Insights into Distribution of Soil Available Heavy Metals in Karst Area and Its Influencing Factors in Guilin, Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection
2.3. Chemical Analysis
2.3.1. Soil Heavy Metals and Environmental Indexes
2.3.2. Sequential Extraction Procedure for Analysis of Ca Fraction
2.4. Data Processing
2.4.1. Activation Rate (AR)
2.4.2. Q Index
2.4.3. Redundancy Analysis (RDA)
3. Results
3.1. Soil Environmental Factors
3.2. Distribution of Ca Fractions
3.3. Distribution of Heavy Metals
3.4. AR of Carbonate and Clastic Area
4. Discussion
4.1. Accumulation of Total Metal Elements in Calcareous Soils
4.2. Principal Controlling Factors for Heavy Metals:Soil pH and Ca Fraction
4.3. Other Controlling Factors for Heavy Metals: AP, C:N, Cation Exchange Capacity (CEC), and Soil Texture
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, D. Modern karstology and global change study. Earth Sci. Front. 1997, 4, 17–25. [Google Scholar]
- Wang, S.; Ji, H. Preliminary study of carbonate rock weathering pedogenesis. Sci. China Ser. D 1999, 29, 441–449. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, K.; Pan, Y.; Zhao, D.; Lu, X. Global Distribution of Phanerozoic Carbonates and Controlling Factors. Geoscience 2013, 27, 637–643. (In Chinese) [Google Scholar]
- Jiang, Z.; Qin, X.; Cao, J.; Jiang, X.; Luo, W. Calculation of atmospheric CO2 sink formed in karst progresses of the karst divided regions in China. Carsol. Sin. 2011, 30, 363–367. (In Chinese) [Google Scholar]
- Yuan, D. Karst of China; China Geological Publishing House: Beijing, China, 1991; p. 224. [Google Scholar]
- Yang, Q.; Yang, Z.; Filippelli, G.M.; Ji, J.; Ji, W.; Liu, X.; Wang, L.; Yu, T.; Wu, T.; Zhuo, X.; et al. Distribution and secondary enrichment of heavy metal elements in karstic soils with high geochemical background in Guangxi, China. Chem. Geol. 2021, 567, 120081. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Yang, Z.; Zhuo, X.; Ji, J. Evaluation of various approaches to predict cadmium bioavailability to rice grown insoils with high geochemical background in the karst region, Southwestern China. Environ. Pollut. 2020, 258, 113645. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Yang, Z.; Zhang, Q.; Ji, J. Enrichment and source identification of Cd and other heavy metals in soils with high geochemical background in the karst region, Southwestern China. Chemosphere 2020, 245, 125620. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Yamasaki, S.I.; Takeda, A.; Nunohara, K.; Tsuchiya, N. Red soils derived from limestone contain higher amounts of trace elements than those derived from various other parent materials. Soil Sci. Plant Nutr. 2013, 59, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Halamić, J.; Peh, Z.; Miko, S.; Galović, L.; Šorša, A. Geochemical Atlas of Croatia: Environmental implications and geodynamical thread. J. Geochem. Explor. 2012, 115, 36–46. [Google Scholar] [CrossRef]
- Zhang, C.; Lalor, G. Multivariate relationships and spatial distribution of geochemical features of soils in Jamaica. Chem. Speciat. Bioavailab. 2002, 14, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Miko, S.; Peh, Z.; Bukovec, D.; Prohić, E.; Kastmüller, Ž. Geochemical baseline mapping and lead pollution assessment of soils on the karst in Western Croatia. Nat. Croat. 2000, 9, 41–59. [Google Scholar]
- Yang, H.; Lu, M.; Cao, J. Trace elements of the soil–plant systems in subtropical karst and clasolite areas in Guilin, Guangxi, China. Environ. Earth. Sci. 2015, 73, 6259–6269. [Google Scholar] [CrossRef]
- Gan, Y.; Miao, Y.; Wang, L.; Yang, G.; Li, Y.; Wang, W.; Dai, J. Source contribution analysis and collaborative assessment of heavy metals in vegetable-growing soils. J. Agric. Food Chem. 2018, 66, 10943–10951. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of China. Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land (GB15618-2018); China Environmental Science Press: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Minstry of Health of China (MHC). National Food Safety Standard: Maximum Levels of Contaminants in Food (GB2762-2017); China Quality and Standards Publishing: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Huang, F.; Cao, J.; Zhu, T.; Fan, M.; Ren, M. CO2 Transfer Characteristics of Calcareous Humid Subtropical Forest Soils and Associated Contributions to Carbon Source and Sink in Guilin, Southwest China. Forests 2020, 11, 219. [Google Scholar] [CrossRef] [Green Version]
- Lu, R. Soil Agricultural Chemical Analysis Method; Agricultural Science and Technology Press of China: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Lu, M.; Cao, J.; He, X. The Comparative Study of Biogeocheistry between Limestone Soil and Red Soil in Maocun, Guilin. Guangxi Sci. 2006, 13, 58–64. (In Chinese) [Google Scholar]
- Dahlqvist, R.; Benedetti, M.F.; Andersson, K.; Turner, D.; Larsson, T.; Stolpe, B.; Ingri, J. Association of calcium with colloidal particles and speciation of calcium in the Kalix and Amazon rivers. Geochim. Cosmochim. Acta 2004, 68, 4059–4075. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Zheng, Z.; Chen, J. Environmental geochemical characteristics of some microelements in the yellow brown soil of Hubei province. Acta Pedol. Sin. 2001, 38, 89–95. [Google Scholar]
- Xie, D.; Yang, Y.; Su, C.; Meng, X. Contrasts of geochemical characteristics and environmental quality between soils of carbonate and clastic matrix. Guizhou Agric. Sci. 2015, 43, 128–132. (In Chinese) [Google Scholar]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Chen, J.R.; Cao, J.H.; Liang, Y.; Yang, H. Relationship of the humus components and the calcium form with the development of limestone soil. Carsol. Sin. 2012, 31, 7–11. (In Chinese) [Google Scholar]
- Rudnick, R.; Gao, S. Composition of the Continental Crust. Treatise Geochem. 2014. [Google Scholar] [CrossRef]
- Lian, B.; Chen, Y.; Zhu, L.; Yang, R. Effect of Microbial Weathering on Carbonate Rocks. Earth Sci. Front. 2008, 15, 90–99. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.M.; Gu, Y.; Kopittke, P.M.; Zhao, F.J.; Wang, P. Iron-Manganese (Oxyhydro) oxides, Rather than Oxidation ofSulfides, Determine Mobilization of Cd during Soil Drainage in Paddy Soil Systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef]
- He, S.; Lu, Q.; Li, W.; Ren, Z.; Zhou, Z.; Feng, X.; Zhang, Y.; Li, Y. Factors controlling cadmium and lead activities in different parent material-derived soils from the Pearl River Basin. Chemosphere 2017, 182, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Hinojosa, R.P.; Föllmi, K.B.; Verrecchia, E.; Adatte, T.; Matera, V. Speciation and multivariable analyses of geogenic cadmium in soils at Le Gurnigel, Swiss Jura Mountains. Catena 2015, 125, 10–32. [Google Scholar] [CrossRef]
- Li, X.; Pan, S. The characteristics of translocation accumulation and distribution about trace elements in the soils of both Guangdong and Hainan provinces Tropical. Geography 1991, 11, 62–70. (In Chinese) [Google Scholar]
- Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Muoz-Rojas, M. Soil, site, and management factors affecting cadmium concentrations in cacao-growing soils. Agronomy 2020, 10, 806. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, R.; Khan, R.; Khan, R.; Shah, R. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, S.; Ouyang, Z.; Zhang, S.; Sun, C.; Liu, X.; Zhou, D. Geochemistry of red residua underlying dolomites in karst terrains of Yunnan-Guizhou Plateau: II. The mobility of rare earth elements during weathering. Chem. Geol. 2004, 203, 29–50. [Google Scholar] [CrossRef]
- Sun, C.X.; Wang, S.J.; Liu, X.M.; Feng, Z.G. Geological characteristics and formation mechanism of rock-soil interface in limestone weathering crust at huaxi, Guizhou Province. Acta Mineral. Sin. 2002, 22, 126–132. (In Chinese) [Google Scholar]
- Ji, H.; Ouyang, Z.; Wang, S.; Zhou, D. Element geochemistry of weathering profile of dolomitite and its implications for the average chemical composition of the upper-continental crust. Sci. China Ser. D. 2000, 43, 23–35. [Google Scholar] [CrossRef]
- Feng, J. Trace elements in ferromanganese concretions, gibbsite spots, and the surrounding terra rossa overlying dolomite: Their mobilization, redistribution and fractionation. J. Geochem. Explor. 2010, 108, 99–111. [Google Scholar] [CrossRef]
- Weerasooriya, R.; Wijesekara, H.; Bandara, A. Surface complexation modeling of cadmium adsorption on gibbsite. Colloids Surf. A 2002, 207, 13–24. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Webster, J.G.; Miskelly, G.M. Goethite adsorption of Cu(II), Pb(II), Cd(II), and Zn(II) in the presence of sulfate: Properties of the ternary complex. Geochim. Cosmochim. Acta 2008, 73, 1548–1562. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, W.F.; Feng, X.H.; Qiu, G.H.; Liu, F. Adsorption of heavy metals on the surface of birnessiterelationship with its Mn average oxidation state and adsorption sites. Environ. Sci. 2011, 32, 3128–3136. (In Chinese) [Google Scholar]
- Zhang, Y.; Vriesc, W.; Thomasd, B.; Hao, X.; Shi, X. Impacts of long-term nitrogen fertilization on acid buffering rates andmechanisms of a slightly calcareous clay soil. Geofis. Int. 2017, 305, 92–99. [Google Scholar]
- Yang, H.; Zhang, P.; Zhu, T.; Li, Q.; Cao, J. The Characteristics of Soil C, N, and P Stoichiometric Ratios as Affected by Geological Background in a Karst Graben Area, Southwest China. Forests 2019, 10, 601. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xiao, T.; Perkins, R.; Zhu, J.; Ning, Z. Geogenic cadmium pollution and potential health risks, with emphasis on black shale. J. Geochem. Explor. 2017, 176, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Najdu, R. Cadmium Sorption and Desorption in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 489–533. [Google Scholar] [CrossRef]
- Silver, W.; Miya, R. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Zhu, T.; Zeng, S.; Qin, H.; Zhou, K.; Yang, H.; Lan, F.; Huang, F.; Cao, J.; Müller, C. Low nitrate retention capacity in calcareous soil under woodland in the karst region of southwestern China. Soil Biol. Biochem. 2016, 97, 99–101. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, Y.; Xu, M.; Luo, L.; Zhang, S. Application of X-ray absorption fine structure spectroscopy to soil science. Acta Pedol. Sin. 2008, 43, 155–164. (In Chinese) [Google Scholar]
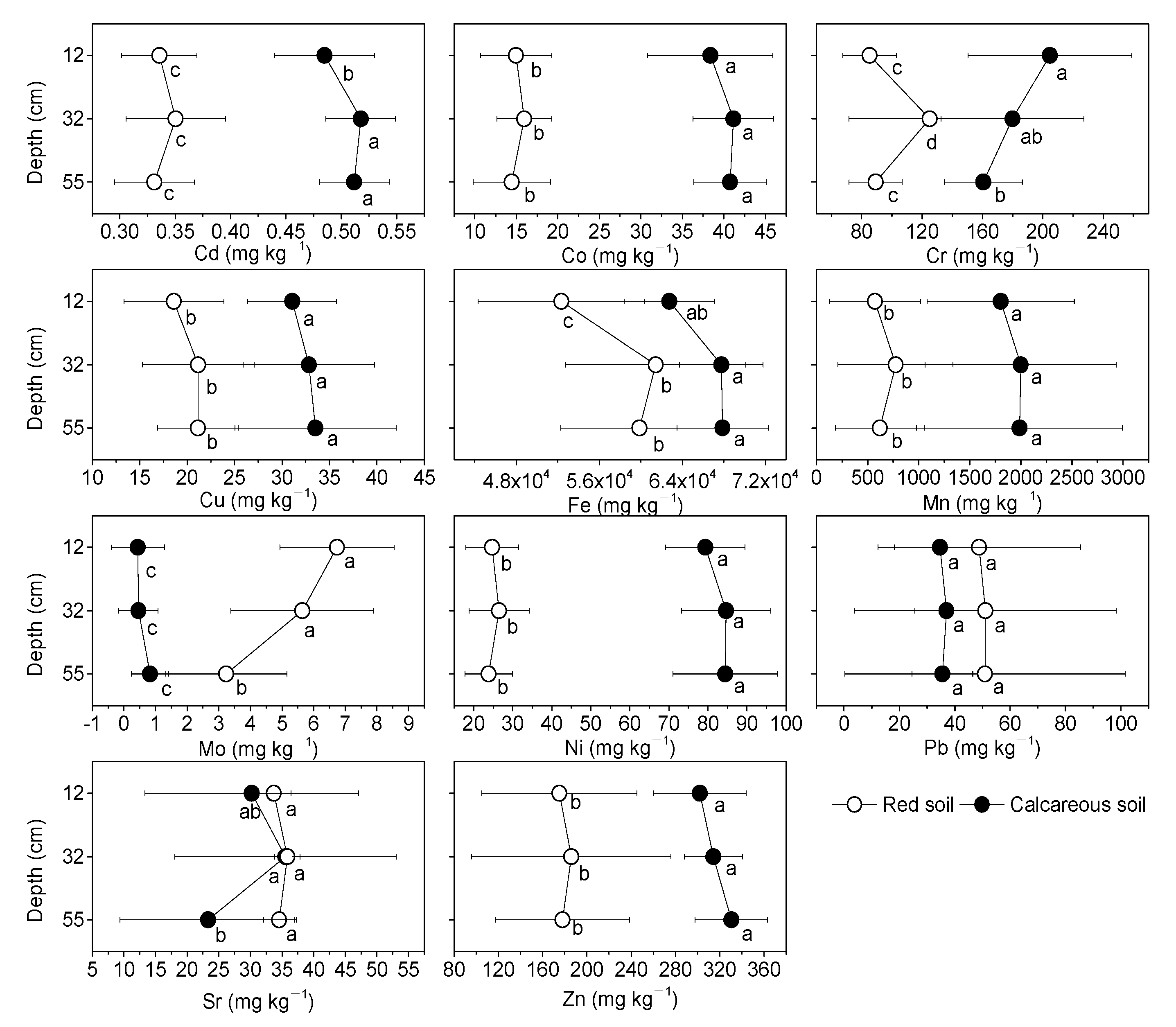
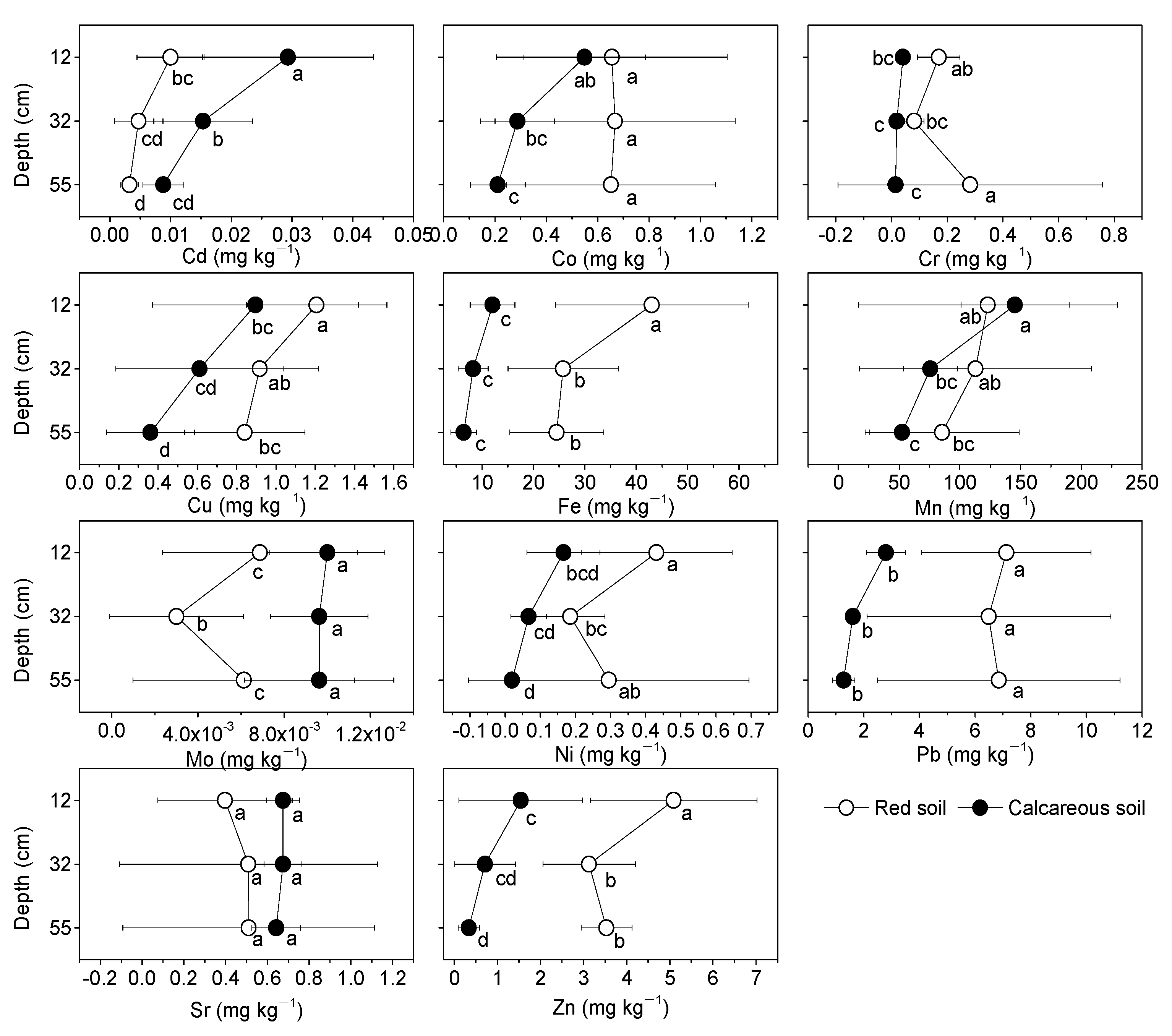
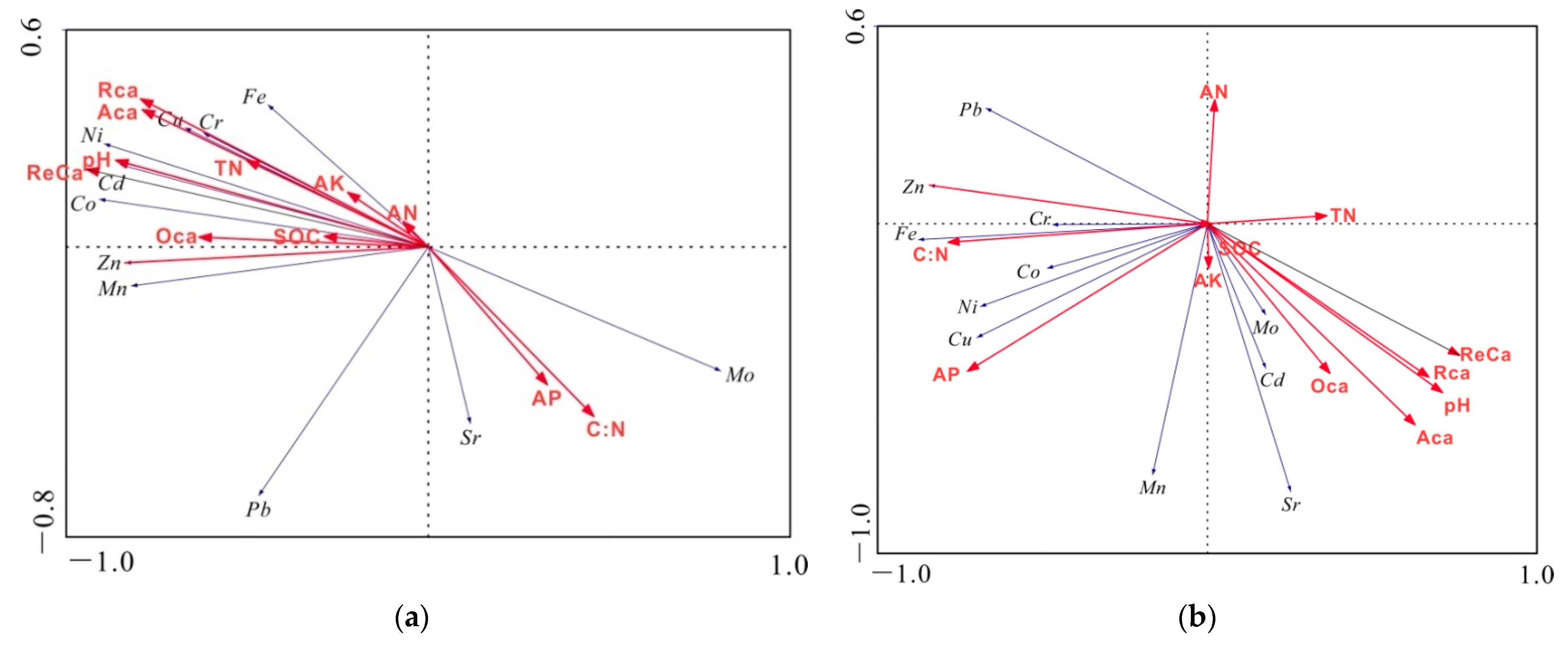
| Soil Depth (cm) | pH Value | SOC (%) | TN (%) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | C:N | |
|---|---|---|---|---|---|---|---|---|
| Calcareous soil | 0–25 | 6.04 ± 0.34 a | 2.66 ± 1.01 a | 0.33 ± 0.19 a | 123.29 ± 56.18 a | 6.22 ± 4.60 ab | 50.22 ± 28.71 a | 8.92 ± 1.05 b |
| 25–40 | 6.10 ± 0.32 a | 1.79 ± 0.67 b | 0.23 ± 0.09 ab | 88.99 ± 21.83 ab | 3.64 ± 1.10 b | 25.66 ± 9.72 b | 7.54 ± 0.77 c | |
| 40–70 | 5.86 ± 0.25 a | 1.22 ± 0.13 c | 0.17 ± 0.02 b | 78.30 ± 23.44 b | 2.63 ± 1.31 b | 23.36 ± 6.62 b | 7.07 ± 0.47 c | |
| mean | 6.00 ± 0.31 A | 1.89 ± 0.91 A | 0.24 ± 0.13 A | 97.67 ± 41.12 A | 4.16 ± 3.01 A | 33.08 ± 21.15 A | 7.84 ± 1.09 B | |
| Red soil | 0–25 | 4.39 ± 0.49 c | 2.14 ± 0.70 b | 0.17 ± 0.05 b | 89.00 ± 28.10 ab | 9.56 ± 5.01 a | 29.11 ± 4.24 b | 12.68 ± 0.51 a |
| 25–40 | 4.78 ± 0.42 bc | 0.99 ± 0.21 c | 0.11 ± 0.03 b | 81.43 ± 24.28 b | 5.75 ± 3.32 ab | 18.80 ± 3.90 b | 9.36 ± 1.26 b | |
| 40–70 | 5.11 ± 0.62 b | 0.86 ± 0.35 c | 0.10 ± 0.04 b | 70.26 ± 37.48 b | 7.09 ± 4.88 ab | 19.56 ± 3.35 b | 8.02 ± 1.34 bc | |
| mean | 4.76 ± 0.58 B | 1.33 ± 0.74 A | 0.13 ± 0.05 A | 80.12 ± 30.65 A | 7.47 ± 4.37 A | 22.49 ± 6.04 A | 10.02 ± 2.27 A |
| Soil Depth(cm) | Aca (mg kg−1) | Rca (mg kg−1) | Oca (mg kg−1) | ReCa (mg kg−1) | Tca (mg kg−1) | |
|---|---|---|---|---|---|---|
| Calcareous soil | 0–25 | 3234 ± 1730 a | 1636 ± 1462 a | 233.5 ± 54.56 a | 275.2 ± 54.85 a | 5291 ± 3427 a |
| 25–40 | 2923 ± 1698 a | 1291 ± 1153 a | 221.9 ± 112.2 a | 284.3 ± 82.60 a | 4720 ± 3249 a | |
| 40–70 | 2458 ± 950.2 a | 962.5 ± 730.3 a | 229.4 ± 90.10 a | 285.8 ± 84.44 a | 3935 ± 1939 a | |
| mean | 2872 ± 1459 A | 1228 ± 1125 A | 227.9 ± 85.64 A | 281.8 ± 73.96 A | 4649 ± 812 A | |
| Red soil | 0–25 | 395.1 ± 293.7 b | 52.33 ± 23.97 b | 198.1 ± 125.1 a | 81.29 ± 15.74 b | 726.8 ± 335.1 b |
| 25–40 | 356.3 ± 399.0 b | 139.2 ± 119.2 b | 108.2 ± 32.85 b | 73.04 ± 28.76 b | 676.7 ± 574.7 b | |
| 40–70 | 339.7 ± 345.6 b | 84.67 ± 76.04 b | 49.50 ± 16.90 b | 105.7 ± 49.73 b | 579.6 ± 464.5 b | |
| mean | 363.7 ± 346.1 B | 92.06 ± 73.08 B | 118.6 ± 58.27 A | 86.68 ± 31.41 B | 661.1 ± 119.9 B |
| Cd | Co | Cr | Cu | Mo | Ni | Pb | Sr | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Calcareous soil | CA (mg kg−1) | 0.0293 | 0.5486 | 0.0408 | 0.8956 | 0.008 | 0.1664 | 2.797 | 0.675 | 1.541 |
| CT (mg kg−1) | 0.485 | 38.38 | 204.7 | 31.08 | 1.526 | 79.33 | 34.63 | 30.22 | 301.9 | |
| AR1 (%) | 6.04 | 1.43 | 0.02 | 2.88 | 0.51 | 0.21 | 8.08 | 2.23 | 0.51 | |
| Red soil | CA (mg kg−1) | 0.0100 | 0.655 | 0.1694 | 1.2063 | 0.0069 | 0.4308 | 7.1226 | 0.3973 | 5.088 |
| CT (mg kg−1) | 0.3356 | 14.95 | 85.27 | 18.61 | 8.790 | 24.72 | 48.83 | 33.73 | 175.1 | |
| AR2 (%) | 2.98 | 4.38 | 0.2 | 6.48 | 0.08 | 1.74 | 14.59 | 1.18 | 2.91 | |
| AR1:AR2 | 2.03 | 0.33 | 0.10 | 0.44 | 6.38 | 0.12 | 0.55 | 1.89 | 0.18 | |
| RSV a (mg kg−1) | 0.4 | - | 150 | 50 | - | 70 | 90 | - | 200 | |
| FQS (mg kg−1) | 0.2 | - | 1.0 | - | - | 1.0 | 0.2 | - | - |
| Ca | Fe | Mn | Cd | Co | Cr | Cu | Mo | Ni | Pb | Sr | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | 0.04 | 51.54 | 51.86 | 0.75 | 29.86 | 3.51 | 10.85 | 6.12 | 52.69 | 2.40 | 0.68 | 2.33 |
| Q2 | 0.19 | 0.58 | 1.09 | 0.81 | 0.30 | 2.24 | 2.54 | 12.1 | 3.71 | 0.22 | 0.99 | 2.95 |
| CT1:CT2 | 7.28 | 1.20 | 3.12 | 1.44 | 2.57 | 1.4 | 1.67 | 0.53 | 2.37 | 0.75 | 0.90 | 1.68 |
| CR1 (mg kg−1) | 142,200 | 1217 | 34.86 | 0.64 | 1.29 | 118.5 | 2.87 | 1.35 | 1.80 | 17.81 | 46.25 | 134.5 |
| CR2 (mg kg−1) | 3900 | 89,500 | 530 | 0.41 | 49.75 | 132.5 | 7.33 | 1.28 | 10.78 | 262.5 | 35.50 | 63.25 |
| CR1:CR2 | 36.46 | 0.01 | 0.07 | 1.56 | 0.03 | 0.89 | 0.39 | 1.05 | 0.17 | 0.07 | 1.30 | 2.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Wei, X.; Zhu, T.; Luo, Z.; Cao, J. Insights into Distribution of Soil Available Heavy Metals in Karst Area and Its Influencing Factors in Guilin, Southwest China. Forests 2021, 12, 609. https://doi.org/10.3390/f12050609
Huang F, Wei X, Zhu T, Luo Z, Cao J. Insights into Distribution of Soil Available Heavy Metals in Karst Area and Its Influencing Factors in Guilin, Southwest China. Forests. 2021; 12(5):609. https://doi.org/10.3390/f12050609
Chicago/Turabian StyleHuang, Fen, Xiaomei Wei, Tongbin Zhu, Zhuanxi Luo, and Jianhua Cao. 2021. "Insights into Distribution of Soil Available Heavy Metals in Karst Area and Its Influencing Factors in Guilin, Southwest China" Forests 12, no. 5: 609. https://doi.org/10.3390/f12050609






