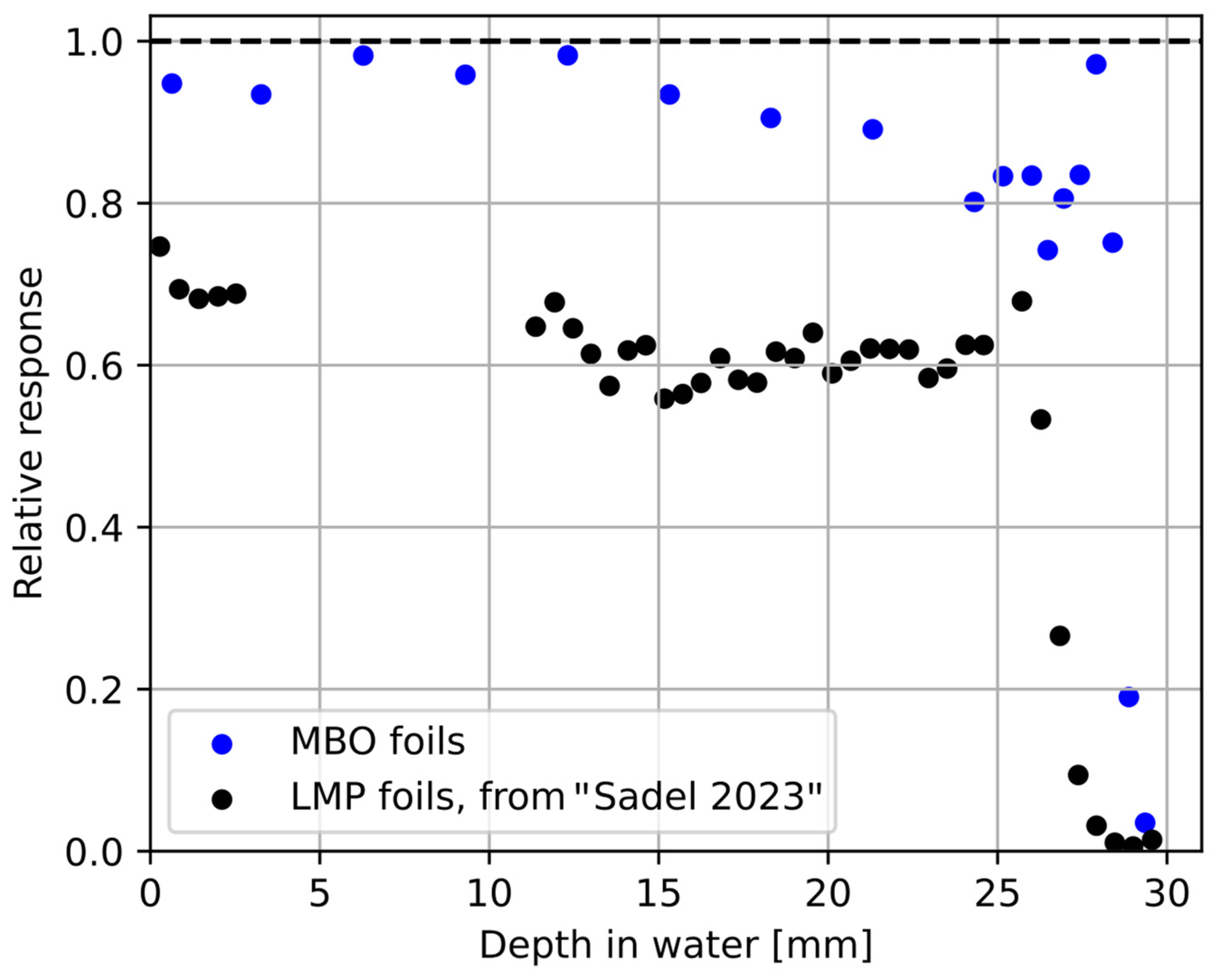
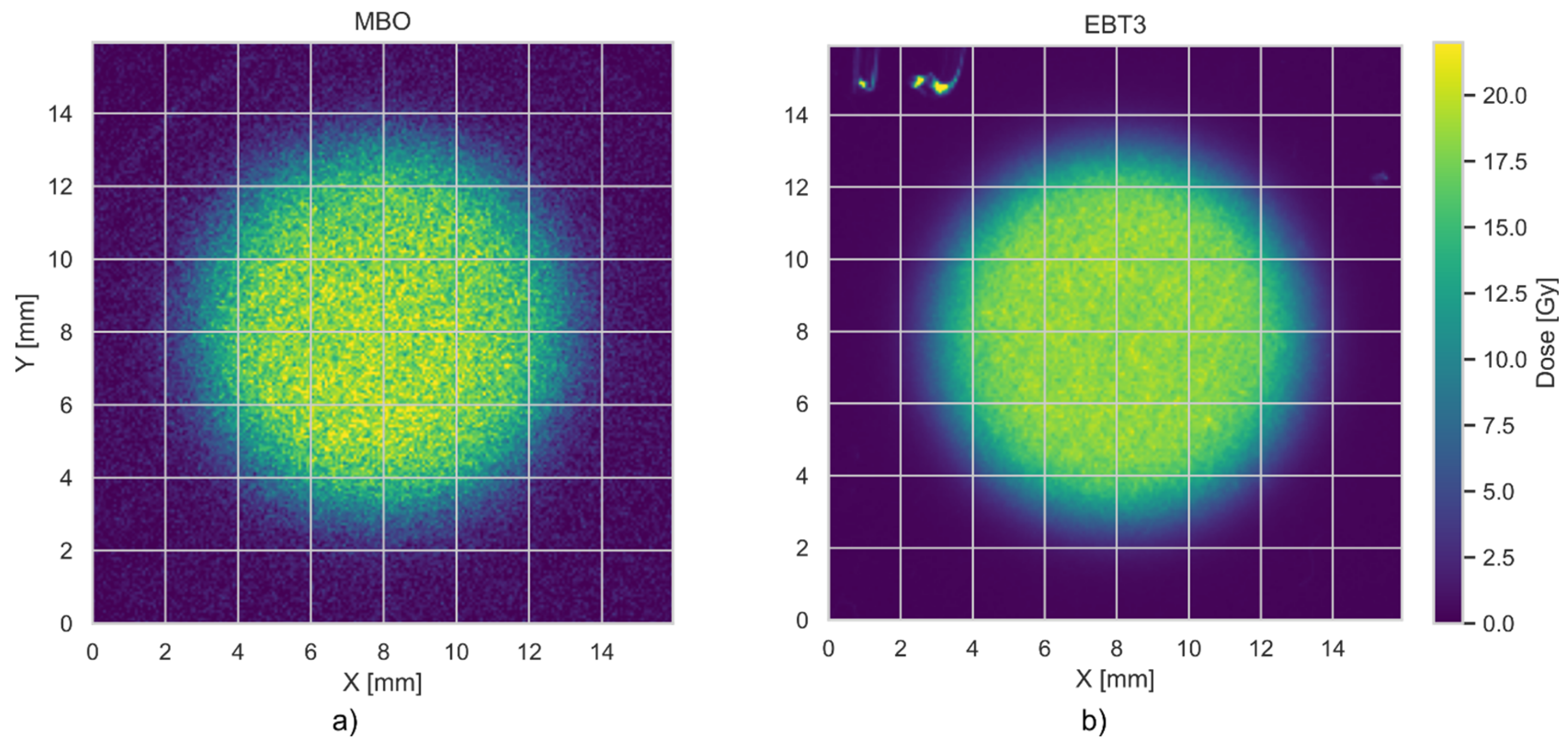
3. Results
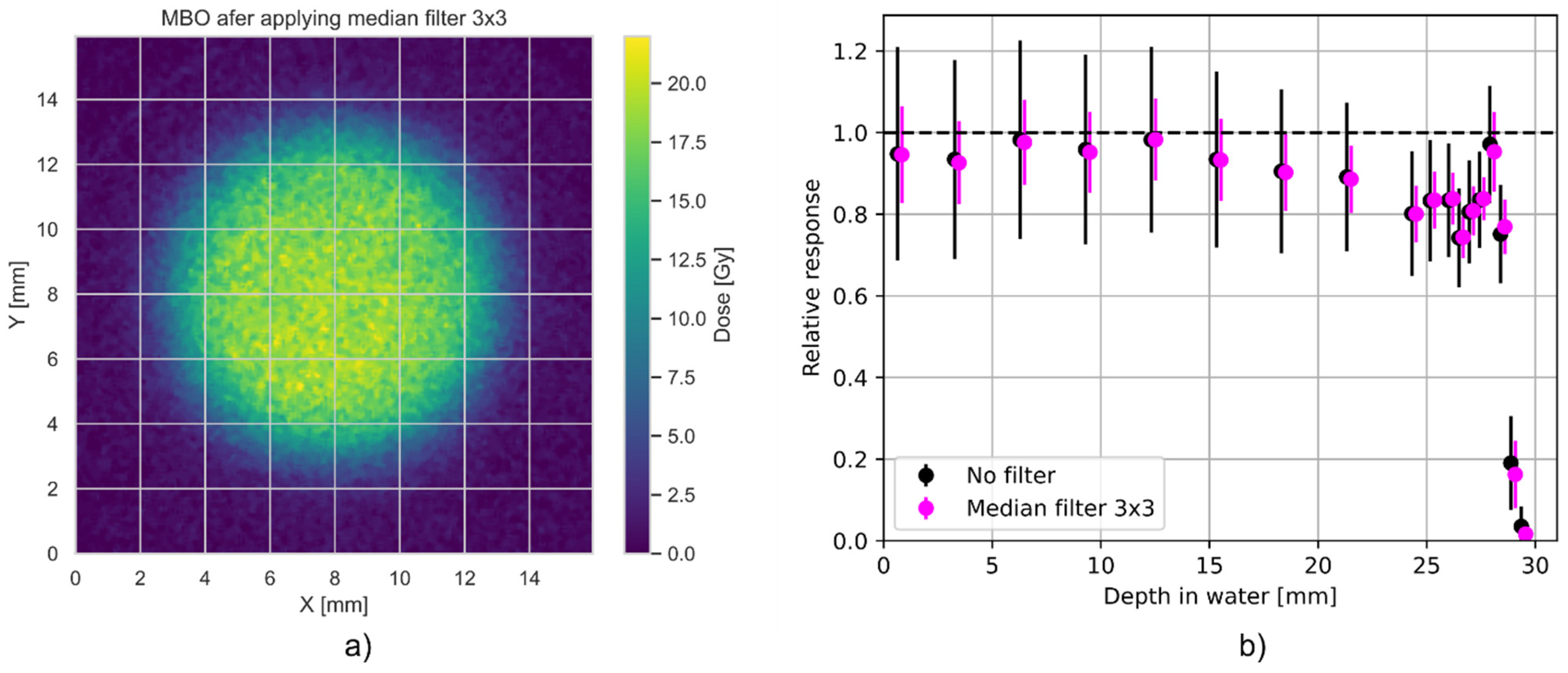
3.1. Relative Luminescence Efficiency Response
3.2. Spatial (Axial) Resolution of MBO Foils vs. EBT3 Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newhauser, W.D.; Zhang, R. The Physics of Proton Therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation Oncology in the Era of Precision Medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef]
- Novotny, J.; Spevacek, V.; Dvorak, P.; Novotny, J.; Cechak, T. Energy and Dose Rate Dependence of BANG-2 Polymer-Gel Dosimeter. Med. Phys. 2001, 28, 2379–2386. [Google Scholar] [CrossRef]
- Heufelder, J.; Stiefel, S.; Pfaender, M.; Lüdemann, L.; Grebe, G.; Heese, J. Use of BANG® Polymer Gel for Dose Measurements in a 68 MeV Proton Beam. Med. Phys. 2003, 30, 1235–1240. [Google Scholar] [CrossRef]
- Gustavsson, H.; Bäck, S.Å.J.; Medin, J.; Grusell, E.; Olsson, L.E. Linear Energy Transfer Dependence of a Normoxic Polymer Gel Dosimeter Investigated Using Proton Beam Absorbed Dose Measurements. Phys. Med. Biol. 2004, 49, 3847–3855. [Google Scholar] [CrossRef]
- Heard, M.; Adamovics, J.; Ibbott, G. Measurement of a 200 MeV Proton Beam Using a Polyurethane Dosimeter. J. Phys. Conf. Ser. 2006, 56, 228–230. [Google Scholar] [CrossRef]
- Gorjiara, T.; Kacperek, A.; Kuncic, Z.; Baldock, C.; Doran, S. Preliminary Characterization of PRESAGE® for 3D Dosimetry of 62 MeV Proton Beam. J. Phys. Conf. Ser. 2013, 444, 012058. [Google Scholar] [CrossRef]
- Doran, S.; Gorjiara, T.; Kacperek, A.; Adamovics, J.; Kuncic, Z.; Baldock, C. Issues Involved in the Quantitative 3D Imaging of Proton Doses Using Optical CT and Chemical Dosimeters. Phys. Med. Biol. 2015, 60, 709–726. [Google Scholar] [CrossRef]
- Al-Nowais, S.; Doran, S.; Kacperek, A.; Krstajic, N.; Adamovics, J.; Bradley, D. A Preliminary Analysis of LET Effects in the Dosimetry of Proton Beams Using PRESAGETM and Optical CT. Appl. Radiat. Isot. 2009, 67, 415–418. [Google Scholar] [CrossRef]
- Scotti, M.; Arosio, P.; Brambilla, E.; Gallo, S.; Lenardi, C.; Locarno, S.; Orsini, F.; Pignoli, E.; Pedicone, L.; Veronese, I. How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA–GTA Fricke Gel Dosimeters: A Spectrophotometric Study. Gels 2022, 8, 204. [Google Scholar] [CrossRef]
- Průšová, H.; Dudáš, D.; Spěváček, V.; Průša, P. Dose-Response Dependencies of Turnbull Blue, Modified Fricke, VIPET, and Presage® Gel Dosimeters in High-Dose-Rate Radiation Fields. Radiat. Meas. 2023, 161, 106910. [Google Scholar] [CrossRef]
- Kozicki, M.; Maras, P. An Optical Reusable 2D Radiochromic Gel-Based System for Ionising Radiation Measurements in Radiotherapy. Molecules 2024, 29, 2558. [Google Scholar] [CrossRef]
- Gallo, S.; Pasquale, S.; Lenardi, C.; Veronese, I.; Gueli, A.M. Effect of Ionizing Radiation on the Colorimetric Properties of PVA-GTA Xylenol Orange Fricke Gel Dosimeters. Dye. Pigment. 2021, 187, 109141. [Google Scholar] [CrossRef]
- Rousseau, A.; Stien, C.; Gouriou, J.; Bordy, J.-M.; Boissonnat, G.; Chabert, I.; Dufreneix, S.; Blideanu, V. End-to-End Quality Assurance for Stereotactic Radiotherapy with Fricke-Xylenol Orange-Gelatin Gel Dosimeter and Dual-Wavelength Cone-Beam Optical CT Readout. Phys. Medica 2023, 113, 102656. [Google Scholar] [CrossRef] [PubMed]
- Stelljes, T.S.; Harmeyer, A.; Reuter, J.; Looe, H.K.; Chofor, N.; Harder, D.; Poppe, B. Dosimetric Characteristics of the Novel 2D Ionization Chamber Array OCTAVIUS Detector 1500. Med. Phys. 1528, 42, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Létourneau, D.; Gulam, M.; Yan, D.; Oldham, M.; Wong, J.W. Evaluation of a 2D Diode Array for IMRT Quality Assurance. Radiother. Oncol. 2004, 70, 199–206. [Google Scholar] [CrossRef]
- Yukihara, E.G.; Gasparian, P.B.R.; Sawakuchi, G.O.; Ruan, C.; Ahmad, S.; Kalavagunta, C.; Clouse, W.J.; Sahoo, N.; Titt, U. Medical Applications of Optically Stimulated Luminescence Dosimeters (OSLDs). Radiat. Meas. 2010, 45, 658–662. [Google Scholar] [CrossRef]
- Yukihara, E.G. Luminescence Properties of BeO Optically Stimulated Luminescence (OSL) Detectors. Radiat. Meas. 2011, 46, 580–587. [Google Scholar] [CrossRef]
- Yukihara, E.G.; McKeever, S.W.S. Optically Stimulated Luminescence; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780470697252. [Google Scholar]
- Polge, G.; Dusseau, L.; Idri, K.; Plattard, D.; Vaille, J.R.; Ranchoux, G.; Fesquet, J.; Gasiot, J.; Iborra-Brassard, N. Characterization of a 63 MeV Proton Beam with Optically Stimulated Luminescent Films. In Proceedings of the RADECS 2001. 2001 6th European Conference on Radiation and Its Effects on Components and Systems (Cat. No.01TH8605), Grenoble, France, 10–14 September 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 1–5. [Google Scholar]
- Clausen, S.J.G.; Jensen, M.L.; Nielsen, C.L.; Jensen, M.F.; Muren, L.; Søndergaard, C.S.; Skyt, P.S.; Stick, L.B.; Yates, E.; Vindbæk, S.H.; et al. Reusable Optically Stimulated Luminescence Dosimetry Films for 2D Dose Verification of Proton Therapy. J. Phys. Conf. Ser. 2024, 2799, 012002. [Google Scholar] [CrossRef]
- Jensen, M.L.; Nielsen, C.L.; Jensen, M.B.; Muren, L.P.; Turtos, R.M.; Julsgaard, B.; Balling, P. A Tissue-Equivalent, Reusable Dosimeter for 3D Verification of Radiotherapy. Adv. Funct. Mater. 2024, 35, 2412587. [Google Scholar] [CrossRef]
- Sądel, M.; Bilski, P.; Kłosowski, M.; Sankowska, M. A New Approach to the 2D Radiation Dosimetry Based on Optically Stimulated Luminescence of LiF:Mg,Cu,P. Radiat. Meas. 2020, 133, 106293. [Google Scholar] [CrossRef]
- Sądel, M.; Gajewski, J.; Sowa, U.; Swakoń, J.; Kajdrowicz, T.; Bilski, P.; Kłosowski, M.; Pędracka, A.; Horwacik, T. 3D Dosimetry Based on LiMgPO4 OSL Silicone Foils: Facilitating the Verification of Eye-Ball Cancer Proton Radiotherapy. Sensors 2021, 21, 6015. [Google Scholar] [CrossRef] [PubMed]
- Bossin, L.; Plokhikh, I.; Christensen, J.B.; Gawryluk, D.J.; Kitagawa, Y.; Leblans, P.; Tanabe, S.; Vandenbroucke, D.; Yukihara, E.G. Addressing Current Challenges in OSL Dosimetry Using MgB4O7:Ce,Li: State of the Art, Limitations and Avenues of Research. Materials 2023, 16, 3051. [Google Scholar] [CrossRef] [PubMed]
- Yukihara, E.G.; Christensen, J.B.; Togno, M. Demonstration of an Optically Stimulated Luminescence (OSL) Material with Reduced Quenching for Proton Therapy Dosimetry: MgB4O7:Ce,Li. Radiat. Meas. 2022, 152, 106721. [Google Scholar] [CrossRef]
- Pradhan, A.; Lee, J.; Kim, J. Recent Developments of Optically Stimulated Luminescence Materials and Techniques for Radiation Dosimetry and Clinical Applications. J. Med. Phys. 2008, 33, 85. [Google Scholar] [CrossRef]
- Shrestha, N.; Vandenbroucke, D.; Leblans, P.; Yukihara, E.G. Feasibility Studies on the Use of MgB4O7:Ce,Li-Based Films in 2D Optically Stimulated Luminescence Dosimetry. Phys. Open 2020, 5, 100037. [Google Scholar] [CrossRef]
- de Souza, L.F.; Souza, D.N.; Rivera, G.B.; Vidal, R.M.; Caldas, L.V.E. Dosimetric Characterization of MgB4O7:Ce,Li as an Optically Stimulated Dosimeter for Photon Beam Radiotherapy. Perspect. Sci. 2019, 12, 100397. [Google Scholar] [CrossRef]
- Souza, L.F.; Novais, A.L.F.; Antonio, P.L.; Caldas, L.V.E.; Souza, D.N. Luminescent Properties of MgB4O7:Ce,Li to Be Applied in Radiation Dosimetry. Radiat. Phys. Chem. 2019, 164, 108353. [Google Scholar] [CrossRef]
- Gustafson, T.D.; Milliken, E.D.; Jacobsohn, L.G.; Yukihara, E.G. Progress and Challenges towards the Development of a New Optically Stimulated Luminescence (OSL) Material Based on MgB4O7:Ce,Li. J. Lumin. 2019, 212, 242–249. [Google Scholar] [CrossRef]
- Plokhikh, I.; Sadykov, I.I.; Safonova, O.V.; Kondracki, Ł.; Yukihara, E.G.; Bossin, L.; Gawryluk, D.J. Role of Dopant Concentration and Starting Reagents in the Dosimetric Performance of MgB4O7:Ce,Li. J. Lumin. 2025, 279, 121019. [Google Scholar] [CrossRef]
- Plokhikh, I.; Kondracki, Ł.; Yukihara, E.G.; Gawryluk, D.J.; Bossin, L. Impact of Sample Preparation Temperature on Li and Ce Co-Doped MgB4O7 Dosimetry Performance: A Plausible Scenario for Controlling Defect Clustering. J. Lumin. 2024, 275, 120784. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Sądel, M.; Grzanka, L.; Swakoń, J.; Baran, J.; Gajewski, J.; Bilski, P. Optically Stimulated Luminescent Response of the LiMgPO4 Silicone Foils to Protons and Its Dependence on Proton Energy. Materials 2023, 16, 1978. [Google Scholar] [CrossRef]
- Xie, Y.; Ji, Q. A New Efficient Ellipse Detection Method. In Proceedings of the Object Recognition Supported by User Interaction for Service Robots; IEEE Computer Society: Washington, DC, USA, 2002; pp. 957–960. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Swakon, J.; Olko, P.; Adamczyk, D.; Cywicka-Jakiel, T.; Dabrowska, J.; Dulny, B.; Grzanka, L.; Horwacik, T.; Kajdrowicz, T.; Michalec, B.; et al. Facility for Proton Radiotherapy of Eye Cancer at IFJ PAN in Krakow. Radiat. Meas. 2010, 45, 1469–1471. [Google Scholar] [CrossRef]
- Sorriaux, J.; Kacperek, A.; Rossomme, S.; Lee, J.A.; Bertrand, D.; Vynckier, S.; Sterpin, E. Evaluation of Gafchromic® EBT3 Films Characteristics in Therapy Photon, Electron and Proton Beams. Phys. Medica 2013, 29, 599–606. [Google Scholar] [CrossRef]
- Palmer, A.L.; Dimitriadis, A.; Nisbet, A.; Clark, C.H. Evaluation of Gafchromic EBT-XD Film, with Comparison to EBT3 Film, and Application in High Dose Radiotherapy Verification. Phys. Med. Biol. 2015, 60, 8741–8752. [Google Scholar] [CrossRef]
- Wouter, C.; Dirk, V.; Paul, L.; Tom, D. A Reusable OSL-Film for 2D Radiotherapy Dosimetry. Phys. Med. Biol. 2017, 62, 8441–8454. [Google Scholar] [CrossRef]
- Zhao, L.; Das, I.J. Gafchromic EBT Film Dosimetry in Proton Beams. Phys. Med. Biol. 2010, 55, N291–N301. [Google Scholar] [CrossRef]
- Lapen, K.; Yamada, Y. The Development of Modern Radiation Therapy. Curr. Phys. Med. Rehabil. Rep. 2023, 11, 131–138. [Google Scholar] [CrossRef]



| Aspect | OSL | Radiochromic Films (e.g., EBT3) | TL |
|---|---|---|---|
| Principle | Luminescence induced by optical stimulation | Polymerization-induced color change | Luminescence induced by thermal stimulation |
| Materials | MgB4O7:Ce,Li | Poly-diacetylene or leuco dye-based films | LiF:Mg,Ti |
| Dose Response Range | Linear from 0.1–several kGy | Typically 0.01–8 Gy | Linear range 0.01–several Gy |
| Fading Characteristics | Minimal fading (<1% over 40 days) | Stable post-irradiation; some variation over long-term storage | Low fading (<3% over 1 year) |
| Advantages | High sensitivity, tissue-equivalent (Zeff ≈ 8.2), fast signal decay | High spatial resolution, no readout stimulation needed | High sensitivity, robust materials |
| Limitations | Requires optical stimulation system; some trap instability | Sensitive to light/temperature; slower scanning process | Requires heating; signal can degrade if mishandled |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sądel, M.; Grzanka, L.; Swakoń, J.; Wróbel, D.; Kusyk, S.; Bossin, L.; Bilski, P. Optically Stimulated Luminescence Silicone Foils for 2D Dose Mapping in Proton Radiotherapy. Materials 2025, 18, 1928. https://doi.org/10.3390/ma18091928
Sądel M, Grzanka L, Swakoń J, Wróbel D, Kusyk S, Bossin L, Bilski P. Optically Stimulated Luminescence Silicone Foils for 2D Dose Mapping in Proton Radiotherapy. Materials. 2025; 18(9):1928. https://doi.org/10.3390/ma18091928
Chicago/Turabian StyleSądel, Michał, Leszek Grzanka, Jan Swakoń, Damian Wróbel, Sebastian Kusyk, Lily Bossin, and Paweł Bilski. 2025. "Optically Stimulated Luminescence Silicone Foils for 2D Dose Mapping in Proton Radiotherapy" Materials 18, no. 9: 1928. https://doi.org/10.3390/ma18091928
APA StyleSądel, M., Grzanka, L., Swakoń, J., Wróbel, D., Kusyk, S., Bossin, L., & Bilski, P. (2025). Optically Stimulated Luminescence Silicone Foils for 2D Dose Mapping in Proton Radiotherapy. Materials, 18(9), 1928. https://doi.org/10.3390/ma18091928






