Comprehensive Bioinformatics and Expression Analysis of TCP Transcription Factors in Liriodendron chinense Reveals Putative Abiotic Stress Regulatory Roles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of TCP Family Genes in L. chinense
2.2. Multiple Sequence Alignments and Phylogenetic Analysis
2.3. RNA-Seq Analysis of LcTCP in Response to Temperature Stress
2.4. Plant Material Treatment and qRT-PCR Expression Analysis
2.5. Gene Structure and Conserved Motif Analysis
2.6. Putative Promoter Cis-Acting Element Analysis
2.7. Chromosomal Location and Gene Duplication
3. Results
3.1. Identification and Physicochemical Properties of L. chinense TCP Factors
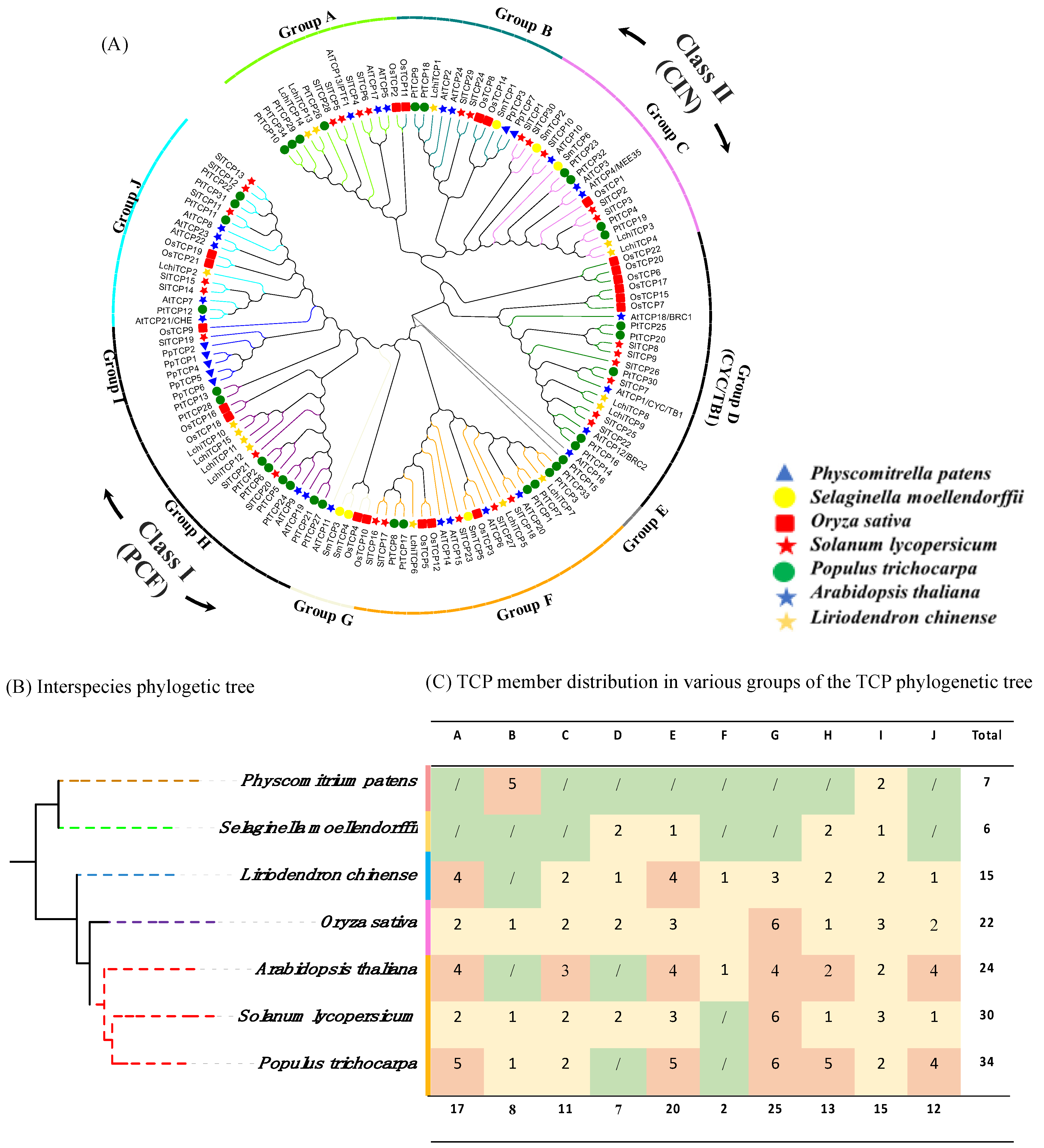
3.2. Phylogenomic and Phylogeny Analysis of the TCP Factors in L. chinense
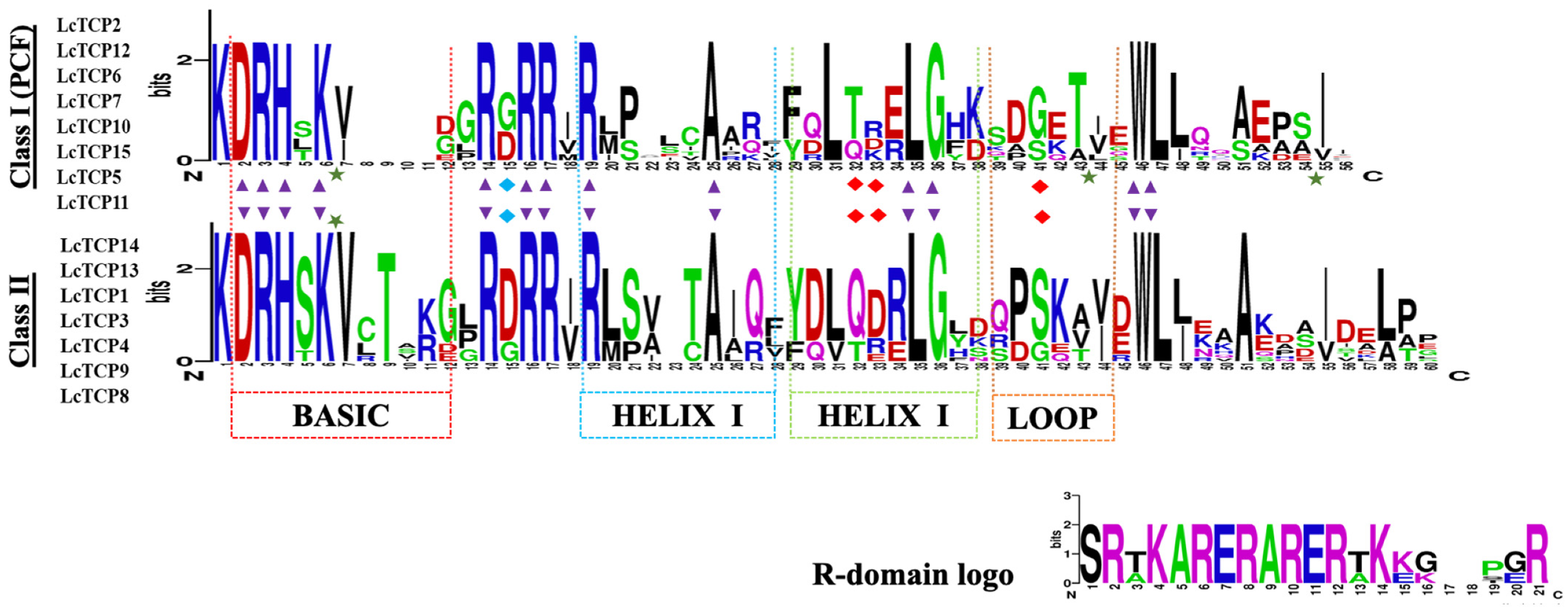
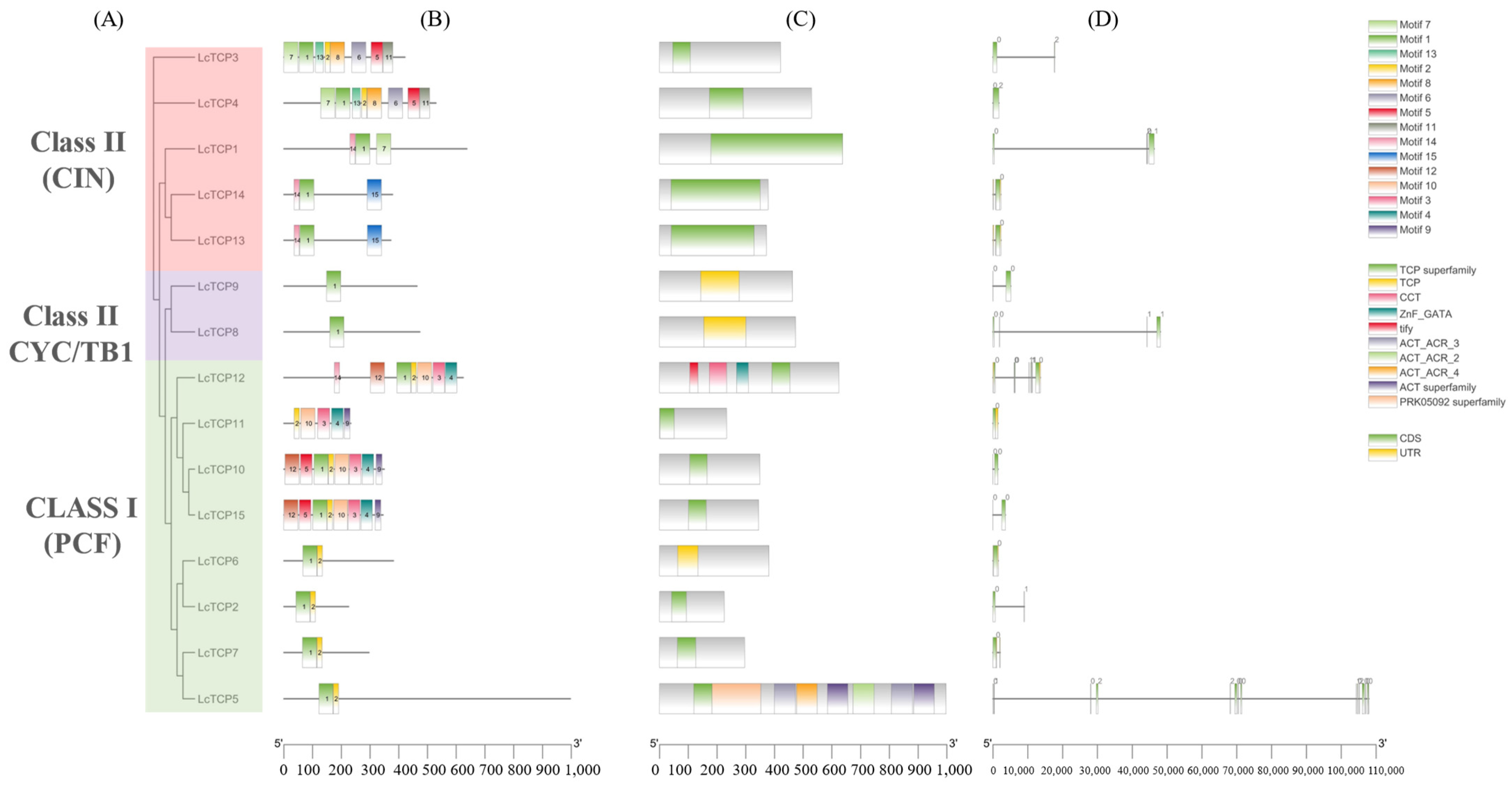
3.3. Conserved Domains and Motif Analysis of TCPs in L. chinense
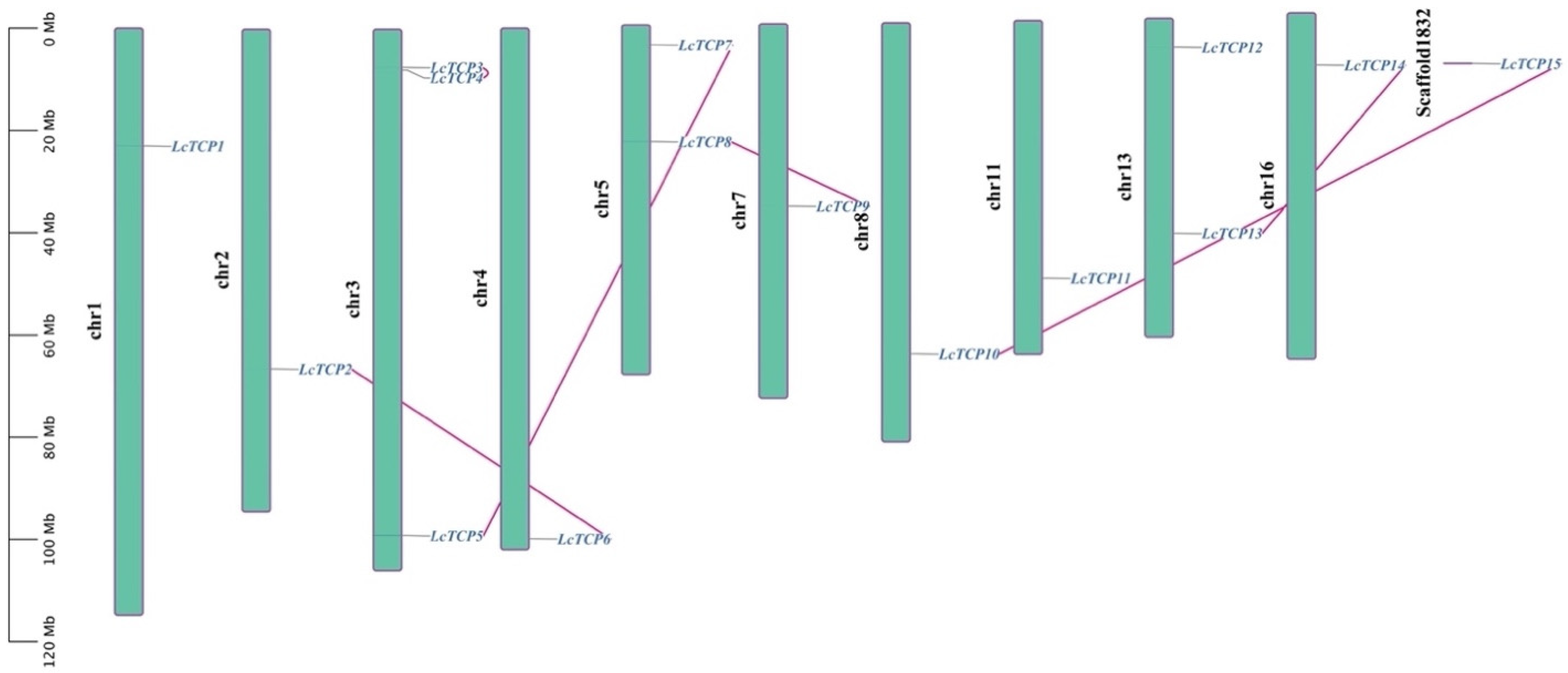
3.4. Exon-Intron Organization and Chromosomal Locations of LcTCP Genes
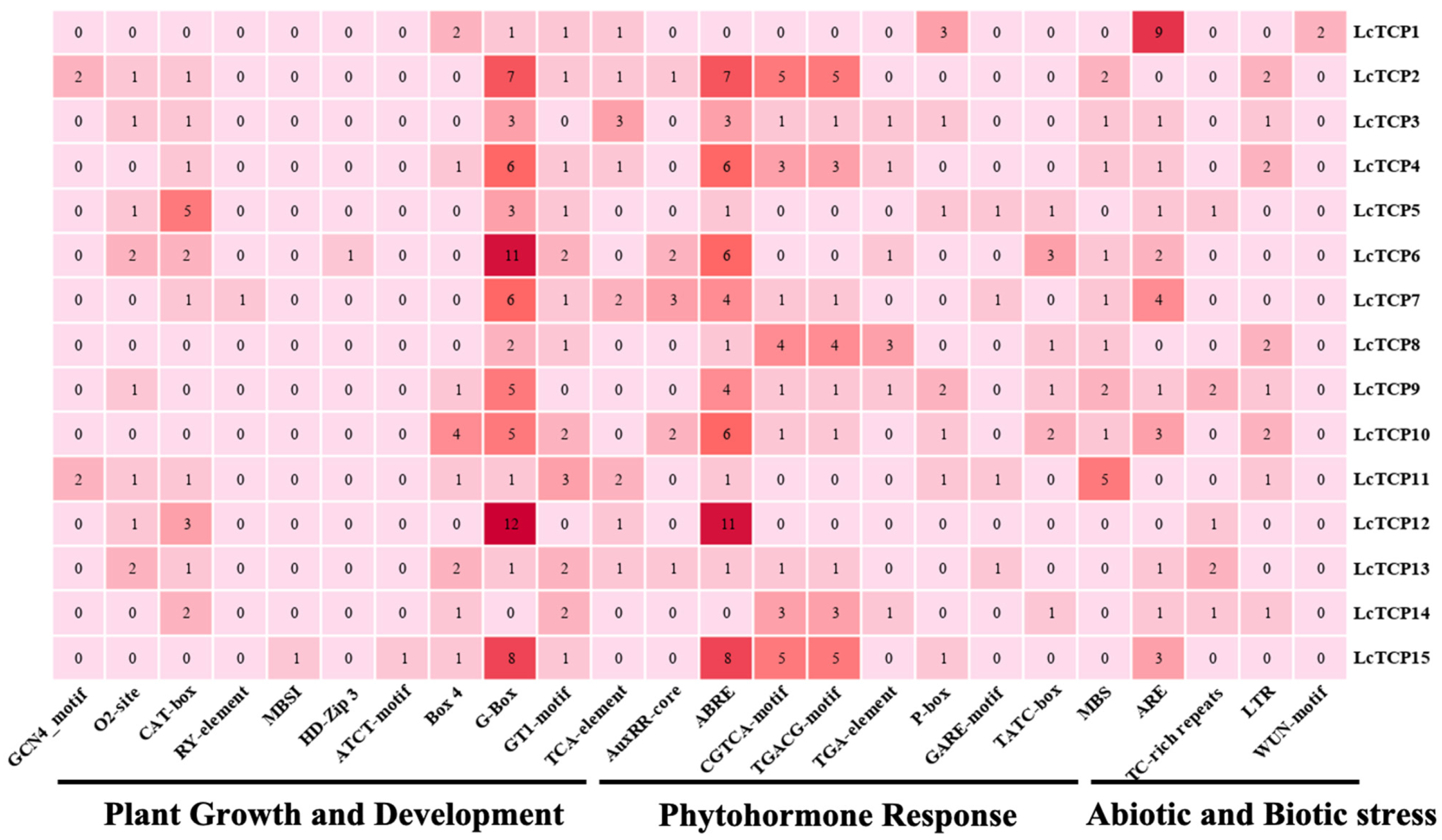
3.5. Identification of the Putative Cis-Elements in the Promoter of LcTCP Genes
3.6. Protein Structure Prediction and PPI Analysis
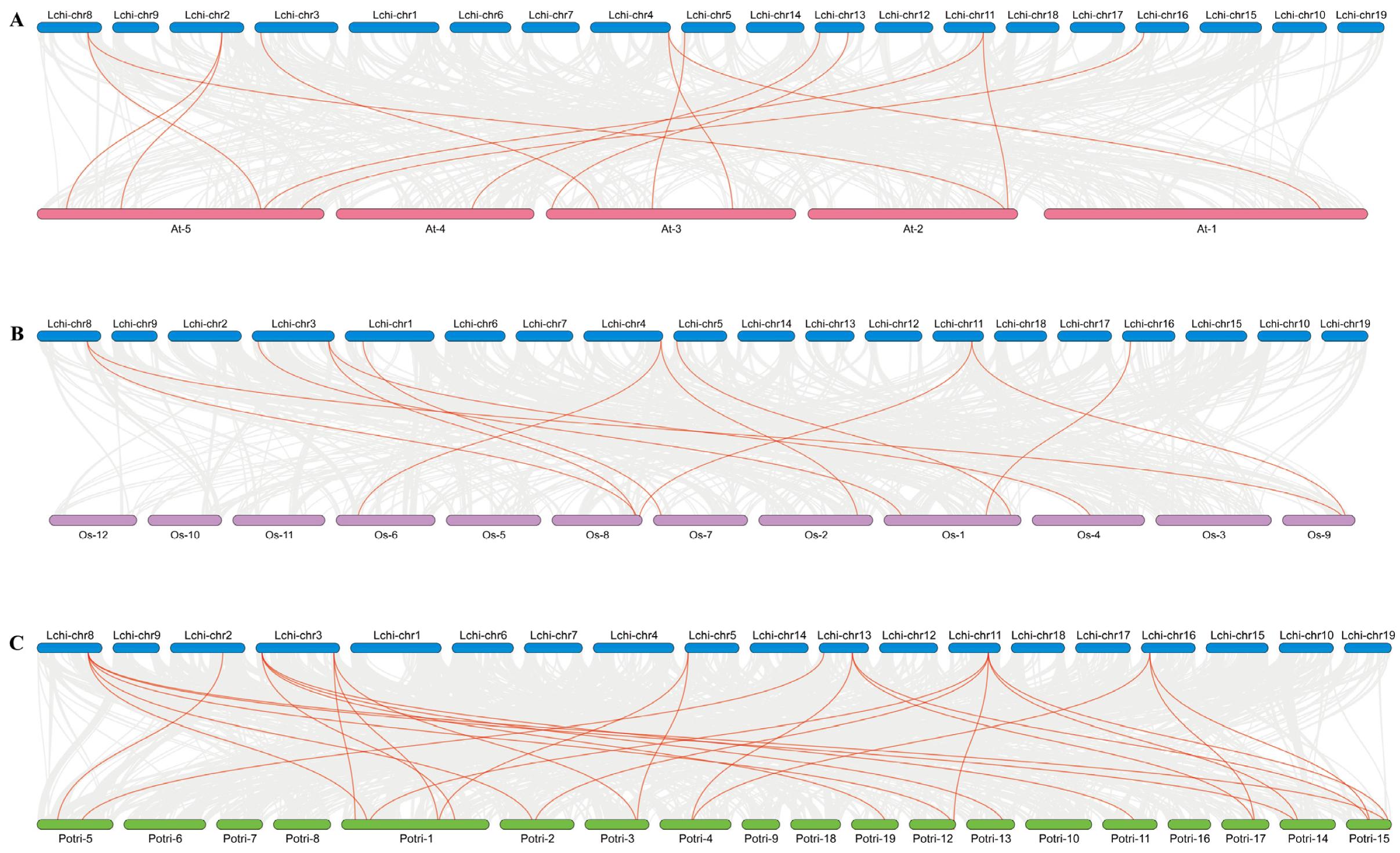
3.7. Collinearity Analysis, Selection Pressure, and Divergence of LcTCPs
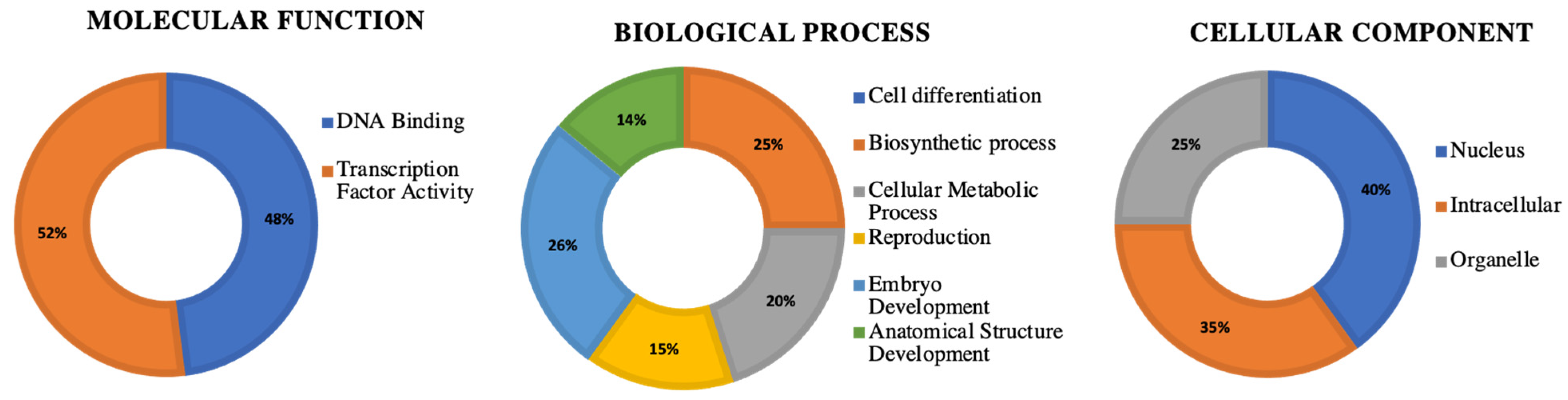
3.8. Gene Ontology Analysis
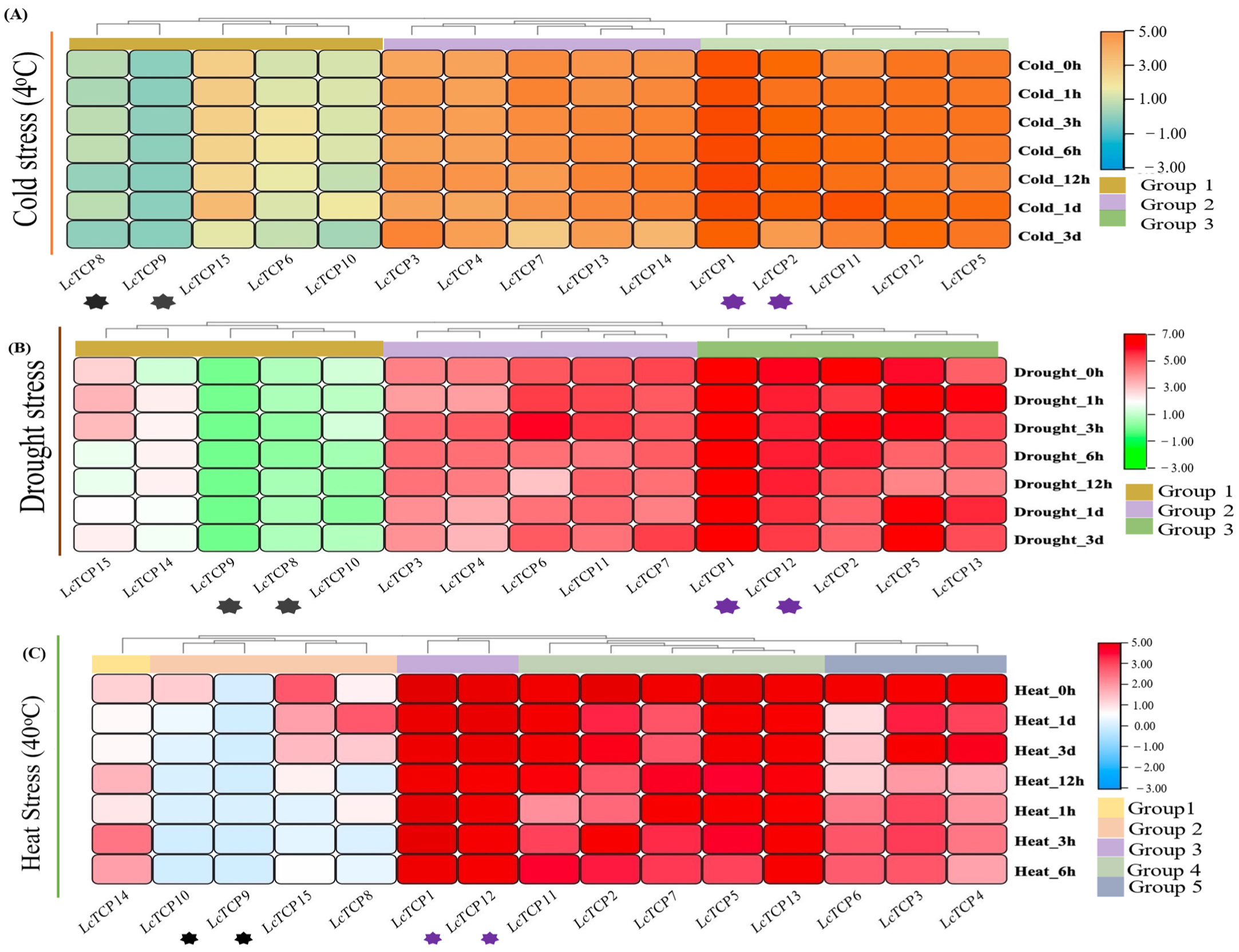
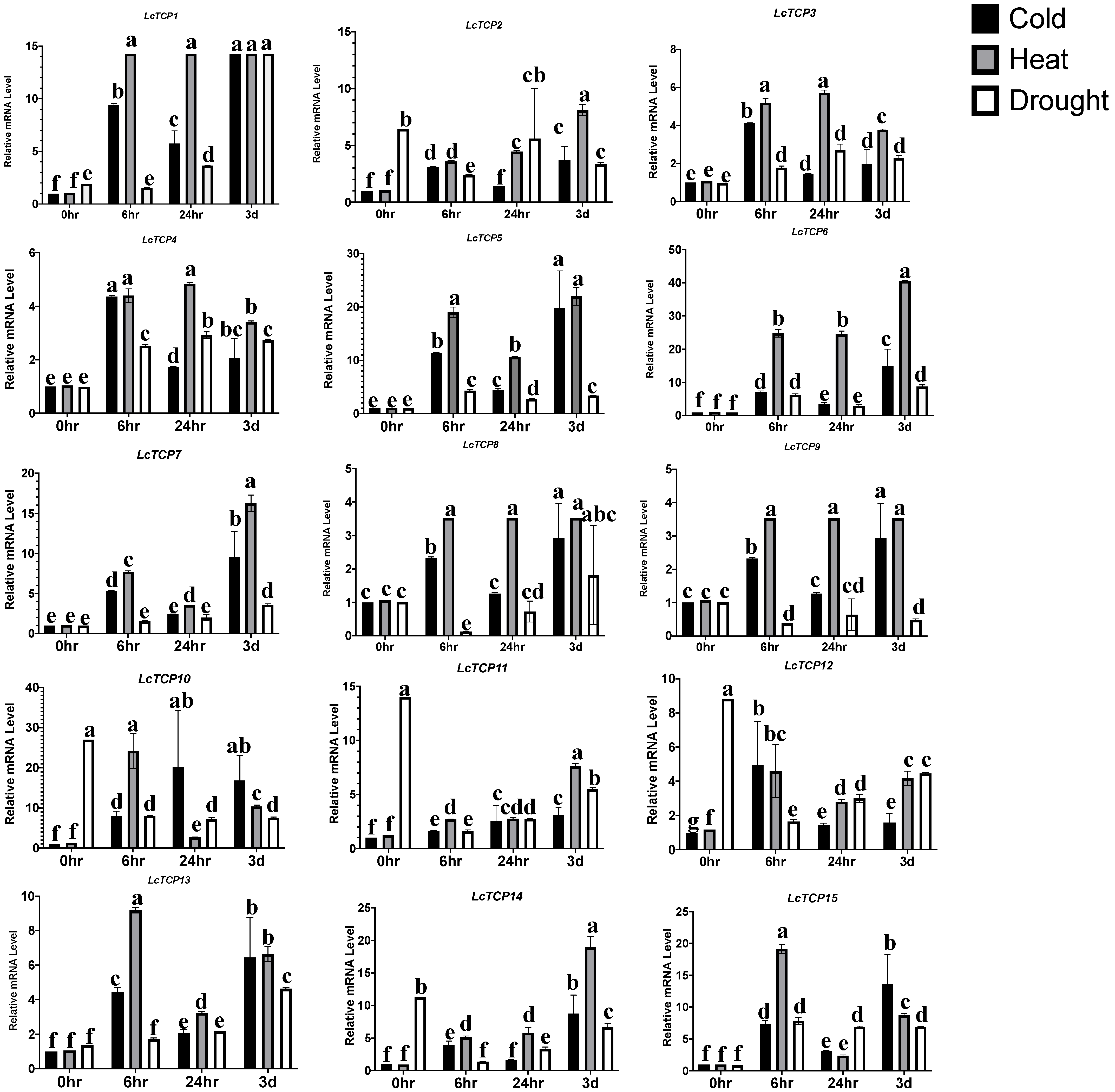
3.9. Abiotic Stresses: Transcriptomic Data Expression Analysis and qPCR Validation of LcTCP Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell 1997, 9, 1607–1619. [Google Scholar] [PubMed]
- Hepworth, J.; Lenhard, M. Regulation of plant lateral-organ growth by modulating cell number and size. Curr. Opin. Plant Biol. 2014, 17, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Das Gupta, M.; Joseph, A.P.; Chatterjee, N.; Srinivasan, N.; Nath, U. Identification of Specific DNA Binding Residues in the TCP Family of Transcription Factors in Arabidopsis. Plant Cell 2010, 22, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Manassero, N.G.U.; Viola, I.L.; Welchen, E.; Gonzalez, D.H. TCP transcription factors: Architectures of plant form. Biomol. Concepts 2013, 4, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Camoirano, A.; Arce, A.L.; Ariel, F.D.; Alem, A.L.; Gonzalez, D.H.; Viola, I.L. Class I TCP transcription factors regulate trichome branching and cuticle development in Arabidopsis. J. Exp. Bot. 2020, 71, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, V.; Lucero, L.E.; Ferrero, L.V.; Ariel, F.D.; Gonzalez, D.H. Class-I TCP Transcription Factors Activate the SAUR63 Gene Subfamily in Gibberellin-Dependent Stamen Filament Elongation. Plant Physiol. 2020, 182, 2096–2110. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, V.; Viola, I.L.; Ariel, F.D.; Gonzalez, D.H. Class I TCP Transcription Factors Target the Gibberellin Biosynthesis Gene GA20ox1 and the Growth-Promoting Genes HBI1 and PRE6 during Thermo-morphogenic Growth in Arabidopsis. Plant Cell Physiol. 2019, 60, 1633–1645. [Google Scholar] [CrossRef]
- Steiner, E.; Yanai, O.; Efroni, I.; Ori, N.; Eshed, Y.; Weiss, D.S. Class I TCPs modulate cytokinin-induced branching and meristematic activity in tomato. Plant Signal. Behav. 2012, 7, 807–810. [Google Scholar] [CrossRef]
- Ferrero, L.V.; Gastaldi, V.; Ariel, F.D.; Viola, I.L.; Gonzalez, D.H. Class I TCP proteins TCP14 and TCP15 are required for elongation and gene expression responses to auxin. Plant Mol. Biol. 2021, 105, 147–159. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, H.; Zhang, C.; Li, X.; Lyu, Y.; Qi, D.; Cui, Y.; Hu, L.; Wang, Z.; Liang, Z.; et al. TCP7 functions redundantly with several Class I TCPs and regulates endoreplication in Arabidopsis. J. Integr. Plant Biol. 2019, 61, 1151–1170. [Google Scholar] [CrossRef]
- Bao, S.; Zhang, Z.; Lian, Q.; Sun, Q.; Zhang, R. Evolution and expression of genes encoding TCP transcription factors in Solanum tuberosum reveal the involvement of StTCP23 in plant defence. BMC Genet. 2019, 20, 91. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Gao, Y.; Xiong, R.; Wu, M.; Zhang, K.; Xiang, Y. The TCP transcription factor PeTCP10 modulates salt tolerance in transgenic Arabidopsis. Plant Cell Rep. 2021, 40, 1971–1987. [Google Scholar] [CrossRef]
- Sun, T. Unexpected Role of a TCP Transcription Factor in Seed Oil Biosynthesis. Plant Physiol. 2020, 184, 550–551. [Google Scholar] [CrossRef]
- Lan, J.; Qin, G. The Regulation of CIN-like TCP Transcription Factors. Int. J. Mol. Sci. 2020, 21, 4498. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Mou, M.; Chen, Y.; Xiang, S.; Chen, L.; Yu, D. Arabidopsis Class II TCP Transcription Factors Integrate with the FT–FD Module to Control Flowering. Plant Physiol. 2019, 181, 97–111. [Google Scholar] [CrossRef]
- He, Z.; Zhou, X.; Chen, J.; Yin, L.; Zeng, Z.; Xiang, J.; Liu, S. Identification of a consensus DNA-binding site for the TCP domain transcription factor TCP2 and its important roles in the growth and development of Arabidopsis. Mol. Biol. Rep. 2021, 48, 2223–2233. [Google Scholar] [CrossRef]
- Danisman, S.; van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.D.; Muino, J.M.; Cutri, L.; Dornelas, M.C.; et al. Arabidopsis Class I and Class II TCP Transcription Factors Regulate Jasmonic Acid Metabolism and Leaf Development Antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar] [CrossRef]
- Crawford, T.; Karamat, F.; Lehotai, N.; Rentoft, M.; Blomberg, J.; Strand, A.; Björklund, S. Specific functions for Mediator complex subunits from different modules in the transcriptional response of Arabidopsis thaliana to abiotic stress. Sci. Rep. 2020, 10, 5073. [Google Scholar] [CrossRef]
- Leng, X.; Wei, H.; Xu, X.; Ghuge, S.A.; Jia, D.; Liu, G.; Wang, Y.; Yuan, Y. Genome-wide identification and transcript analysis of TCP transcription factors in grapevine. BMC Genom. 2019, 20, 786. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-L.; Wu, M.; Li, F.; Gao, Y.-M.; Chen, F.; Xiang, Y. TCP Transcription Factors in Moso Bamboo (Phyllostachys edulis): Genome-Wide Identification and Expression Analysis. Front. Plant Sci. 2018, 9, 1263. [Google Scholar] [CrossRef]
- Feng, Z.-J.; Xu, S.-C.; Liu, N.; Zhang, G.-W.; Hu, Q.-Z.; Gong, Y.-M. Soybean TCP transcription factors: Evolution, classification, protein interaction and stress and hormone responsiveness. Plant Physiol. Biochem. 2018, 127, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Parapunova, V.; Busscher, M.; Busscher-Lange, J.; Lammers, M.; Karlova, R.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. Identification, cloning and characterization of the tomato TCP transcription factor family. BMC Plant Biol. 2014, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hao, Z.; Guang, X.; Zhao, C.; Wang, P.; Xue, L.; Zhu, Q.; Yang, L.; Sheng, Y.; Zhou, Y.; et al. Author Correction: Liriodendron genome sheds light on angiosperm phylogeny and species–pair differentiation. Nat. Plants 2019, 5, 328. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Sun, P.; Jia, F.; Lu, L.; Li, Y.; Zhang, S.; Huang, J. Genome-wide analysis of TCP transcription factor gene family in Malus domestica. J. Genet. 2014, 93, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting Subcellular Localization of Proteins Based on their N-terminal Amino Acid Sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Liu, M.-M.; Wang, M.-M.; Yang, J.; Wen, J.; Guo, P.-C.; Wu, Y.-W.; Ke, Y.-Z.; Li, P.-F.; Li, J.-N.; Du, H. Evolutionary and Comparative Expression Analyses of TCP Transcription Factor Gene Family in Land Plants. Int. J. Mol. Sci. 2019, 20, 3591. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Q.; Zheng, J.; Xu, F.; Ye, J.; Zhang, W.; Liao, Y.; Yang, X. Genome-wide identification and expression pattern analysis of the TCP transcription factor family in Ginkgo biloba. Plant Signal. Behav. 2022, 17, 1994248. [Google Scholar] [CrossRef]
- Danisman, S. TCP Transcription Factors at the Interface between Environmental Challenges and the Plant’s Growth Responses. Front. Plant Sci. 2016, 7, 1930. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Koyama, T.; Ohme-Takagi, M.; Sato, F. Generation of serrated and wavy petals by inhibition of the activity of TCP transcription factors in Arabidopsis thaliana. Plant Signal. Behav. 2011, 6, 697–699. [Google Scholar] [CrossRef]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 Acts as an Integrator of Branching Signals within Axillary Buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef]
- Moreno-Pachon, N.M.; Mutimawurugo, M.-C.; Heynen, E.; Sergeeva, L.; Benders, A.; Blilou, I.; Hilhorst, H.W.M.; Immink, R.G.H. Role of Tulipa gesneriana TEOSINTE BRANCHED1 (TgTB1) in the control of axillary bud outgrowth in bulbs. Plant Reprod. 2018, 31, 145–157. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef]
- Wang, D.; Liu, F.; Wang, L.; Huang, S.; Yu, J. Nonsynonymous substitution rate (Ka) is a relatively consistent parameter for defining fast-evolving and slow-evolving protein-coding genes. Biol. Direct 2011, 6, 13. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.K.; Jaiswal, A.K.; Taj, G.; Jaiswal, J.P.; Qureshi, M.I.; Singh, N.K. DREB1/CBF transcription factors: Their structure, function and role in abiotic stress tolerance in plants. J. Genet. 2012, 91, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Liu, S.; Wu, W.; Hong, K.; Li, R.; Zhu, L.; Liu, Y.; Lu, Y.; Chen, J.; Yang, L.; et al. Genome-wide identification and cold stress-induced expression analysis of the CBF gene family in Liriodendron chinense. J. For. Res. 2021, 32, 2531–2543. [Google Scholar] [CrossRef]
- Bowers, J.E.; Bachlava, E.; Brunick, R.L.; Rieseberg, L.H.; Knapp, S.J.; Burke, J.M. Development of a 10,000 Locus Genetic Map of the Sunflower Genome Based on Multiple Crosses. G3 Genes|Genomes|Genet. 2012, 2, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Zhang, W.; An, Y.; Du, B.; Wang, D.; Guo, C. Genome-wide analysis of the TCP transcription factor genes in five legume genomes and their response to salt and drought stresses. Funct. Integr. Genom. 2020, 20, 537–550. [Google Scholar] [CrossRef]
- Karaaslan, E.S.; Wang, N.; Faiß, N.; Liang, Y.; Montgomery, S.A.; Laubinger, S.; Berendzen, K.W.; Berger, F.; Breuninger, H.; Liu, C. Marchantia TCP transcription factor activity correlates with three-dimensional chromatin structure. Nat. Plants 2020, 6, 1250–1261. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, X.; Liu, S.; Yang, M.; Ren, J.; Guo, M.; Huang, Z.; Zhang, Y. Genome-Wide Identification and Analysis of TCP Transcription Factors Involved in the Formation of Leafy Head in Chinese Cabbage. Int. J. Mol. Sci. 2018, 19, 847. [Google Scholar] [CrossRef]
- Yao, X.; Ma, H.; Wang, J.; Zhang, D. Genome-Wide Comparative Analysis and Expression Pattern of TCP Gene Families in Arabidopsis thaliana and Oryza sativa. J. Integr. Plant Biol. 2007, 49, 885–897. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Guo, L.; Tan, L.; Ye, X.; Yang, Y.; Zhao, X.; Nie, Y.; Deng, D.; Liu, S.; et al. Innovation and Emerging Roles of Populus trichocarpa TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR Transcription Factors in Abiotic Stresses by Whole-Genome Duplication. Front. Plant Sci. 2022, 13, 850064. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis Transcription Factors: Genome-Wide Comparative Analysis among Eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family in Arabidopsis and Rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Oliver, K.R.; McComb, J.A.; Greene, W. Transposable Elements: Powerful Contributors to Angiosperm Evolution and Diversity. Genome Biol. Evol. 2013, 5, 1886–1901. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Paterson, A.H. Genome and gene duplications and gene expression divergence: A view from plants. Ann. N. Y. Acad. Sci. 2012, 1256, 1–14. [Google Scholar] [CrossRef]
- Xu, R.; Gao, H.; Zhang, S.; Liu, P.; Wang, X.; Hao, Y. Genome-wide identification and phylogenetic, comparative genomic, alternative splicing, and expression analyses of TCP genes in plants. Plant Gene 2017, 12, 23–32. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Sun, R.; Xie, F.; Jones, D.C.; Zhang, B. Genome-wide identification and expression analysis of TCP transcription factors in Gossypium raimondii. Sci. Rep. 2014, 4, 6645. [Google Scholar] [CrossRef]
- Lei, N.; Yu, X.; LiangPing, Z.; Zeng, C.; Zou, L.; Liao, W.; Peng, M. Phylogeny and expression pattern analysis of TCP transcription factors in cassava seedlings exposed to cold and/or drought stress. Sci. Rep. 2017, 7, 10016. [Google Scholar] [CrossRef]
- Finlayson, S.A. Arabidopsis TEOSINTE BRANCHED1-LIKE 1 Regulates Axillary Bud Outgrowth and is Homologous to Monocot TEOSINTE BRANCHED1. Plant Cell Physiol. 2007, 48, 667–677. [Google Scholar] [CrossRef]
- Seven, M.; Akdemir, H. DOF, MYB and TCP transcription factors: Their possible roles on barley germination and seedling establishment. Gene Expr. Patterns 2020, 37, 119116. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, D.; Liu, C.; Zhao, J.; Liu, J.; Liu, N.; Tang, D.; Hu, Y. TCP transcription factors interact with ZED1-related kinases as components of the temperature-regulated immunity. Plant Cell Environ. 2019, 42, 2045–2056. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Li, H. Transcriptomic and microstructural analyses in Liriodendron tulipifera Linn. reveal candidate genes involved in nectary development and nectar secretion. BMC Plant Biol. 2019, 19, 531. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cellular Phosphorylation Signaling and Gene Expression in Drought Stress Responses: ABA-Dependent and ABA-Independent Regulatory Systems. Plants 2021, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, O.; Botha, C.E.; Bradley, G. In silico analysis of cis-acting regulatory elements in 5′ regulatory regions of sucrose transporter gene families in rice (Oryza sativa Japonica) and Arabidopsis thaliana. Comput. Biol. Chem. 2010, 34, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, A.; Vernoux, T. Comparison of plant hormone signalling systems. Essays Biochem. 2015, 58, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Tian, C.; Zhai, L.; Zhu, W.; Qi, X.; Yu, Z.; Wang, H.; Chen, F.; Wang, L.; Chen, S. Characterization of the TCP Gene Family in Chrysanthemum nankingense and the Role of CnTCP4 in Cold Tolerance. Plants 2022, 11, 936. [Google Scholar] [CrossRef]
- Pandey, G.K.; Kanwar, P.; Singh, A.; Steinhorst, L.; Pandey, A.; Yadav, A.K.; Tokas, I.; Sanyal, S.K.; Kim, B.-G.; Lee, S.-C.; et al. Calcineurin B-Like Protein-Interacting Protein Kinase CIPK21 Regulates Osmotic and Salt Stress Responses in Arabidopsis. Plant Physiol. 2015, 169, 780–792. [Google Scholar] [CrossRef]
- Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. Int. J. Mol. Sci. 2019, 20, 2762. [Google Scholar] [CrossRef]
- Horn, S.; Pabon-Mora, N.; Theuß, V.S.; Busch, A.; Zachgo, S. Analysis of the CYC/TB1 class of TCP transcription factors in basal angiosperms and magnoliids. Plant J. 2015, 81, 559–571. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Chromosome Name | Chromosome Location | Protein Length (aa) | Gravy | Molecular Weight | pl | Type | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|
| LcTCP1 | Lchi18883 | chr1 | 23,064,045; 23,077,089 | 637 | −0.808 | 70.20 | 6.67 | CIN | Nucleus |
| LcTCP2 | Lchi01522 | chr2 | 66,472,087,66,485,144 | 225 | −0.628 | 24.40 | 9.51 | PCF | Nucleus |
| LcTCP3 | Lchi09931 | chr3 | 7,460,930; 7,478,685 | 421 | −0.627 | 45.77 | 5.88 | CIN | Nucleus |
| LcTCP4 | Lchi33835 | chr3 | 7,987,289; 7,988,968 | 529 | −0.878 | 58.19 | 6.36 | CIN | Nucleus |
| LcTCP5 | Lchi22568 | chr3 | 99,062,754; 99,080,087 | 997 | −0.366 | 110.90 | 7.55 | PCF | Nucleus |
| LcTCP6 | Lchi14258 | chr4 | 99,940,245; 99,953,735 | 381 | −0.714 | 41.32 | 7.28 | PCF | Nucleus |
| LcTCP7 | Lchi02489 | chr5 | 3,878,180; 3,890,511 | 296 | −0.532 | 30.93 | 9.33 | PCF | Mitochondrion |
| LcTCP8 | Lchi13620 | chr5 | 22,632,970; 22,710,832 | 473 | −0.377 | 52.42 | 9.44 | CYC/TB1 | Nucleus |
| LcTCP9 | Lchi22938 | chr7 | 35,598,950; 35,603,834 | 463 | −0.719 | 51.41 | 7.33 | CYC/TB1 | Nucleus |
| LcTCP10 | Lchi11973 | chr8 | 64,788,838; 64,804,229 | 349 | −0.544 | 37.08 | 9.43 | PCF | Nucleus |
| LcTCP11 | Lchi04918 | chr11 | 50,312,837; 50,322,292 | 233 | 233 | 24.07 | 9.65 | PCF | Nucleus |
| LcTCP12 | Lchi13044 | chr13 | 5,648,594; 5,648,968 | 624 | −0.585 | 67.72 | 6.10 | PCF | Nucleus |
| LcTCP13 | Lchi29056 | chr13 | 41,935,099; 41,938,169 | 372 | −0.581 | 40.97 | 7.30 | CIN | Nucleus |
| LcTCP14 | Lchi14648 | chr16 | 10,135,105; 10,154,118 | 378 | −0.635 | 42.05 | 8.97 | CIN | Nucleus |
| LcTCP15 | Lchi35464 | scaffold1832 | 27,950; 31,519 | 345 | −0.517 | 36.65 | 9.05 | PCF | Nucleus |
| Seq_1 | Seq_2 | ka | ks | ka/ks |
|---|---|---|---|---|
| LcTCP2 | LcTCP6 | 0.201 | 0.518 | 0.387 |
| LcTCP5 | LcTCP7 | 0.168 | 0.433 | 0.388 |
| LcTCP3 | LcTCP3 | 0.018 | 0.032 | 0.565 |
| LcTCP9 | LcTCP8 | 0.191 | 0.326 | 0.587 |
| LcTCP13 | LcTCP14 | 0.347 | 0.432 | 0.802 |
| LcTCP10 | LcTCP15 | 0.104 | 0.373 | 0.279 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwarari, D.; Guan, Y.; Li, R.; Movahedi, A.; Chen, J.; Yang, L. Comprehensive Bioinformatics and Expression Analysis of TCP Transcription Factors in Liriodendron chinense Reveals Putative Abiotic Stress Regulatory Roles. Forests 2022, 13, 1401. https://doi.org/10.3390/f13091401
Hwarari D, Guan Y, Li R, Movahedi A, Chen J, Yang L. Comprehensive Bioinformatics and Expression Analysis of TCP Transcription Factors in Liriodendron chinense Reveals Putative Abiotic Stress Regulatory Roles. Forests. 2022; 13(9):1401. https://doi.org/10.3390/f13091401
Chicago/Turabian StyleHwarari, Delight, Yuanlin Guan, Rongxue Li, Ali Movahedi, Jinhui Chen, and Liming Yang. 2022. "Comprehensive Bioinformatics and Expression Analysis of TCP Transcription Factors in Liriodendron chinense Reveals Putative Abiotic Stress Regulatory Roles" Forests 13, no. 9: 1401. https://doi.org/10.3390/f13091401






