Optimization of Ni-B-Mo Electroless Coating on GCr15 Steel: Effects of Main Salt Concentration and Deposition Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ni-B-Mo Coatings
2.3. Ni-B-Mo Coating Characterization
- The surface morphology, cross-sectional morphology, and elemental distribution of the coatings were characterized using a Japanese optical microscope (OLYMPUS-DSX510, Olympus Corporation, Yijingtong Optical Technology (Shanghai) Co., Shanghai, China) and a German scanning electron microscope (Zeiss Sigma 300, Carl Zeiss AG, Oberkochen, Germany). The coating thickness (µm) was measured, and the deposition rate (µm/h) was calculated.
- Using the Dutch Empyrean X-ray diffractometer (Malvern Panalytical, Almelo, The Netherlands), the voltage was set to 40 kV and the current to 40 mA. Gr-Cu radiation was used, with a 2θ scanning range from 10° to 80°, for a qualitative analysis of the elemental composition of the coating surface.
- The Japanese EDX-7200 energy-dispersive X-ray spectrometer (Shimadzu Corporation, Kyoto, Japan) was used to analyze the elemental content of the coating. Since the spectrometer could not detect elements lighter than oxygen, this experiment mainly used EDX spectrometry to determine the relative contents of Ni and Mo. The instrument had an error margin of ±0.3%, and the measurements were conducted under ambient air conditions at room temperature.
- Using the Chinese HV-1000 microhardness tester (Shanghai Lianer Test Equipment Co., Ltd., Shanghai, China), a load of 100 g was applied along the depth direction of the coating with a dwell time of 10 s. The Vickers microhardness of the coating was measured at seven different points, and the average value was taken.
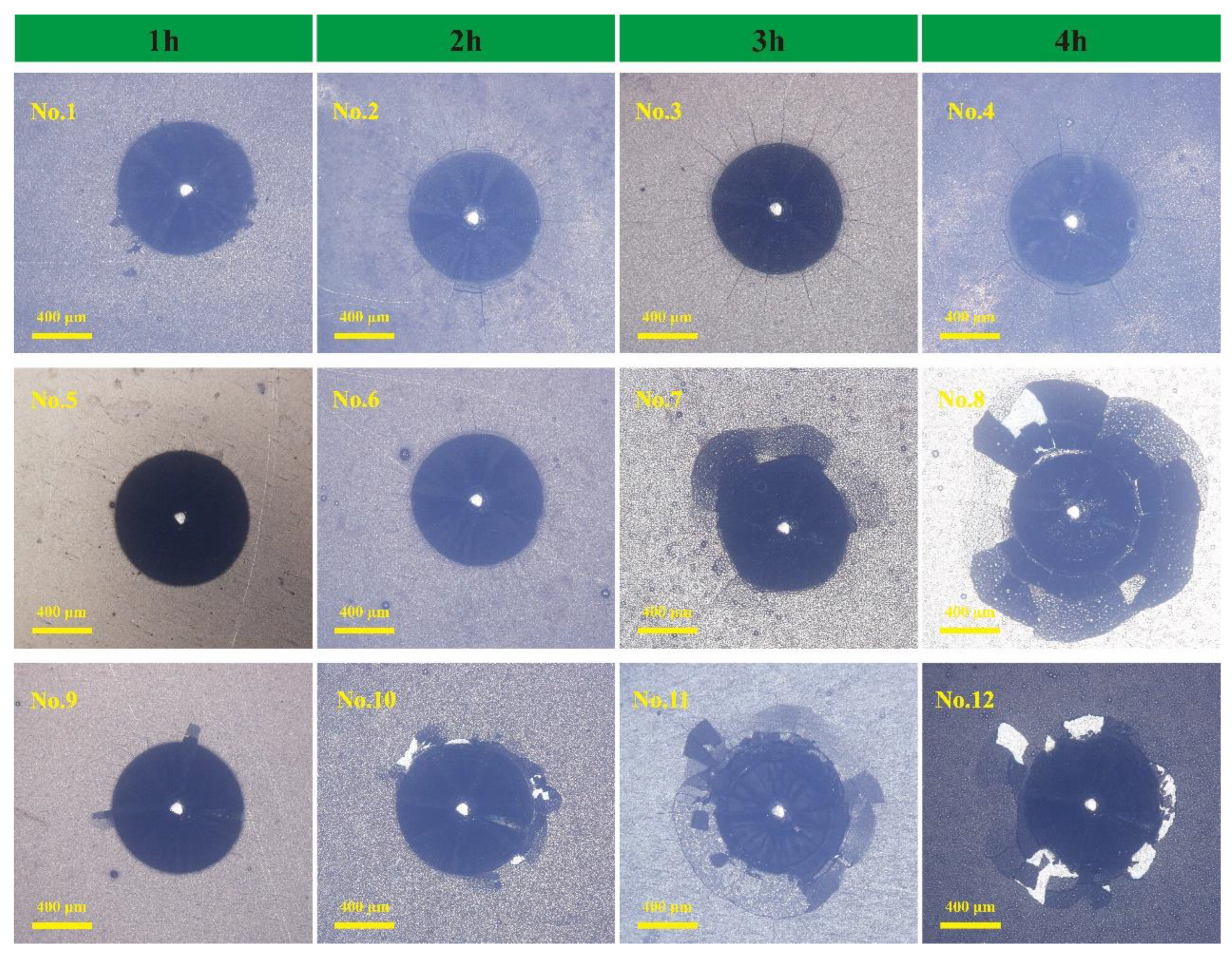
- The Chinese HBE-3000A adhesion tester (Shanghai Lianer Test Equipment Co., Ltd.) was used to perform indentation testing, and the adhesion grade of the coating was qualitatively evaluated according to the VDI 3198 standard [20].
- The American UMT-3 friction and wear tester (Bruker Nano Surfaces Division, Santa Barbara, CA, USA) was used to measure the tribological properties of the samples. Under room temperature and unlubricated conditions, the linear reciprocating mode was selected. The sample was cut into a thin 10 mm × 10 mm square sheet. The testing parameters were set as follows: a load of 10 N, a frequency of 2 Hz, a friction stroke of 5 mm, and a friction time of 30 min were used, and the friction pair used was a GCr15 steel ball with a diameter of 10 mm [21].
3. Results
3.1. The Effect of the Main Salt Concentration and Deposition Time on the Deposition Rate and Hardness of the Coating
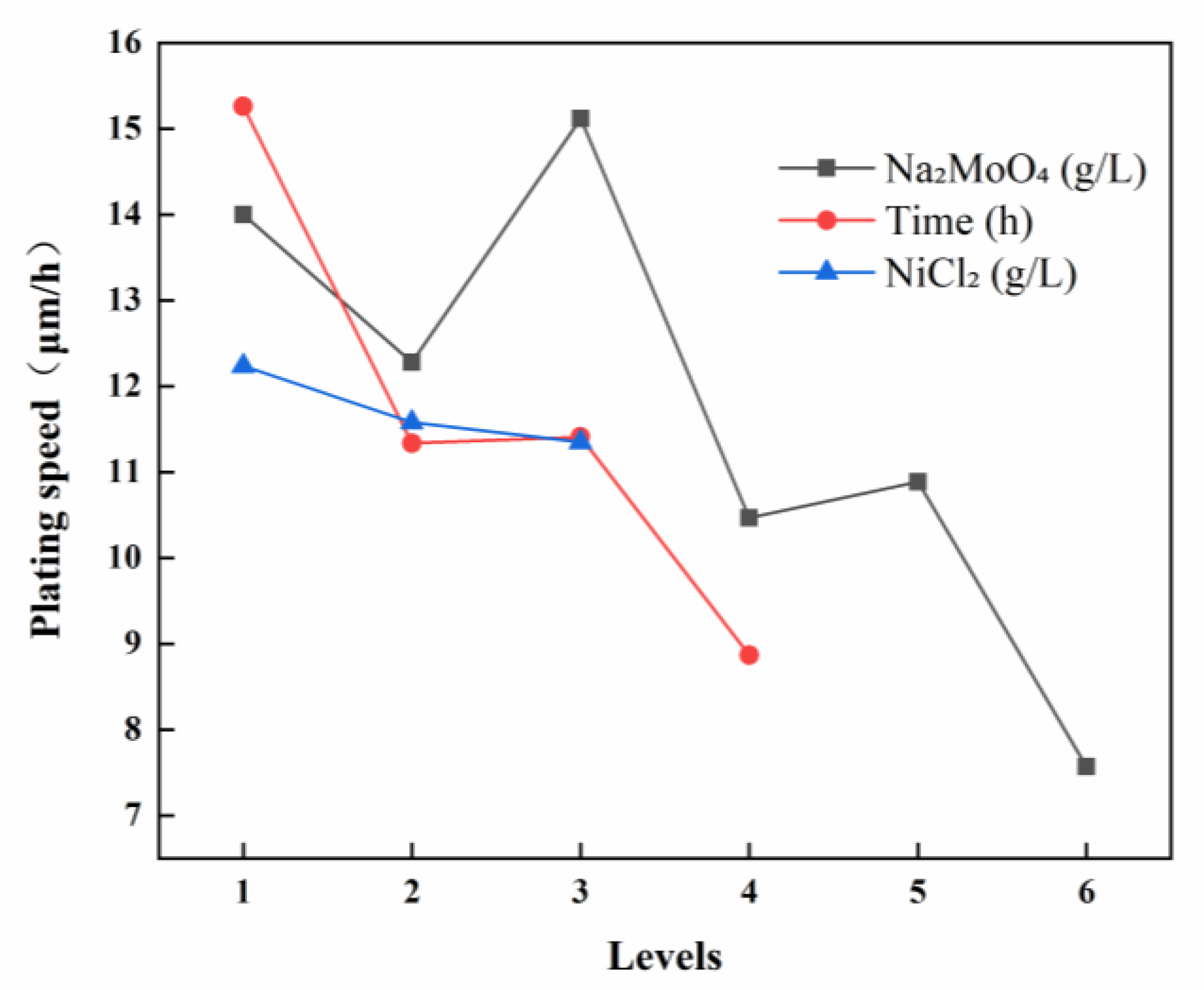
3.1.1. Deposition Rate of Ni-B-Mo Coatings
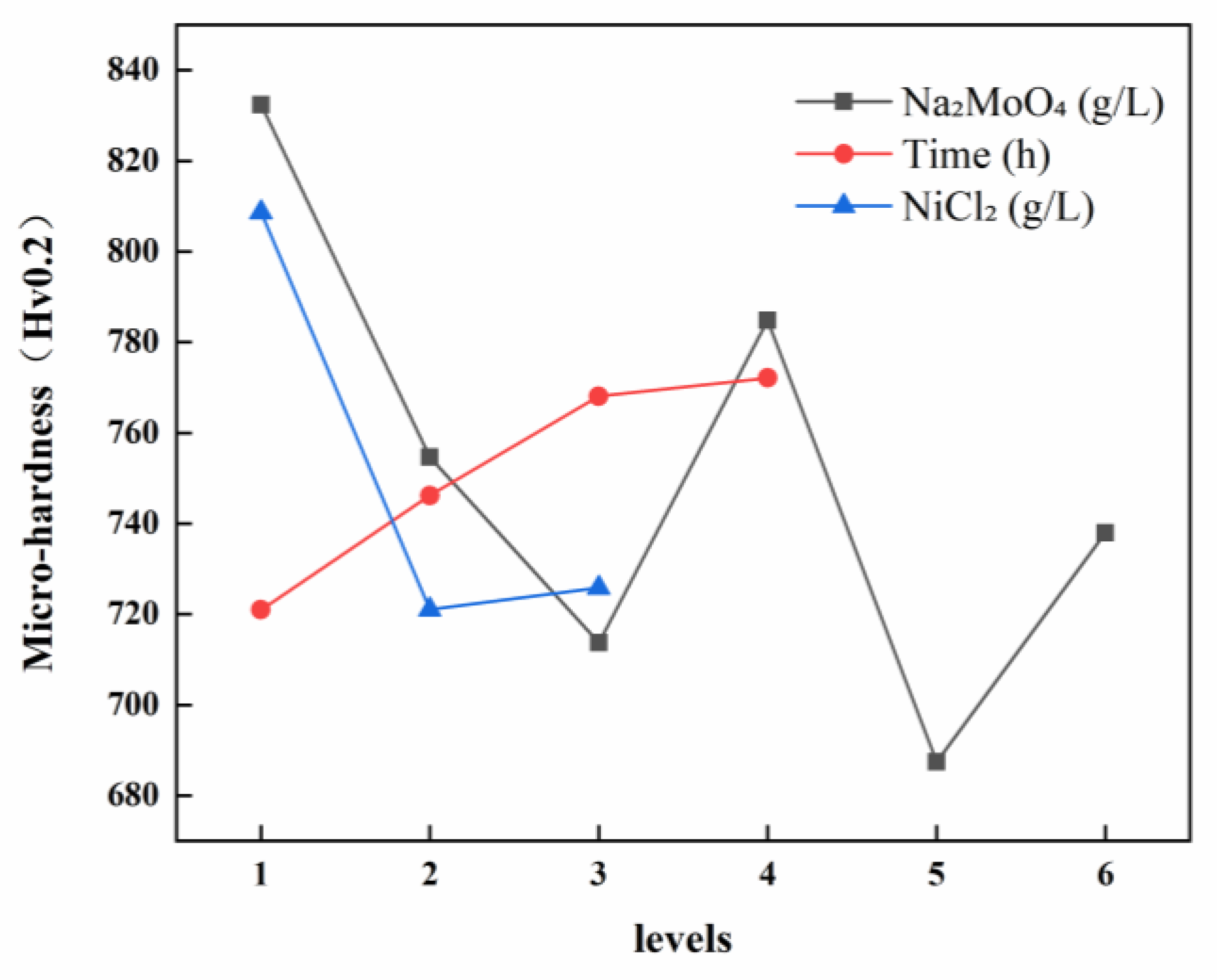
3.1.2. Hardness of Ni-B-Mo Coatings
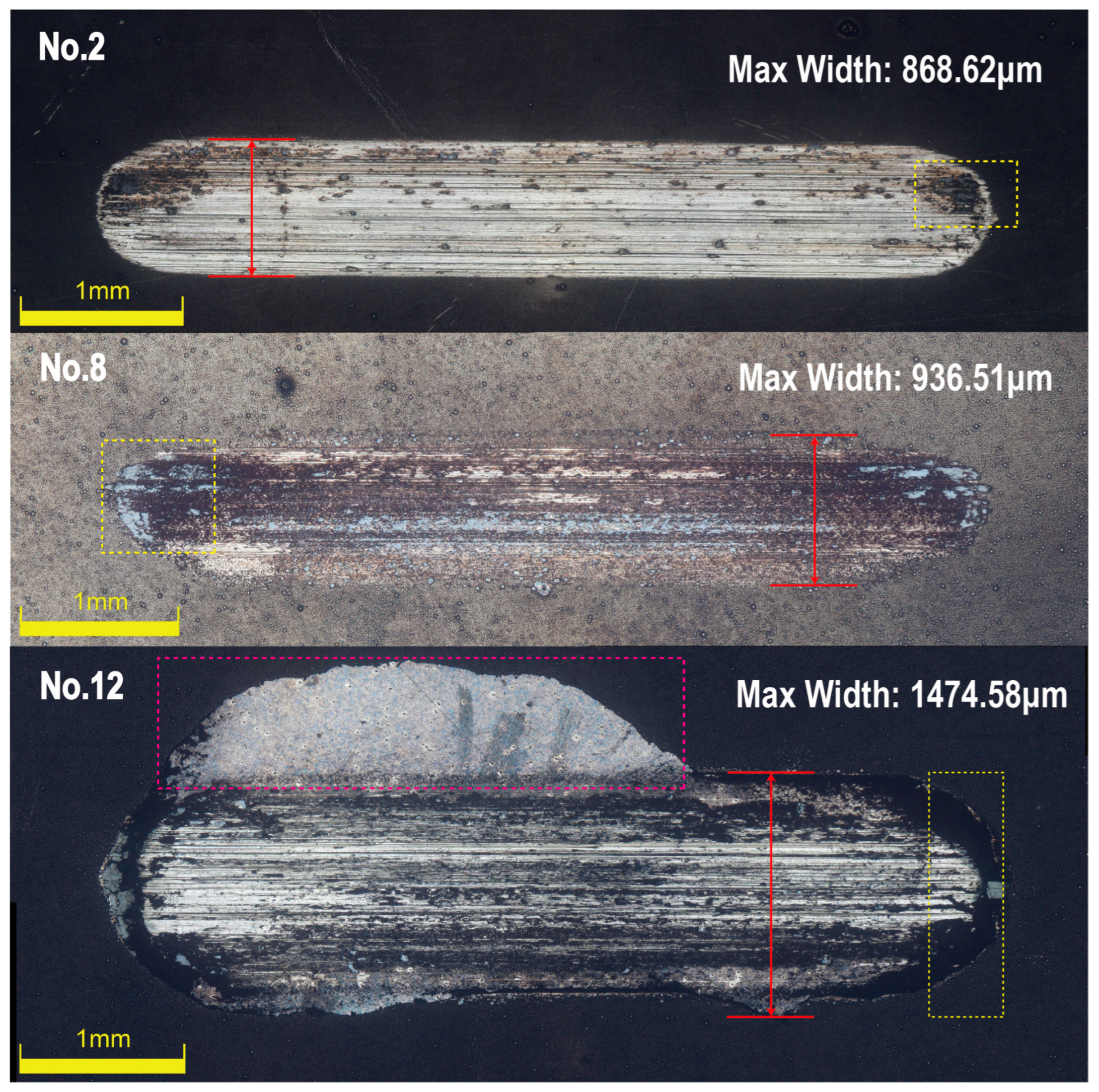
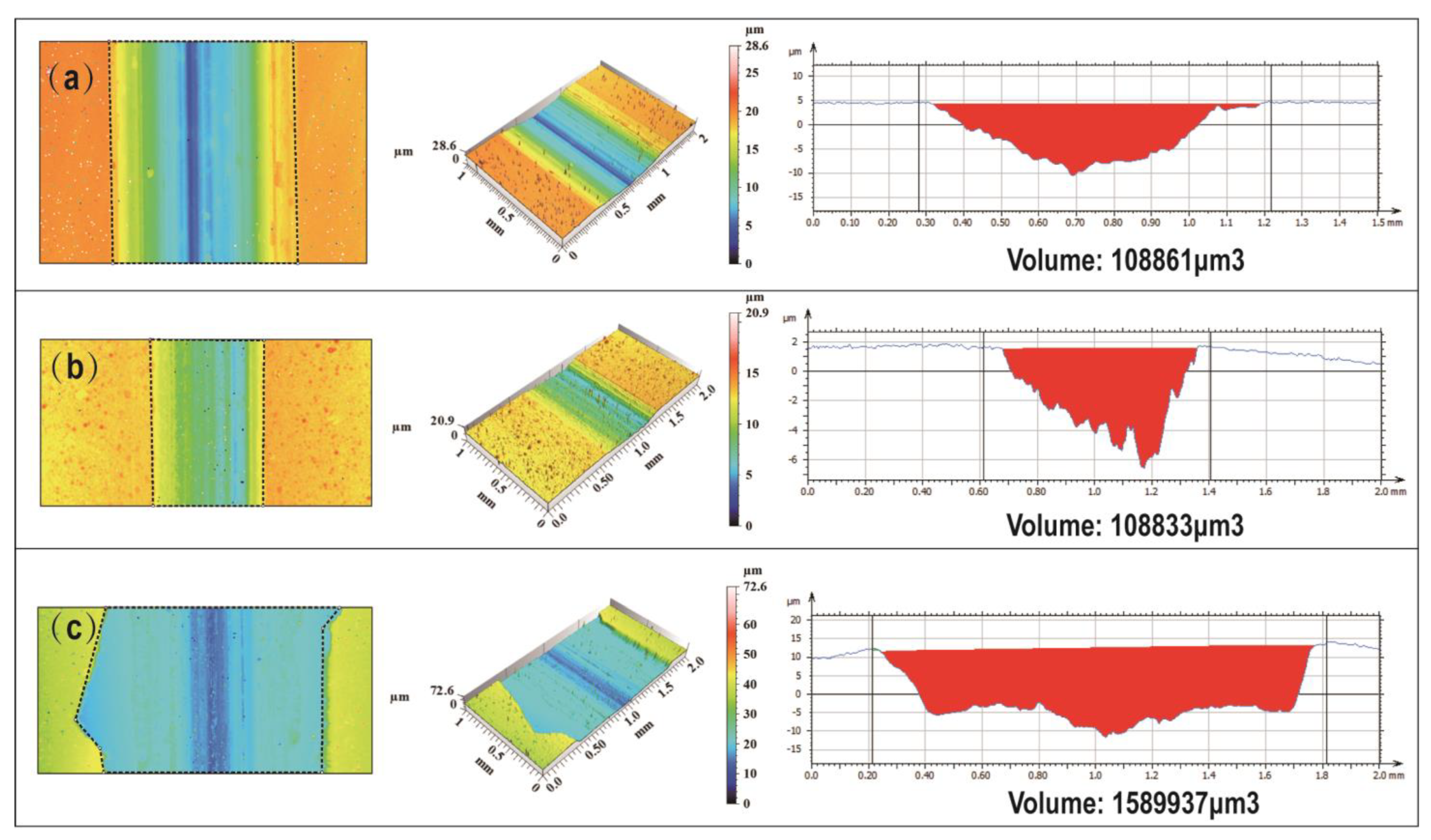
3.2. Ni-B-Mo Plating Bonding Strength
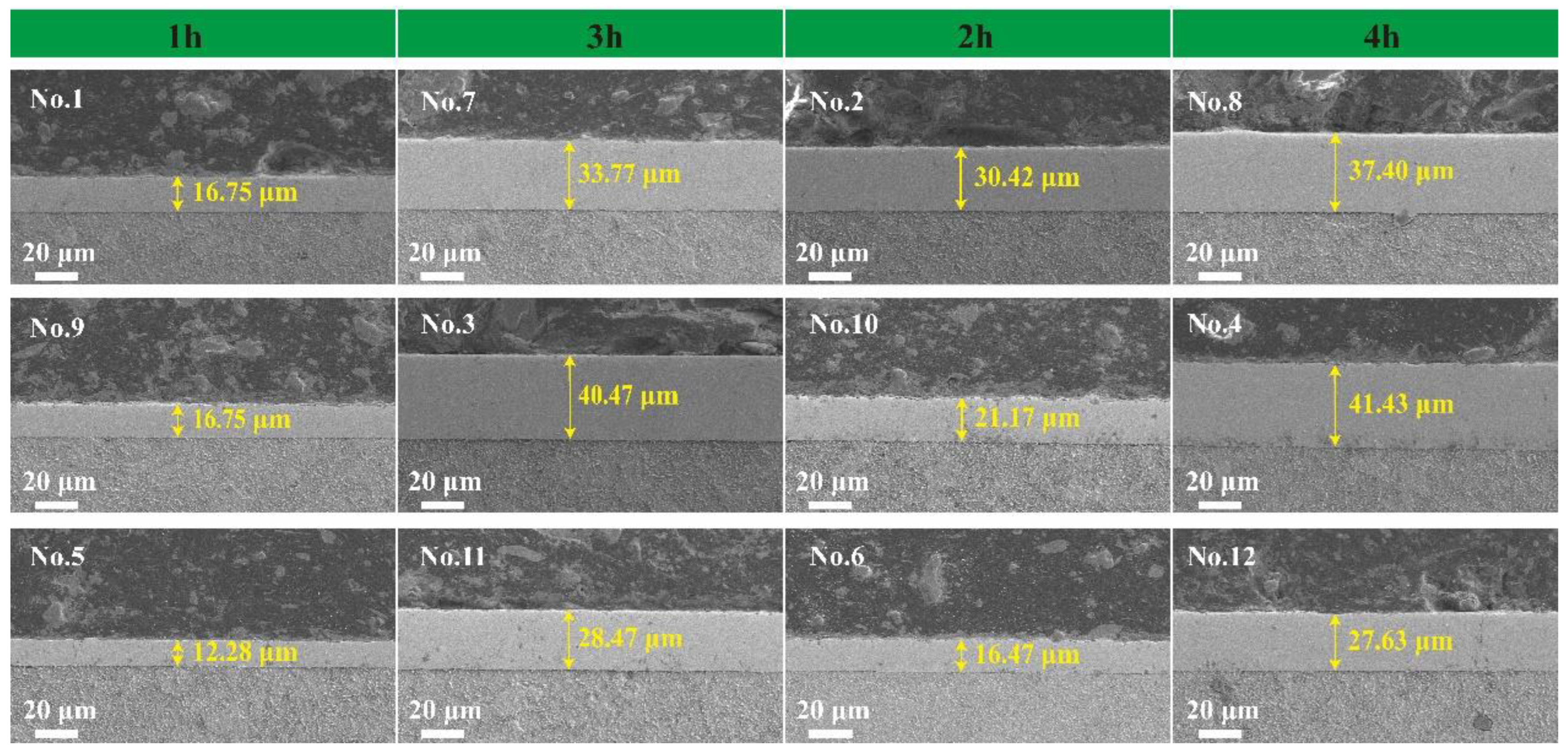
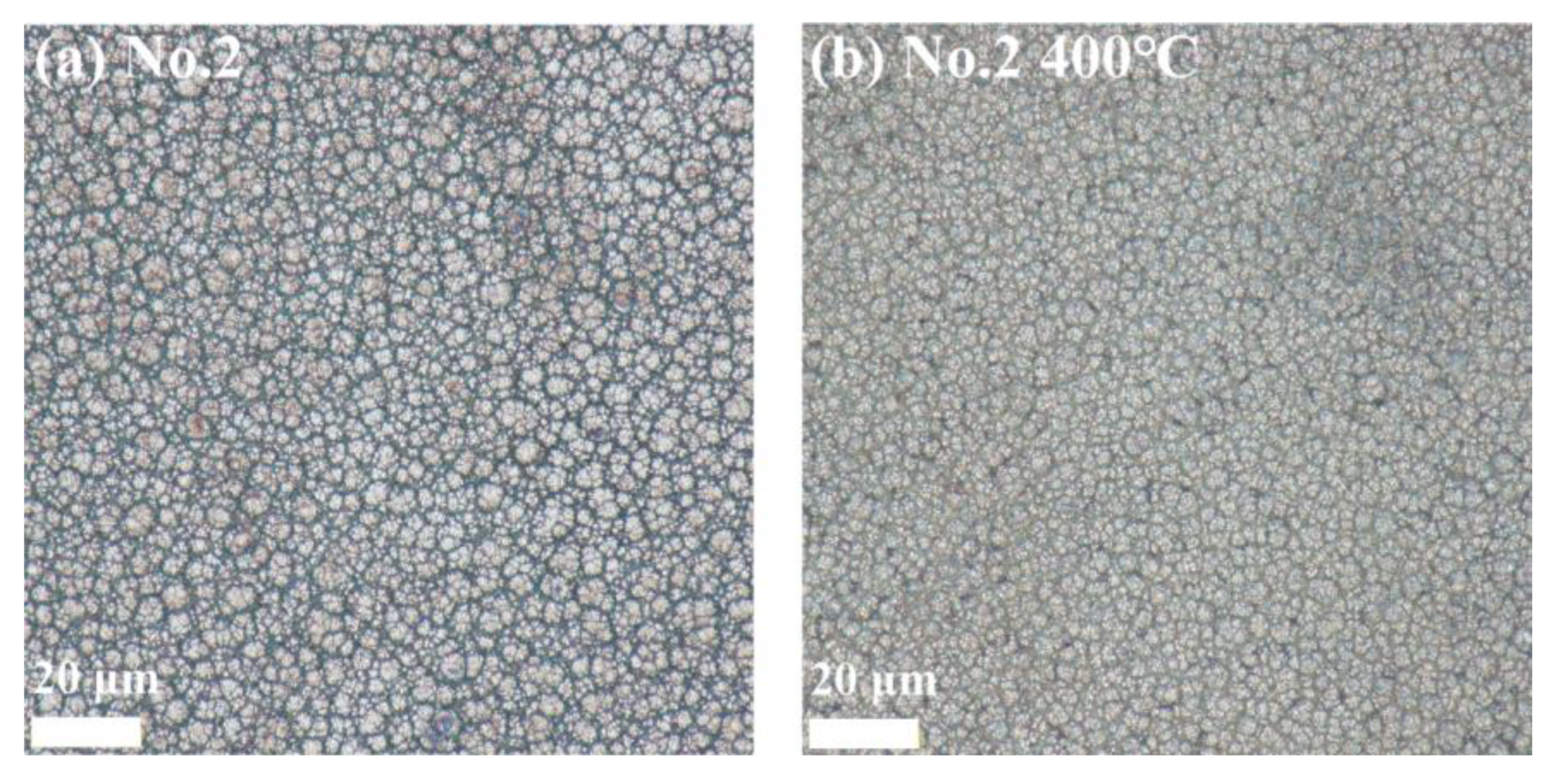
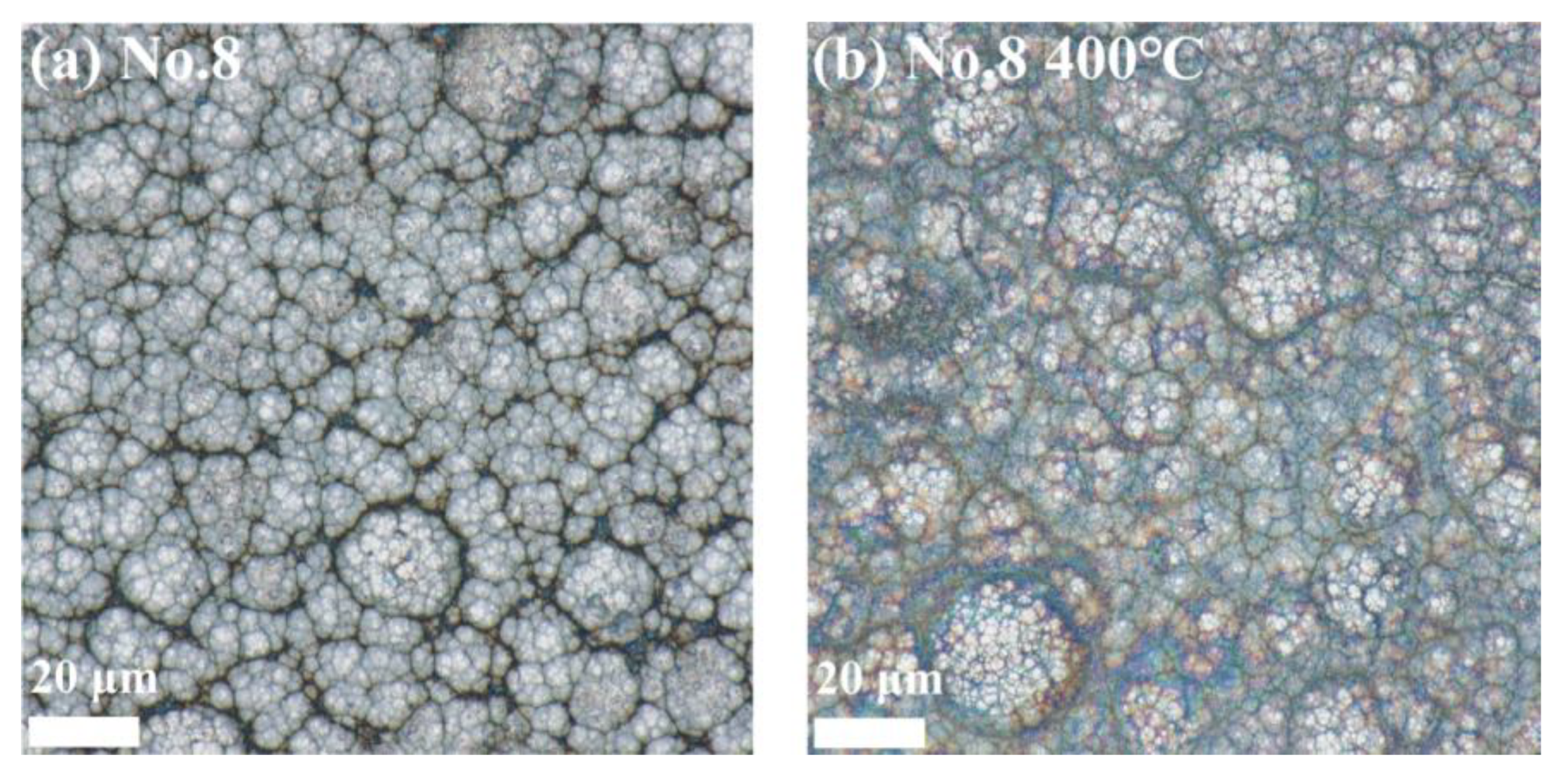
3.3. Ni-B-Mo Surface Morphology
3.4. Component Characterization and Analysis
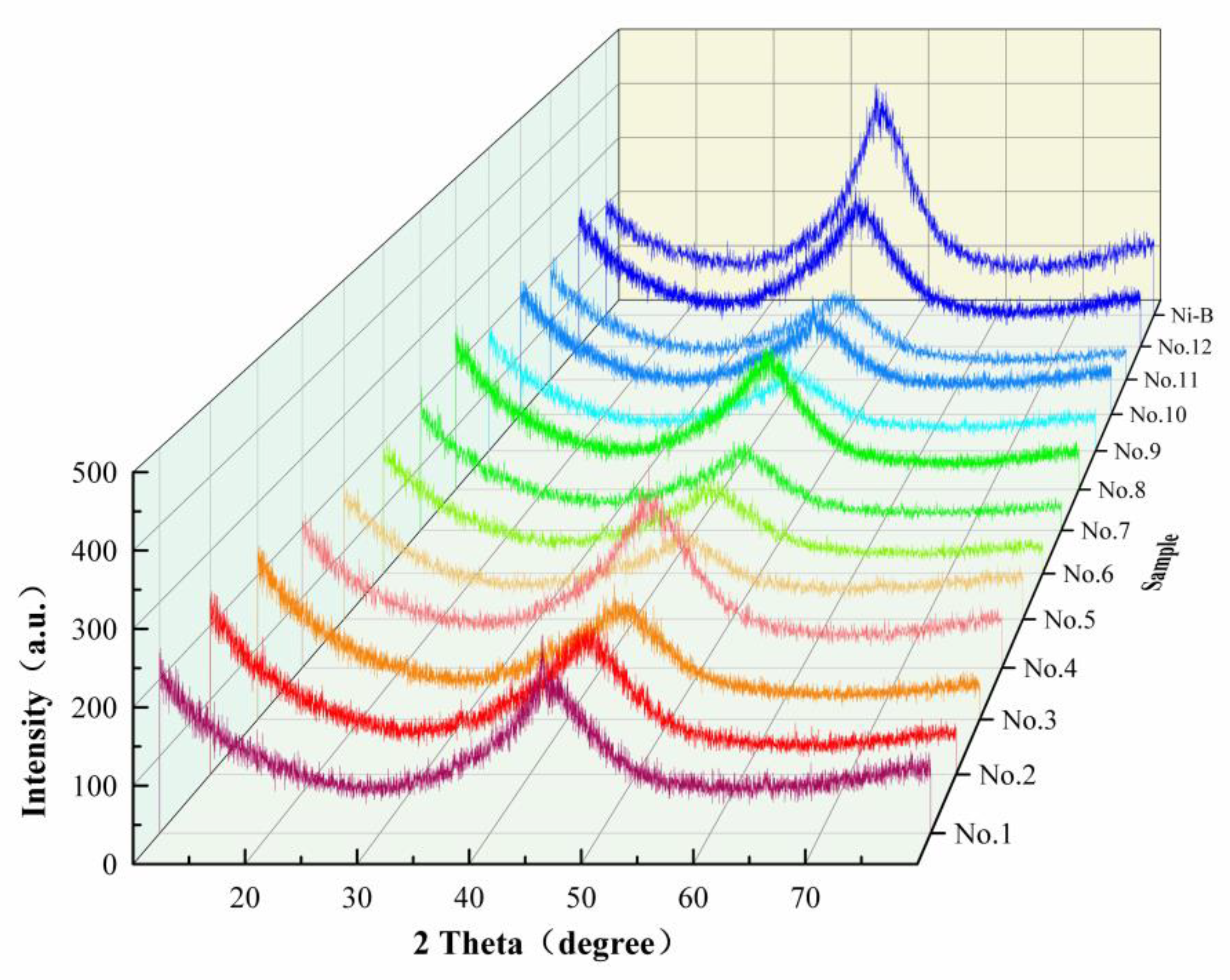
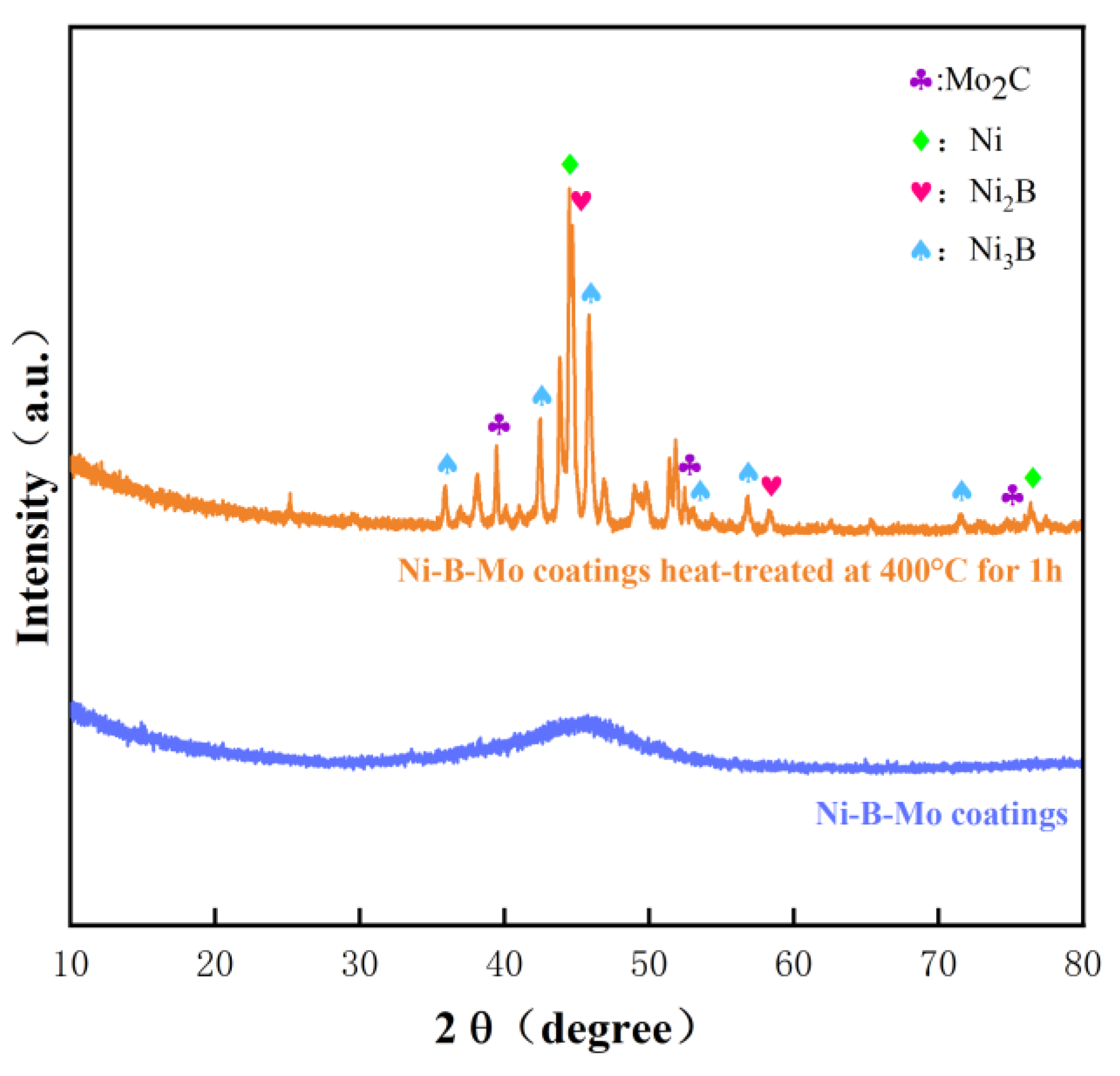
3.4.1. XRD Characterization and Analysis
3.4.2. SEM Characterization and Analysis
3.5. Friction and Wear Analysis and Determination of Optimal Ni-B-Mo Coating Parameters
3.5.1. Preliminary Selection of Optimal Ni-B-Mo Coating
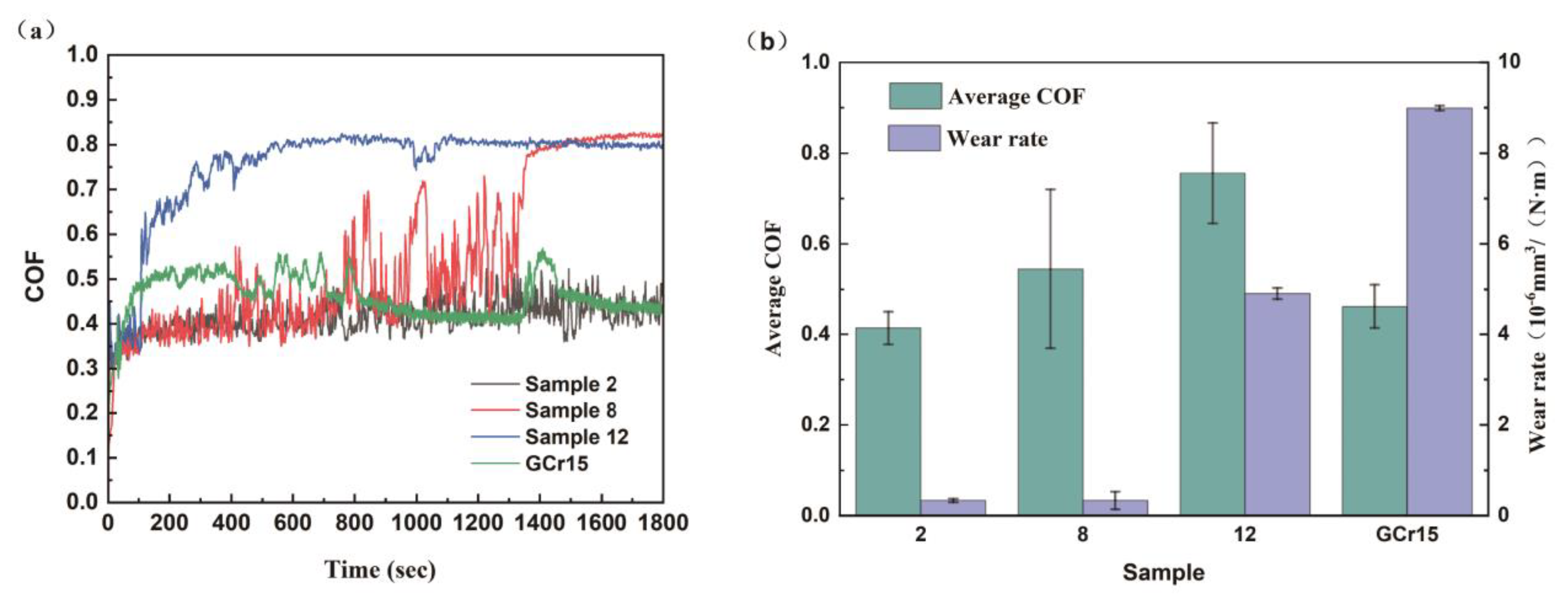
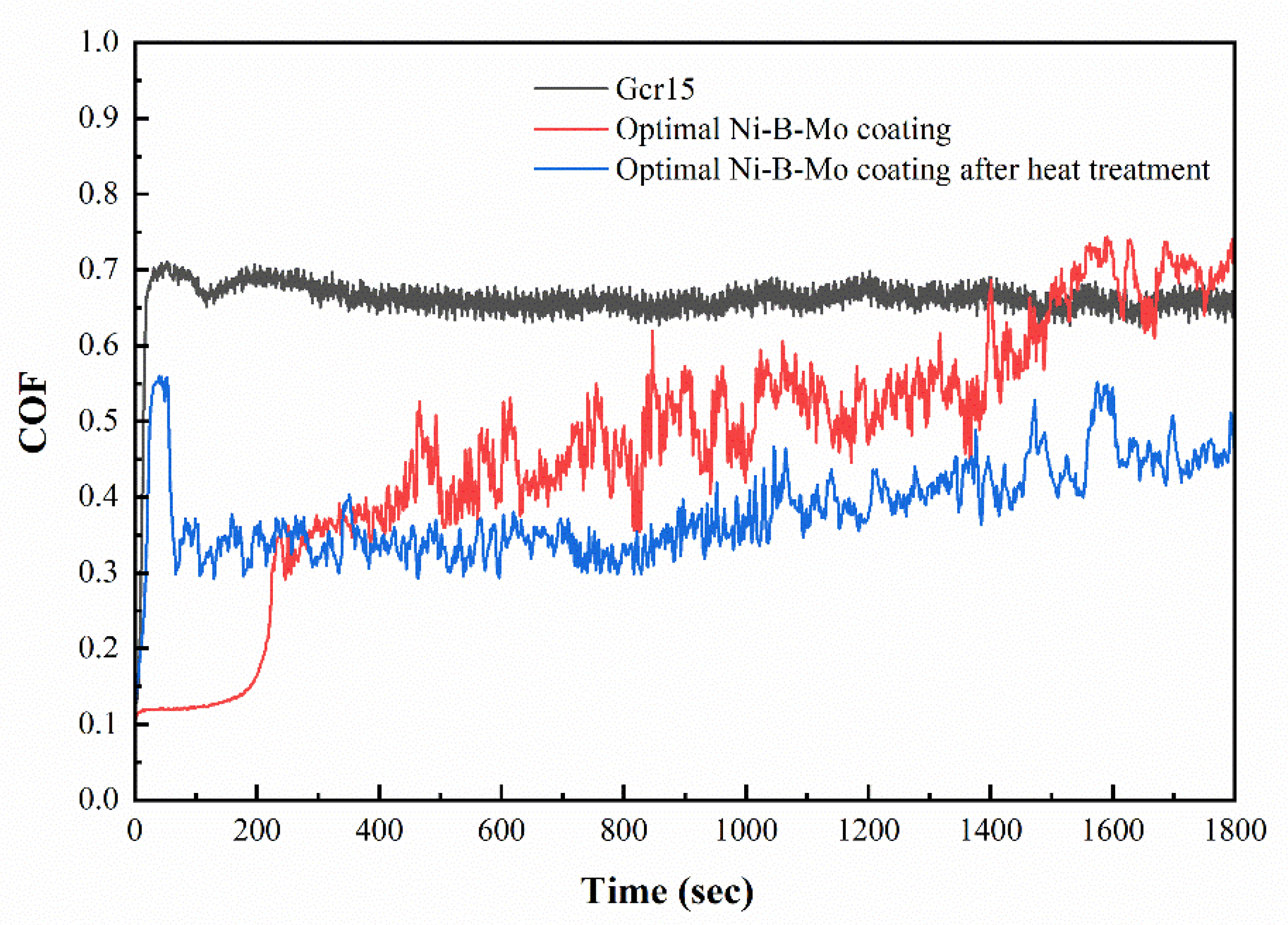
3.5.2. Tribological Behavior Analysis of Coating Samples
3.5.3. Optimization of Ni-B-Mo Coating Process Parameters for GCr15 Steel
3.6. Best Ni-B-Mo Coating Heat Treatment Analysis
3.6.1. Surface Morphology Results and Analysis
3.6.2. XRD Spectrum Results and Analysis
3.6.3. Bonding Strength Results and Analysis
3.6.4. Microhardness Results and Analysis
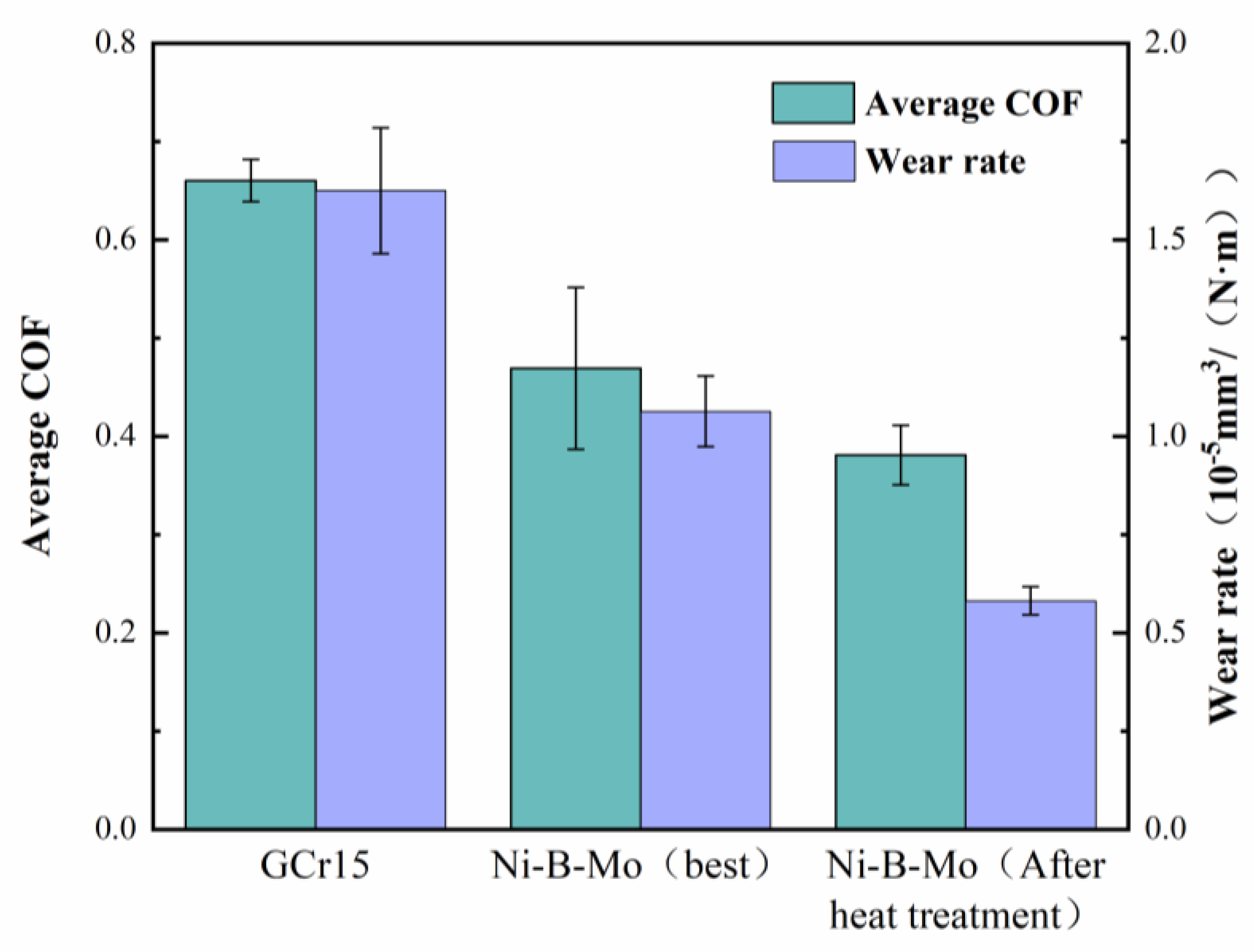
3.6.5. Friction and Wear Performance Comparison and Analysis
4. Conclusions
- (1)
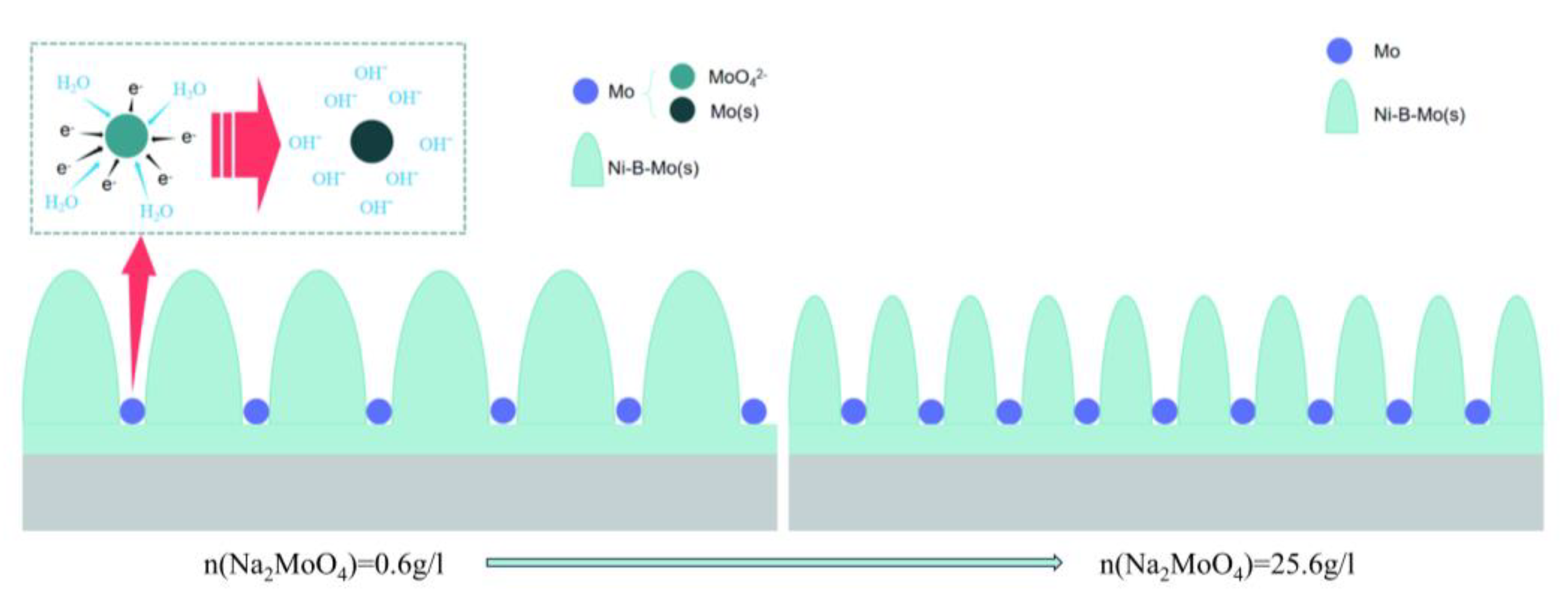
- An appropriate concentration of Na2MoO4 (5.6 g/L) significantly promotes the co-deposition of Ni-B-Mo, improving both the deposition rate and coating performance. However, excessive Na2MoO4 (>15.6 g/L) decreases the deposition rate, induces embrittlement, and reduces the coating adhesion.
- (2)
- A deposition time of 1–2 h maintains a relatively high deposition rate, while prolonged plating (>3 h) leads to bath depletion, a reduced deposition rate, and increased coating defects.
- (3)
- With an increasing Na2MoO4 concentration, the coating surface morphology evolves from blackberry-like and fish egg-like structures to cauliflower-like and broccoli-like structures.
- (4)
- An NiCl2 concentration in the range of 20–30 g/L has a relatively minor effect on the deposition rate and coating properties, primarily acting to stabilize the Ni2+ supply and improve the coating compactness.
- (5)
- Based on orthogonal experimental analysis, the optimal plating parameters were determined as follows: Na2MoO4 = 5.6 g/L, NiCl2 = 25 g/L, deposition time = 2 h, pH > 13, temperature = 90 °C, and stirring speed = 100 rpm. Under these conditions, the coating exhibited high hardness, excellent adhesion, and superior wear resistance.
- (6)
- Post-deposition annealing at 400 °C for 1 h transformed the coating structure from amorphous to nanocrystalline (Ni2B, Ni3B, and Mo2C phases), increasing the hardness from 737.49 HV to 916.19 HV. The friction coefficient decreased from 0.66 to 0.38, and the wear rate was reduced by 64% compared to that of the GCr15 steel substrate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Ying, S.; Zhao, Y.; Li, Z.; Cao, J.; Wu, X.; Du, P.; Wu, T.; Yu, C.; Xie, G. Study on Fretting Friction Properties Based on GCr15 Alloy and Liquid Epoxy Resin Protective Coatings on GCr15 Alloy. Colloids Surf. Physicochem. Eng. Asp. 2024, 696, 134364. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Yang, Y.; Si, Y.; Zhou, Z.; Zhou, X.; Jung, D. Modeling and Simulation of Dynamic Recrystallization Microstructure Evolution for GCr15 Steel Using the Level Set Method. Materials 2025, 18, 342. [Google Scholar] [CrossRef]
- Cui, L.; Wang, D.; Zhang, C.; Wang, X. A Study on the Influence of the Rotating Speed and Load on the Grain Structure and Wear Properties of Bearing Steel GCr15 During Bearing Service. Metals 2024, 14, 1408. [Google Scholar] [CrossRef]
- Li, D.; Zhou, Y.; Liu, X.; Simpson, B.G.; Xiao, J. Experimental Testing of GCr15 Bearing Steel with Different Surface Treatments as Passive Friction Energy-Dissipative Shims. Constr. Build. Mater. 2023, 408, 133628. [Google Scholar] [CrossRef]
- Muench, F. Electroless Plating of Metal Nanomaterials. ChemElectroChem 2021, 8, 2993–3012. [Google Scholar] [CrossRef]
- Molaei, M.; Atapour, M. Nickel-Based Coatings as Highly Active Electrocatalysts for Hydrogen Evolution Reaction: A Review on Electroless Plating Cost-Effective Technique. Sustain. Mater. Technol. 2024, 40, e00991. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P and Ni-P-Al2O3 Nanocomposite Coatings and Their Corrosion and Wear Resistance. J. Mater. Eng. Perform. 2013, 22, 176–183. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N.; Seshadri, S.K. Formation and Characterization of Borohydride Reduced Electroless Nickel Deposits. J. Alloys Compd. 2004, 365, 197–205. [Google Scholar] [CrossRef]
- Baskaran, I.; Sakthi Kumar, R.; Sankara Narayanan, T.S.N.; Stephen, A. Formation of Electroless Ni–B Coatings Using Low Temperature Bath and Evaluation of Their Characteristic Properties. Surf. Coat. Technol. 2006, 200, 6888–6894. [Google Scholar] [CrossRef]
- Oraon, B.; Majumdar, G.; Ghosh, B. Improving Hardness of Electroless Ni–B Coatings Using Optimized Deposition Conditions and Annealing. Mater. Des. 2008, 29, 1412–1418. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Hassan, H.B.; Attyia, A.M. Influence of Deposition Temperature and Heat Treatment on the Performance of Electroless Ni–B Films. Surf. Coat. Technol. 2010, 205, 2348–2354. [Google Scholar] [CrossRef]
- Decomposition of Sodium Borohydride in an Electroless Nickel Bath|Industrial and Engineering Chemistry Research. Available online: https://pubs.acs.org/doi/pdf/10.1021/ie00025a008 (accessed on 3 April 2025).
- Drovosekov, A.B.; Ivanov, M.V.; Krutskikh, V.M.; Loubnin, E.N.; Polukarov, Y.M. The Forming Conditions and Structure Characteristics of the Nickel-Molybdenum-Boron Chemico-Catalytic Coatings. Prot. Met. 2005, 41, 415–421. [Google Scholar] [CrossRef]
- Serin, I.G.; Göksenli, A.; Yüksel, B.; Yildiz, R.A. Effect of Annealing Temperature on the Corrosion Resistance of Electroless Ni-B-Mo Coatings. J. Mater. Eng. Perform. 2015, 24, 3032–3037. [Google Scholar] [CrossRef]
- Serin, I.G.; Göksenli, A. Effect of Annealing Temperature on Hardness and Wear Resistance of Electroless Ni-B-Mo Coatings. Surf. Rev. Lett. 2015, 22, 1550058. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Effect of Heat Treatment on Tribological Behavior of Electroless Ni-B-Mo Coatings at Different Operating Temperatures. Silicon 2018, 10, 1203–1215. [Google Scholar] [CrossRef]
- Barman, M.; Barman, T.K.; Sahoo, P. Tribo-Mechanical Behaviour of Electroless Ni-B-Mo Coatings with Varying Bath Composition. Eng. Res. Express 2023, 5, 025072. [Google Scholar] [CrossRef]
- Doshibekova, A.; Jurinskaya, I.; Tashpulatov, S.; Zhilisbayeva, R.; Sarttarova, L.; Akbarov, R.; Kalmakhanova, M. Effect of Technological Parameters on the Process of Copper Deposition on Chemically and Chemical-Galvanically Nickel-Plated Fibers. Res. J. Text. Appar. 2023, 29, 162–183. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, J. An Approach of Constructing Mixed-Level Orthogonal Arrays of Strength ≥ 3. Sci. China Math. 2013, 56, 1109–1115. [Google Scholar] [CrossRef]
- Vidakis, N.; Antoniadis, A.; Bilalis, N. The VDI 3198 Indentation Test Evaluation of a Reliable Qualitative Control for Layered Compounds. J. Mater. Process. Technol. 2003, 143–144, 481–485. [Google Scholar] [CrossRef]
- Mei, S.; Xiao, Z.; Chen, Z.; Hu, Z.; Zou, X.; Xu, Q.; Zheng, Q. Prediction and Optimization of Aluminum Content in Ni-P-Al2O3 Composite Coatings on GCr15 Steel Based on PSO-BP Neural Network. Surf. Interfaces 2025, 64, 106319. [Google Scholar] [CrossRef]
- Mei, S.; Hu, Z.; Yang, J.; Chen, J.; Zheng, Q.; Lygdenov, B.; Alexey, G. Ni-B-PTFE Nanocomposite Co-Deposition on the Surface of 2A12 Aluminum Alloy. Materials 2024, 17, 3294. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Bi, G.; Lu, Q.; Wang, H.; Fan, X. Microstructure Evolution of Electroless Ni-B Film during Its Depositing Process. Appl. Surf. Sci. 2005, 240, 28–33. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Priyadarshi, A.; Kumar, V.; Manikandanath, N.T.; Kumar, P.P.; Ravisankar, B. Hardness and Wear Behaviour of Electroless Ni–B Coatings. Mater. Sci. Technol. 2016, 32, 1654–1665. [Google Scholar] [CrossRef]
- Vitry, V.; Bonin, L. Increase of Boron Content in Electroless Nickel-Boron Coating by Modification of Plating Conditions. Surf. Coat. Technol. 2017, 311, 164–171. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Tribological Characteristics of Electroless Ni–B–Mo Coatings at Different Operating Temperatures. Surf. Rev. Lett. 2019, 26, 1850175. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Electroless Ni–B Coatings: Preparation and Evaluation of Hardness and Wear Resistance. Surf. Coat. Technol. 2005, 190, 115–121. [Google Scholar] [CrossRef]
- Mei, S.; Zhou, C.; Hu, Z.; Xiao, Z.; Zheng, Q.; Chai, X. Preparation of a Ni-P-nanoPTFE Composite Coating on the Surface of GCr15 Steel for Spinning Rings via a Defoamer and Transition Layer and Its Wear and Corrosion Resistance. Materials 2023, 16, 4427. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Wear and Friction Characteristics of Electroless Ni-B-W Coatings at Different Operating Temperatures. Mater. Res. Express 2018, 5, 026526. [Google Scholar] [CrossRef]
- Bowden, F.P.; Tabor, D. The Friction and Lubrication of Solids; Oxford University Press: New York, NY, USA, 2001; Volume 1, ISBN 0-19-850777-1. [Google Scholar]
- Liu, H.; Yao, H.L.; Thompson, G.E.; Liu, Z.; Harrison, G. Correlation between Structure and Properties of Annealed Electroless Ni–W–P Coatings. Surf. Eng. 2015, 31, 412–419. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, H.; Yu, J.; Lai, J.; He, H.; Zhang, Y.; Qi, H.; Wang, D. Mechanical and Tribological Properties of Ni-B and Ni-B-W Coatings Prepared by Electroless Plating. Lubricants 2023, 11, 42. [Google Scholar] [CrossRef]
- Oriňáková, R.; Oriňák, A.; Kupková, M.; Sabalová, M.; Fedorková, A.S.; Kabátová, M.; Kaľavský, F.; Sedlaříková, M. Effect of Heat Treatment on the Corrosion and Mechanical Properties of Electrolytical Composite Ni-B Coatings. Int. J. Electrochem. Sci. 2014, 9, 4268–4286. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]



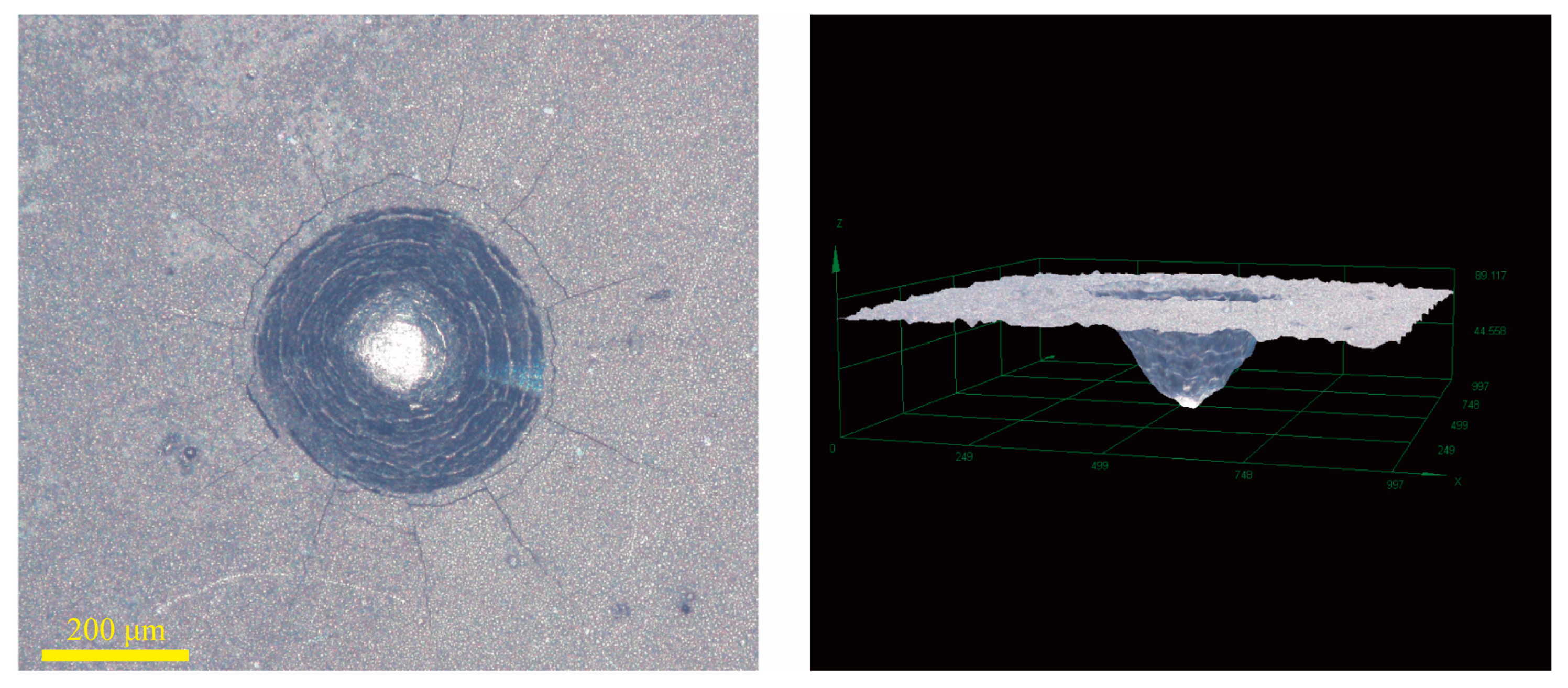
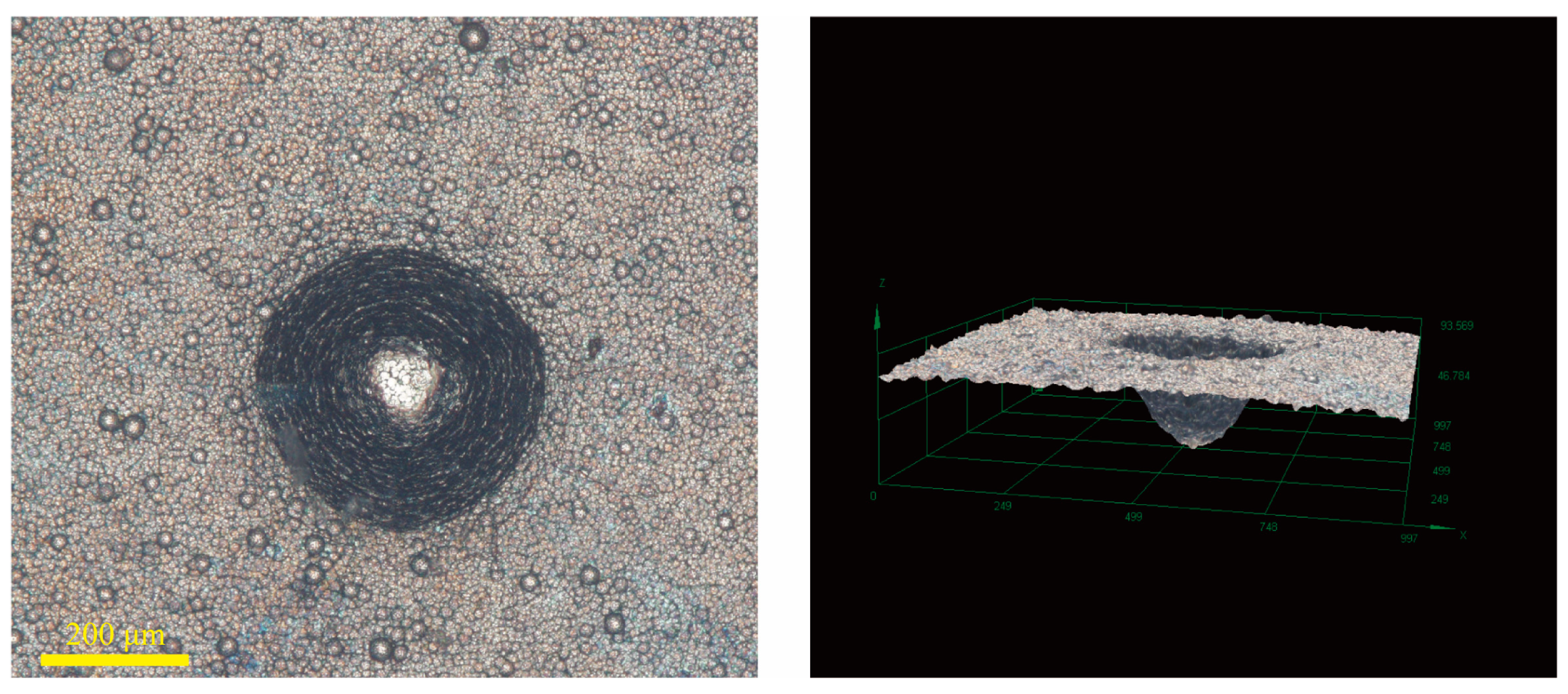



| C | Cr | Mn | Si | Ni | Cu | P | S | O |
|---|---|---|---|---|---|---|---|---|
| 0.95 | 1.44 | 0.36 | 0.27 | 0.06 | 0.07 | 0.13 | 0.004 | 0.0004 |
| Chemical Composition and Process Conditions | Concentration (g/L) and Parameters |
|---|---|
| NiCl2·6H2O (nickel chloride hexahydrate) | 20~30 |
| NaBH4 (sodium borohydride) | 1 |
| C2H8N2 (ethylenediamine) | 60 |
| Na2MoO4 (sodium molybdate) | 0.6~25.6 |
| Pb(NO3)2 (lead nitrate) | 0.03 |
| NaOH (sodium hydroxide) | 40 |
| PH | >13 |
| Deposition time (h) | 1~4 |
| Stirring speed (rpm) | 100 |
| Temperature (°C) | 90 |
| Experimental Group | Na2MoO4 (g/L) | t (h) | NiCl2 (g/L) |
|---|---|---|---|
| 1 | 0.6 | 1 | 20 |
| 2 | 5.6 | 2 | 25 |
| 3 | 10.6 | 3 | 30 |
| 4 | 15.6 | 4 | 20 |
| 5 | 20.6 | 1 | 25 |
| 6 | 25.6 | 2 | 30 |
| 7 | 0.6 | 3 | 20 |
| 8 | 5.6 | 4 | 25 |
| 9 | 10.6 | 1 | 30 |
| 10 | 15.6 | 2 | 20 |
| 11 | 20.6 | 3 | 25 |
| 12 | 25.6 | 4 | 30 |
| Serial No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Deposition Rate (μm/h) | 16.75 | 15.21 | 13.49 | 110.36 | 12.28 | 10.59 |
| Serial No. | 7 | 8 | 9 | 10 | 11 | 12 |
| Deposition Rate (μm/h) | 11.26 | 9.35 | 16.75 | 8.24 | 9.49 | 6.91 |
| Factor | Sodium Molybdate (g/L) | Time (h) | Nickel Chloride (g/L) | ||
|---|---|---|---|---|---|
| Evaluation Index | |||||
| Deposition Rate (μm/h) | K1 | 28.01 | 45.78 | 48.95 | |
| K2 | 24.56 | 34.03 | 46.33 | ||
| K3 | 30.24 | 34.24 | 45.38 | ||
| K4 | 20.94 | 26.62 | |||
| K5 | 21.77 | ||||
| K6 | 15.14 | ||||
| 14.00 | 15.26 | 12.24 | |||
| 12.28 | 11.34 | 11.58 | |||
| 15.12 | 11.41 | 11.35 | |||
| 10.47 | 8.87 | ||||
| 10.89 | |||||
| 7.57 | |||||
| R (Range) | 7.55 | 6.39 | 0.89 | ||
| Primary and Secondary Factors | ABC | ||||
| Optimal Combination | A3B1C1 | ||||
| Serial No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Hardness | 881.00 | 737.49 | 727.76 | 814.08 | 582.30 | 745.51 |
| Serial No. | 7 | 8 | 9 | 10 | 11 | 12 |
| Hardness | 783.91 | 771.94 | 699.61 | 755.57 | 792.67 | 730.34 |
| Factor | Sodium Molybdate (g/L) | Time (h) | Nickel Chloride (g/L) | ||
|---|---|---|---|---|---|
| Evaluation Index | |||||
| Hardness (HV0.2) | K1 | 1664.90 | 2162.91 | 3234.55 | |
| K2 | 1509.43 | 2238.57 | 2884.40 | ||
| K3 | 1427.37 | 2304.34 | 2903.22 | ||
| K4 | 1569.65 | 2316.36 | |||
| K5 | 1374.97 | ||||
| K6 | 1475.85 | ||||
| 832.45 | 720.97 | 808.64 | |||
| 754.71 | 746.19 | 721.10 | |||
| 713.69 | 768.11 | 725.81 | |||
| 784.83 | 772.12 | ||||
| 687.48 | |||||
| 737.93 | |||||
| R (Range) | 144.97 | 51.15 | 87.54 | ||
| Primary and Secondary Factors | ACB | ||||
| Optimal Combination | A1B4C1 | ||||
| Chemical Composition and Process Conditions | Concentration (g/L) and Parameters |
|---|---|
| NiCl2·6H2O (nickel chloride hexahydrate) | 25 |
| NaBH4 (sodium borohydride) | 1 |
| C2H8N2 (ethylenediamine) | 60 |
| Na2MoO4 (sodium molybdate) | 5.6 |
| Pb(NO3)2 (lead nitrate) | 0.03 |
| NaOH (sodium hydroxide) | 40 |
| PH | >13 |
| Deposition time (h) | 2 |
| Stirring speed (rpm) | 100 |
| Temperature (°C) | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, S.; Zou, X.; Hu, Z.; Yang, J.; Zheng, Q.; Huang, W.; Guryev, A.; Lygdenov, B. Optimization of Ni-B-Mo Electroless Coating on GCr15 Steel: Effects of Main Salt Concentration and Deposition Time. Materials 2025, 18, 1981. https://doi.org/10.3390/ma18091981
Mei S, Zou X, Hu Z, Yang J, Zheng Q, Huang W, Guryev A, Lygdenov B. Optimization of Ni-B-Mo Electroless Coating on GCr15 Steel: Effects of Main Salt Concentration and Deposition Time. Materials. 2025; 18(9):1981. https://doi.org/10.3390/ma18091981
Chicago/Turabian StyleMei, Shunqi, Xiaohui Zou, Zekui Hu, Jinyu Yang, Quan Zheng, Wei Huang, Alexey Guryev, and Burial Lygdenov. 2025. "Optimization of Ni-B-Mo Electroless Coating on GCr15 Steel: Effects of Main Salt Concentration and Deposition Time" Materials 18, no. 9: 1981. https://doi.org/10.3390/ma18091981
APA StyleMei, S., Zou, X., Hu, Z., Yang, J., Zheng, Q., Huang, W., Guryev, A., & Lygdenov, B. (2025). Optimization of Ni-B-Mo Electroless Coating on GCr15 Steel: Effects of Main Salt Concentration and Deposition Time. Materials, 18(9), 1981. https://doi.org/10.3390/ma18091981





