Lean Pattern in an Altitude Range Shift of a Tree Species: Abies pinsapo Boiss.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species
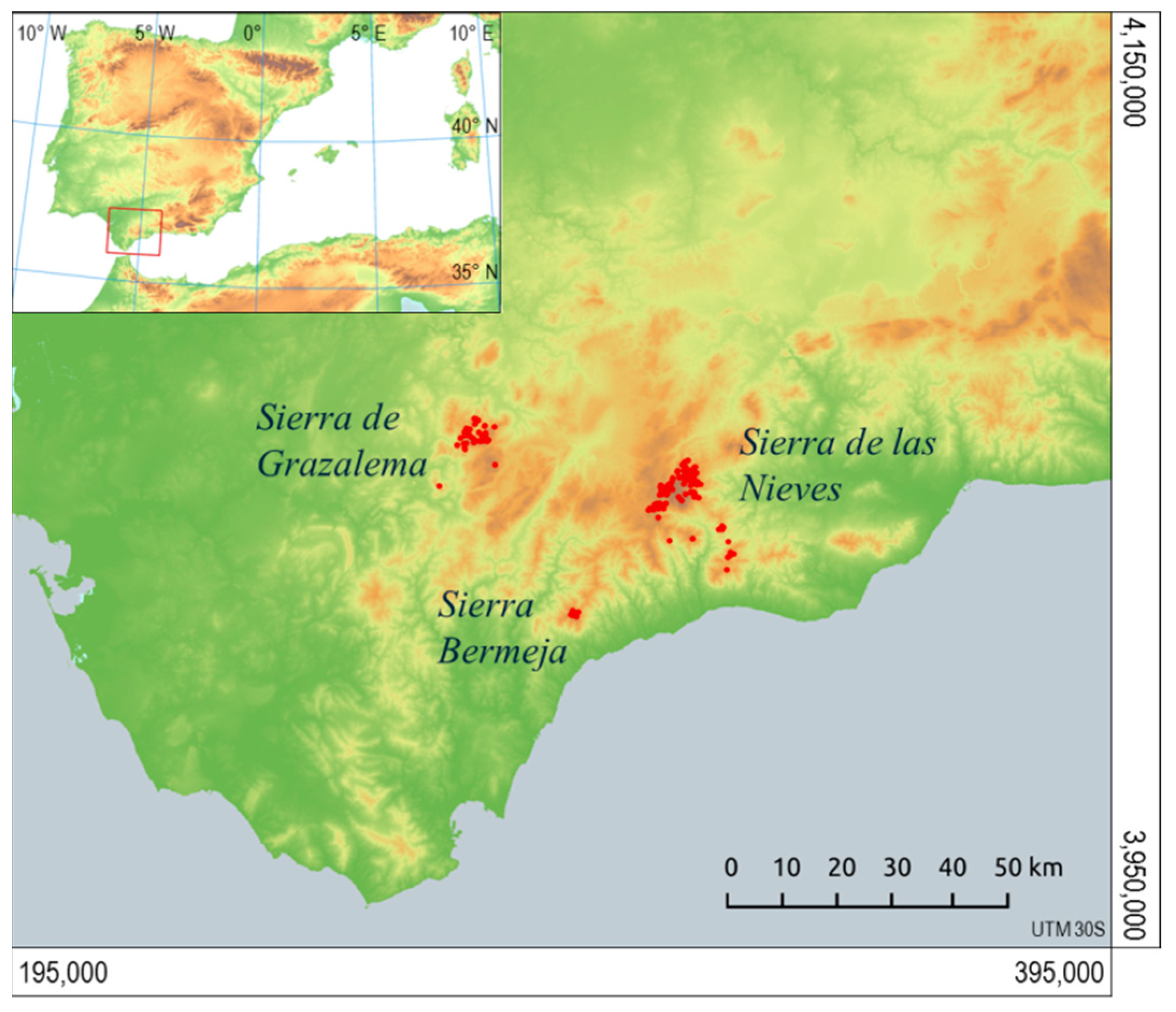
2.2. Area of Study
2.3. Observations of Presence
- Sapling: stands that included the presence of young individuals whose height did not exceed 40 cm; n = 41.
- Mature: stands that included the presence of individuals whose diameter at 130 cm above the ground (dbh) exceeded 20 cm; n = 134.
- Whole: included all of the stands of natural origin, regardless of the life stages they included; n = 141.
2.4. Predictor Variables
2.5. Species Distribution Models
2.6. Altitudinal Distribution Curves
2.7. Persistence/Migration Map
3. Results
3.1. Species Distribution Models
3.2. Altitudinal Distribution Curves
3.3. Persistence/Migration Map
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Appendix A
| GDD | AP | WQP | |
|---|---|---|---|
| GDD | 1 | ||
| AP | −0.209 | 1 | |
| WQP | −0.296 | 0.531 | 1 |
| Training | Evaluation | |||
|---|---|---|---|---|
| Subset | Mature | Sapling | Mature | Sapling |
| 1 | 66 | 19 | 67 | 22 |
| 2 | 68 | 21 | 65 | 20 |
| 3 | 68 | 23 | 65 | 18 |
| 4 | 67 | 23 | 66 | 18 |
| 5 | 65 | 21 | 68 | 19 |
| 6 | 67 | 23 | 66 | 18 |
| 7 | 67 | 23 | 66 | 18 |
| 8 | 65 | 22 | 68 | 19 |
| 9 | 64 | 17 | 69 | 24 |
| 10 | 68 | 19 | 65 | 22 |
References
- Ackerly, D.D.; Schwilk, D.W.; Webb, C.O. Niche evolution and adaptive radiation: Testing the order of trait divergence. Ecology 2006, 87, S50–S61. [Google Scholar] [CrossRef]
- Benito, B.; Lorite, J.; Pérez-Pérez, R.; Gómez-Aparicio, L.; Peñas, J. Forecasting plant range collapse in a Mediterranean hotspot: When dispersal uncertainties matter. Divers. Distrib. 2014, 20, 72–83. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Pfennig, D.W.; Servedio, M.R. Migration, local adaptation and the evolution of plasticity. Trends Ecol. Evol. 2002, 17, 540–541. [Google Scholar] [CrossRef]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; van Kleunen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef]
- Benito, B.; Lorite, J.; Peñas, J. Simulating potential effects of climatic warming on altitudinal patterns of key species in Mediterranean-Alpine ecosystems. Clim. Change 2011, 108, 471–483. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Richardson, A.J.; Molinos, J.G.; Hoffmann, A.; Buckley, L.B.; Moore, P.J.; Brown, C.J.; Bruno, J.F.; Duarte, C.M.; et al. Geographical limits to species-range shifts are suggested by climate velocity. Nature 2014, 507, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Bell, D.M.; Bradford, J.B.; Lauenroth, W.K. Early indicators of change: Divergent climate envelopes between tree life stages imply range shifts in the Western United States. Glob. Ecol. Biogeogr. 2014, 23, 168–180. [Google Scholar] [CrossRef]
- Jump, A.S.; Mátyás, C.; Peñuelas, J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Change Biol. 2012, 18, 1042–1052. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.-C.; Pierrat, J.-C.; Bontemps, J.-D.; Dhôte, J.-F. Differences between tree species seedling and adult altitudinal distribution in mountain forests during the Recent Warm Period (1986–2006). Ecography 2009, 32, 765–777. [Google Scholar] [CrossRef]
- Matías, L.; Jump, A.S. Asymmetric changes of growth and reproductive investment herald altitudinal and latitudinal range shifts of two woody species. Glob. Change Biol. 2015, 21, 882–896. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Ogaya, R.; Boada, M.; Jump, A. Migration, invasion and decline: Changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 2007, 30, 829–837. [Google Scholar] [CrossRef]
- Peñuelas, J.; Boada, M. A global change-induced biome shift in the Montseny Mountains (NE Spain). Glob. Change Biol. 2003, 9, 131–140. [Google Scholar] [CrossRef]
- Urli, M.; Delzon, S.; Eyermann, A.; Couallier, V.; García-Valdés, R.; Zavala, M.A.; Porté, A.J. Inferring shifts in tree species distribution using asymmetric distribution curves: A case study in the Iberian Mountains. J. Veg. Sci. 2014, 25, 147–159. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G. Equilibrium of species’ distributions with climate. Ecography 2005, 28, 693–695. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Sandel, B. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 2013, 100, 1266–1286. [Google Scholar] [CrossRef]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef]
- Bykova, O.; Chuine, I.; Morin, X.; Higgins, S.I. Temperature dependence of the reproduction niche and its relevance for plant species distributions. J. Biogeogr. 2012, 39, 2191–2200. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S.J. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Thuiller, W.; Albert, C.; Araújo, M.B.; Berry, P.M.; Cabeza, M.; Guisan, A.; Hickler, T.; Midgley, G.F.; Paterson, J.; Schurr, F.M.; et al. Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 2008, 9, 137–152. [Google Scholar] [CrossRef]
- Schurr, F.M.; Pagel, J.; Cabral, J.S.; Groeneveld, J.; Bykova, O.; O’Hara, R.B.; Hartig, F.; Kissling, W.D.; Linder, H.P.; Midgley, G.F.; et al. How to understand species’ niches and range dynamics: A demographic research agenda for biogeography. J. Biogeogr. 2012, 39, 2146–2162. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.-C. Latitudinal and elevational range shifts under contemporary climate change. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 599–611. ISBN 978-0-12-384720-1. [Google Scholar]
- Lenoir, J.; Svenning, J.-C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Arista, M.; Knees, S.; Gardner, M. Abies Pinsapo Var. Pinsapo. IUCN Red List of Threatened Species 2011; IUCN Global Species Programme Red List Unit: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Junta de Andalucía/Consejería de Medio Ambiente. Plan de Recuperación del Pinsapo. Available online: http://www.juntadeandalucia.es/boja/2011/25/boletin.25.pdf (accessed on 23 January 2013).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report; Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2014. [Google Scholar]
- Farjon, A. Pinaceae: Drawing and Descriptions of the Genera Abies, Cedrus, Pseudolarix, Keteleeria, Nothotsuga, Tsuga, Cathaya, Pseudotsuga, Larix and Picea; Koeltz Scientific Books: Königstein, Germany, 1990; ISBN 978-1-878762-04-7. [Google Scholar]
- Linares, J.C.; Carreira, J.A. El pinsapo, abeto endémico andaluz. O, ¿Qué hace un tipo como tú en un sitio como éste? Ecosistemas 2006, 15, 171–191. [Google Scholar]
- Govaerts, R.; Farjon, A. Pinaceae. Available online: http://wcsp.science.kew.org (accessed on 2 February 2021).
- Balao, F.; Lorenzo, M.T.; Sánchez-Robles, J.M.; Paun, O.; García-Castaño, J.L.; Terrab, A. Early diversification and permeable species boundaries in the Mediterranean firs. Ann. Bot. 2020, 125, 495–507. [Google Scholar] [CrossRef]
- Sękiewicz, K.; Sękiewicz, M.; Jasińska, A.K.; Boratyńska, K.; Iszkuło, G.; Romo, A.; Boratyński, A. Morphological diversity and structure of West Mediterranean Abies species. Plant Biosyst. 2013, 147, 125–134. [Google Scholar] [CrossRef]
- Terrab, A.; Talavera, S.; Arista, M.; Paun, O.; Stuessy, T.F.; Tremetsberger, K. Genetic diversity at chloroplast microsatellites (CpSSRs) and geographic structure in endangered West Mediterranean firs (Abies spp., Pinaceae). Taxon 2007, 56, 409–416. [Google Scholar] [CrossRef]
- Guzmán Álvarez, J.R.; Catalina, M.A.; Navarro Cerrillo, R.M.; López Quintanilla, J.; Sánchez-Salguero, R. Los paisajes del pinsapo a través del tiempo. In Los Pinsapares en Andalucia (Abies pinsapo Boiss.): Conservación y Sostenibilidad en el Siglo XXI; López Quintanilla, J., Ed.; Junta de Andalucía-Universidad de Córdoba: Cordoba, Spain, 2013; pp. 111–149. ISBN 978-84-9927-137-8. [Google Scholar]
- Arista, M.; Herrera, F.J.; Talavera, S. Biología del Pinsapo; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 1997. [Google Scholar]
- Valladares, A. Abetales de Abies pinsapo Boiss. In Bases Ecológicas Preliminares para la Conservación de los Tipos de Hábitat de Interés Comunitario en España; Ministerio Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2009; Volume 9520, ISBN 978-84-491-0911-9. [Google Scholar]
- Skov, F.; Svenning, J.-C. Potential impact of climatic change on the distribution of forest herbs in Europe. Ecography 2004, 27, 366–380. [Google Scholar] [CrossRef]
- Aussenac, G. Ecology and ecophysiology of circum-Mediterranean firs in the context of climate change. Ann. For. Sci. 2002, 59, 823–832. [Google Scholar] [CrossRef]
- REDIAM Red de Información Ambiental de Andalucía. Available online: http://www.juntadeandalucia.es/medioambiente/site/rediam (accessed on 17 January 2012).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Nix, H.A. A biogeographic analysis of Australian elapid snakes. In Atlas of Elapid Snakes of Australia: Australian Flora and Fauna; Longmore, R., Ed.; Bureau of Flora and Fauna: Camberra, Australia, 1986; pp. 4–15. [Google Scholar]
- Hijmans, R.J.; Phillips, S.J.; Leathwick, J.; Elith, J. Package ‘Dismo’. Species Distribution Modeling. R Package, Version 0.7-17; Institute for Statistics and Mathematics: Vienna, Austria, 2014. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.r-project.org/ (accessed on 21 January 2013).
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Lechuga, V.; Carraro, V.; Viñegla, B.; Carreira, J.A.; Linares, J.C. Carbon limitation and drought sensitivity at contrasting elevation and competition of Abies pinsapo forests. Does experimental thinning enhance water supply and carbohydrates? Forests 2019, 10, 1132. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Interacting effects of changes in climate and forest cover on mortality and growth of the southernmost European fir forests. Glob. Ecol. Biogeogr. 2009, 18, 485–497. [Google Scholar] [CrossRef]
- Linares, J.C.; Delgado-Huertas, A.; Camarero, J.J.; Merino, J.; Carreira, J.A. Competition and drought limit the response of water-use efficiency to rising atmospheric carbon dioxide in the Mediterranean fir Abies pinsapo. Oecologia 2009, 161, 611–624. [Google Scholar] [CrossRef]
- Linares, J.C.; Covelo, F.; Carreira, J.A.; Merino, J.Á. Phenological and water-use patterns underlying maximum growing season length at the highest elevations: Implications under climate change. Tree Physiol. 2012, 32, 161–170. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Ortíz, C.; Covelo, F.; Ochoa, V.; García-Ruíz, R.; Seco, J.I.; Carreira, J.A.; Merino, J.Á.; Linares, J.C. Regulation of water use in the southernmost European fir (Abies pinsapo Boiss.): Drought avoidance matters. Forests 2015, 6, 2241–2260. [Google Scholar] [CrossRef]
- Körner, C. A re-assessment of high elevation treeline positions and their explanation. Oecologia 1998, 115, 445–459. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Linares, J.C.; Viñegla, B.; Carreira, J.A. Caracterización estructural de poblaciones de Abies pinsapo Boiss. en la sierra de Yunquera (Málaga). Iniciación Investig. 2010, 2, a7. [Google Scholar]
- Arista, M. Germinación de las semillas y supervivencia de las plántulas de Abies pinsapo Boiss. Acta Bot. Malacit. 1993, 18, 173–177. [Google Scholar] [CrossRef]
- Arista, M. Supervivencia de las plántulas de Abies pinsapo Boiss. en su hábitat natural. An. Jardín Bot. Madr. 1994, 51, 193–198. [Google Scholar]
- Holt, R.D. Bringing the hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. USA 2009, 106, 19659–19665. [Google Scholar] [CrossRef] [PubMed]
- Catalina, M.A. Incendios en el pinsapar. In Los Pinsapares en Andalucia (Abies pinsapo Boiss.): Conservación y Sostenibilidad en el Siglo XXI; López Quintanilla, J., Ed.; Junta de Andalucía-Universidad de Córdoba: Córdoba, Spain, 2013; pp. 371–373. ISBN 978-84-9927-137-8. [Google Scholar]
- García Esteban, L.; de Palacios, P.; Rodríguez-Losada Aguado, L. Abies Pinsapo forests in Spain and Morocco: Threats and conservation. Oryx 2010, 44, 276–284. [Google Scholar] [CrossRef]




| AUC | |||
|---|---|---|---|
| Whole | Mature | Sapling | |
| MaxEnt | 0.9819 | 0.9815 | 0.9880 |
| Bioclim | 0.9201 | 0.9234 | 0.8483 |
| MaxEnt | Bioclim | |||||
|---|---|---|---|---|---|---|
| Mature | Sapling | ΔQ | Mature | Sapling | ΔQ | |
| Q.05 | 652 | 780 | 128 | 540 | 631 | 91 |
| Q.95 | 1368 | 1423 | 55 | 1281 | 1335 | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Hernández, A.; Nieto-Lugilde, D.; Peñas, J.; Alba-Sánchez, F. Lean Pattern in an Altitude Range Shift of a Tree Species: Abies pinsapo Boiss. Forests 2021, 12, 1451. https://doi.org/10.3390/f12111451
González-Hernández A, Nieto-Lugilde D, Peñas J, Alba-Sánchez F. Lean Pattern in an Altitude Range Shift of a Tree Species: Abies pinsapo Boiss. Forests. 2021; 12(11):1451. https://doi.org/10.3390/f12111451
Chicago/Turabian StyleGonzález-Hernández, Antonio, Diego Nieto-Lugilde, Julio Peñas, and Francisca Alba-Sánchez. 2021. "Lean Pattern in an Altitude Range Shift of a Tree Species: Abies pinsapo Boiss." Forests 12, no. 11: 1451. https://doi.org/10.3390/f12111451
APA StyleGonzález-Hernández, A., Nieto-Lugilde, D., Peñas, J., & Alba-Sánchez, F. (2021). Lean Pattern in an Altitude Range Shift of a Tree Species: Abies pinsapo Boiss. Forests, 12(11), 1451. https://doi.org/10.3390/f12111451







