Turkey Oak (Quercus cerris L.) Resilience to Climate Change: Insights from Coppice Forests in Southern and Central Europe
Abstract
:1. Introduction
2. Methodology
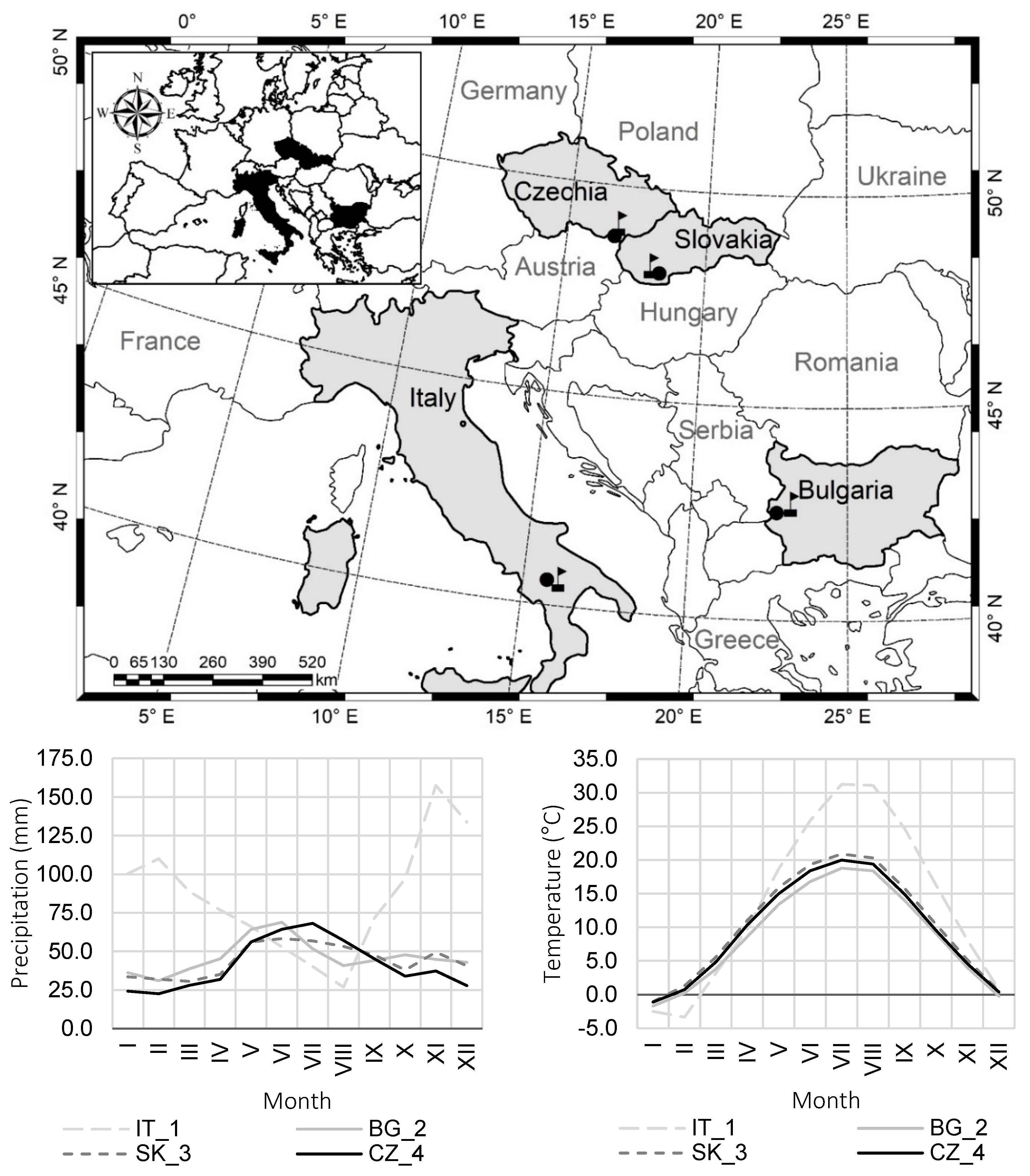
2.1. Study Area
2.2. Data Collection
2.3. Data Processing
2.3.1. Stand Structure and Analysis
2.3.2. Dendrochronological Processing and Analysis
2.3.3. Tree Rings and Climatic Analysis
3. Results
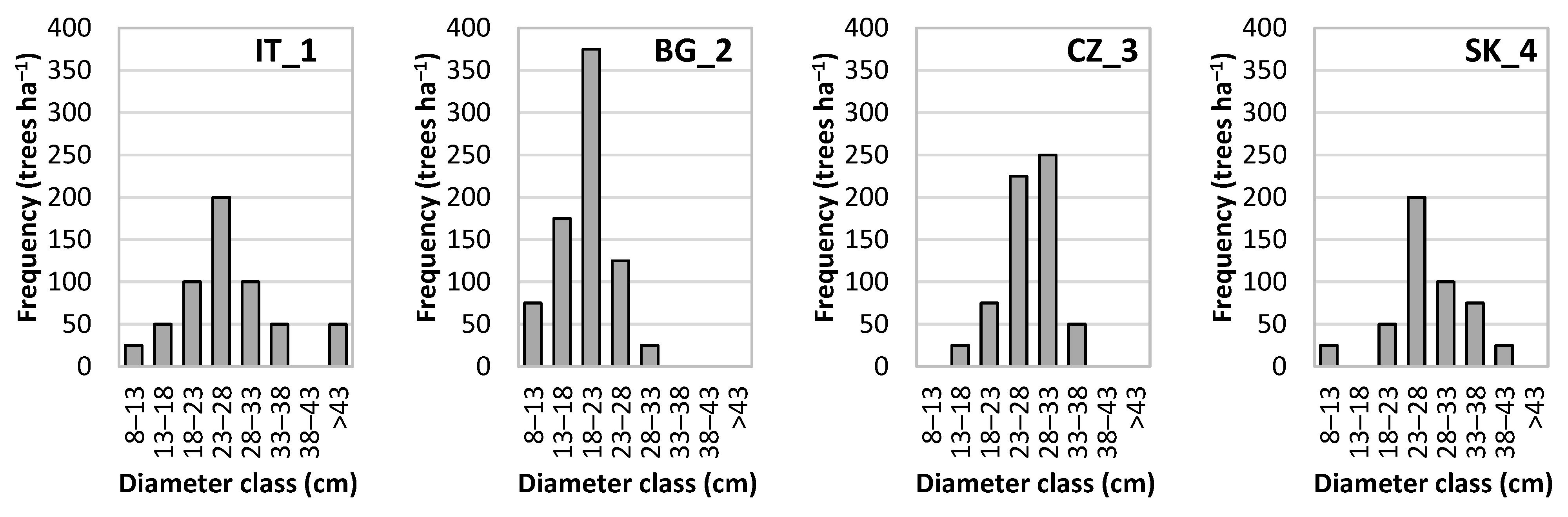
3.1. Stand Characteristics, Production, and Diversity
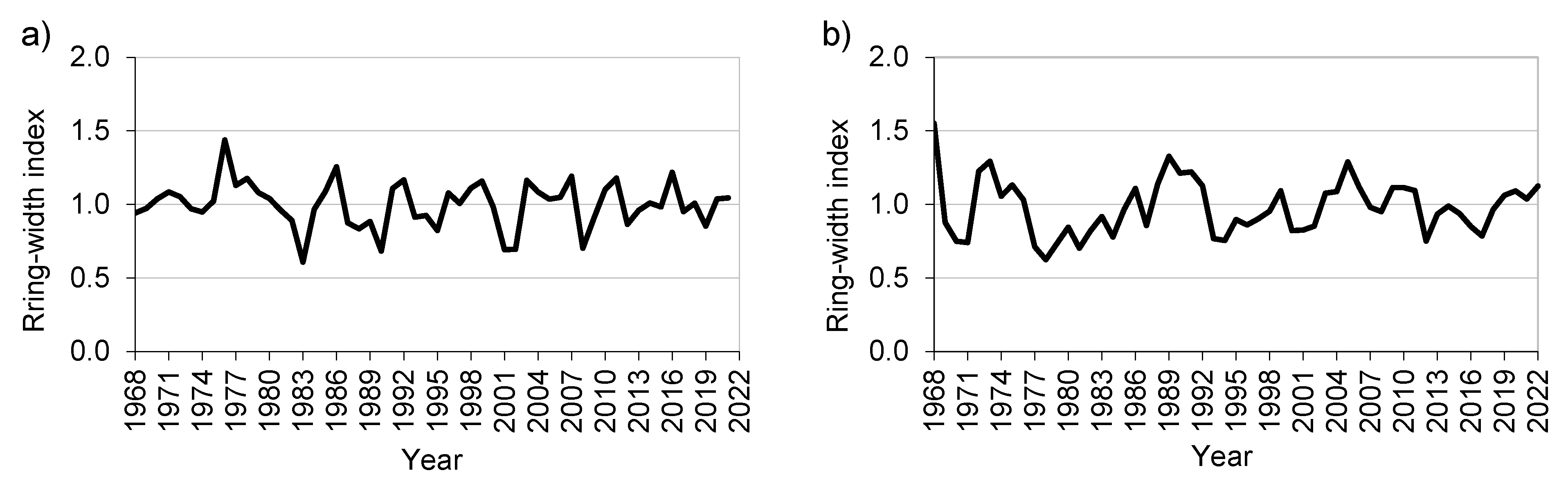
3.2. Tree-Ring Growth
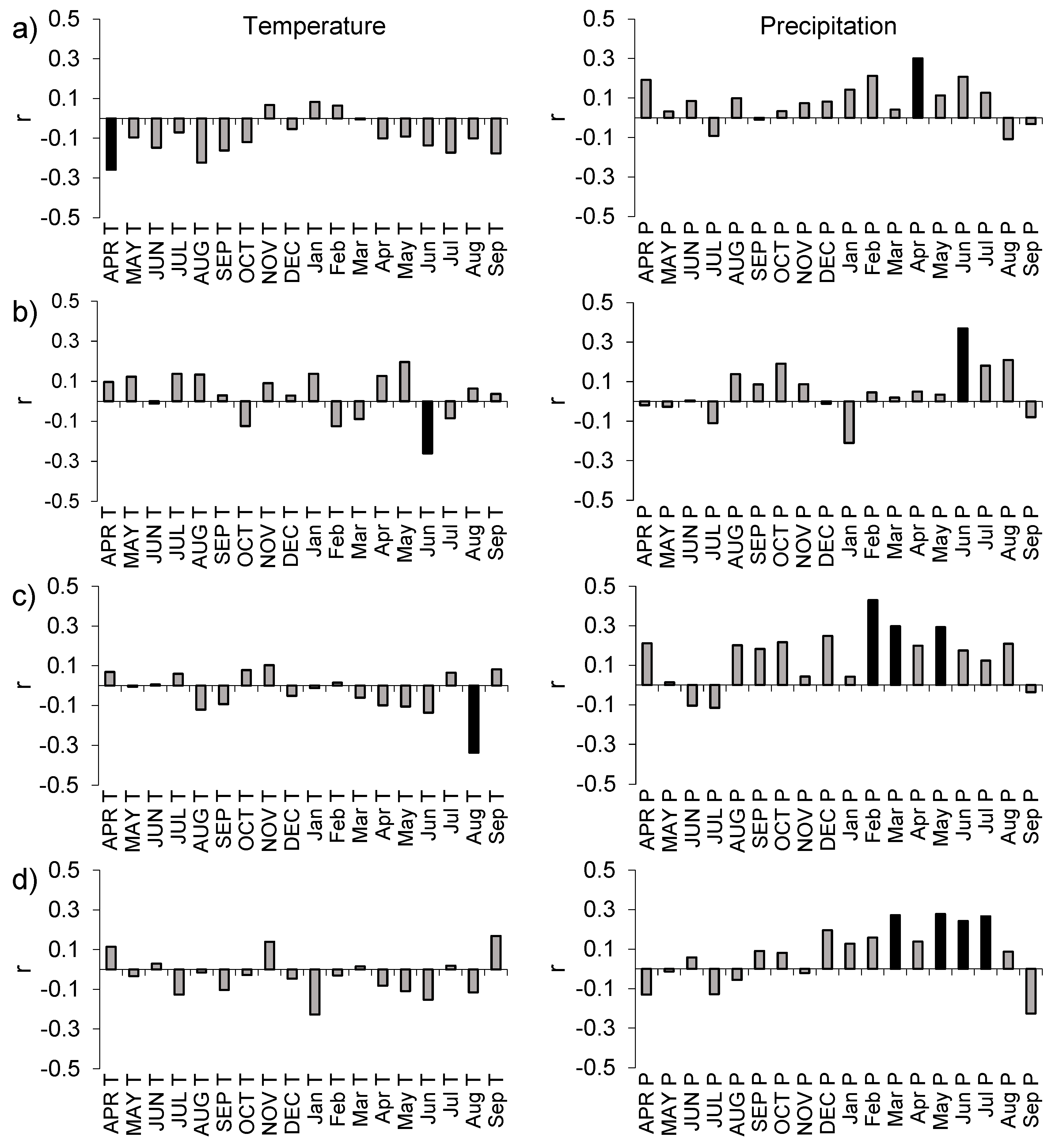
3.3. Turkey Oak’s RWI with Monthly Precipitation and Temperature
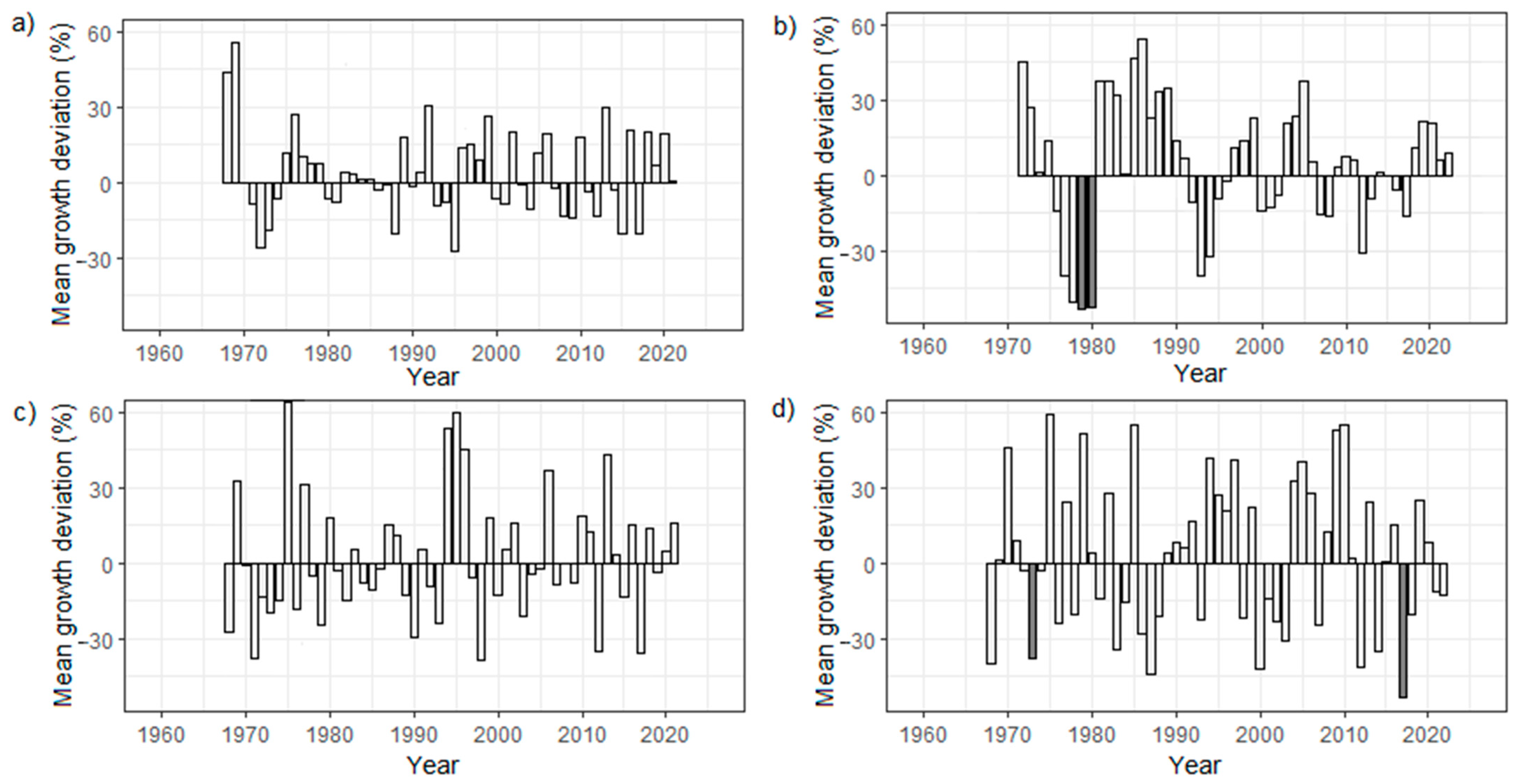
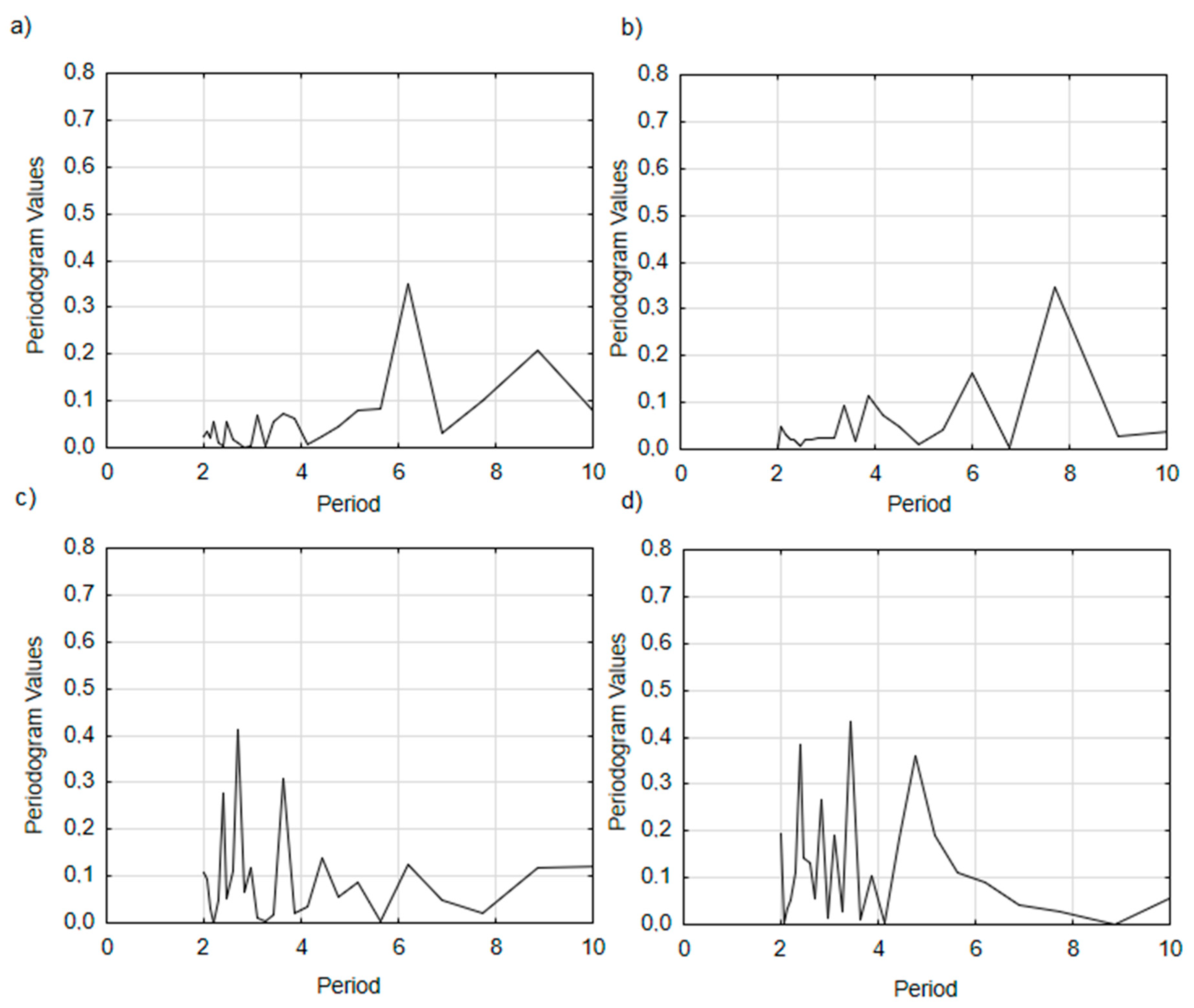
3.4. Turkey Oak’s RWI Growth Cycles
3.5. Interaction between Stand Structure, Production Parameters, and Growth
4. Discussion
4.1. Production Potential and Stand Density
4.2. Stand Biodiversity and Structure
4.3. Turkey Oak Coppices and Climate
4.4. Tree-Ring Growth Cycles across the Studied Plots
4.5. Potential for Coppice Forests
4.6. Study Limitations and Ideas
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadell, J.G.; Raupach, M.R. Managing Forests for Climate Change Mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Fitzgerald, J.; Zimmerman, N.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.-J.; Lasch, P.; Eggers, J.; van der Maaten-Thunissen, M.; et al. Climate Change and European Forests: What Do We Know, What Are the Uncertainties, and What Are the Implications for Forest Management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Vacek, Z.; Vacek, S.; Cukor, J.; Bulušek, D.; Slávik, M.; Lukáčik, I.; Štefančík, I.; Sitková, Z.; Eşen, D.; Ripullone, F.; et al. Dendrochronological Data from Twelve Countries Proved Definite Growth Response of Black Alder ([L.] Gaertn.) to Climate Courses across Its Distribution Range. Cent. Eur. For. J. 2022, 68, 139–153. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Cukor, J. European Forests under Global Climate Change: Review of Tree Growth Processes, Crises and Management Strategies. J. Environ. Manag. 2023, 332, 117353. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil Carbon Sequestration to Mitigate Climate Change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Prietzel, J.; Barthold, F.; Spörlein, P.; Geuß, U.; Hangen, E.; Reischl, A.; Schilling, B.; von Lützow, M.; Kögel-Knabner, I. Storage and Drivers of Organic Carbon in Forest Soils of Southeast Germany (Bavaria)—Implications for Carbon Sequestration. For. Ecol. Manag. 2013, 295, 162–172. [Google Scholar] [CrossRef]
- Cukor, J.; Vacek, Z.; Vacek, S.; Linda, R.; Podrázský, V. Biomass Productivity, Forest Stability, Carbon Balance, and Soil Transformation of Agricultural Land Afforestation: A Case Study of Suitability of Native Tree Species in the Submontane Zone in Czechia. Catena 2022, 210, 105893. [Google Scholar] [CrossRef]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought Response of Five Conifer Species under Contrasting Water Availability Suggests High Vulnerability of Norway Spruce and European Larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- González de Andrés, E.; Seely, B.; Blanco, J.A.; Imbert, J.B.; Lo, Y.-H.; Castillo, F.J. Increased Complementarity in Water-Limited Environments in Scots Pine and European Beech Mixtures under Climate Change. Ecohydrology 2017, 10, e1810. [Google Scholar] [CrossRef]
- Niemelä, J.; Young, J.; Alard, D.; Askasibar, M.; Henle, K.; Johnson, R.; Kurttila, M.; Larsson, T.-B.; Matouch, S.; Nowicki, P.; et al. Identifying, Managing and Monitoring Conflicts between Forest Biodiversity Conservation and Other Human Interests in Europe. For. Policy Econ. 2005, 7, 877–890. [Google Scholar] [CrossRef]
- Agnoletti, M. The Conservation of Cultural Landscapes; CAB International: Chatham, UK, 2006; ISBN 1845931548. [Google Scholar]
- Agnoletti, M. The Degradation of Traditional Landscape in a Mountain Area of Tuscany during the 19th and 20th Centuries: Implications for Biodiversity and Sustainable Management. For. Ecol. Manag. 2007, 249, 5–17. [Google Scholar] [CrossRef]
- McGrath, M.J.; Luyssaert, S.; Meyfroidt, P.; Kaplan, J.O.; Bürgi, M.; Chen, Y.; Erb, K.; Gimmi, U.; McInerney, D.; Naudts, K.; et al. Reconstructing European Forest Management from 1600 to 2010. Biogeosciences 2015, 12, 4291–4316. [Google Scholar] [CrossRef]
- Poleno, Z.; Vacek, S.; Podrázský, V.; Remeš, J.; Štefančík, I.; Mikeska, M.; Kobliha, J.; Kupka, I.; Malík, V.; Turčáni, M.; et al. Pěstování Lesů III—Praktické Postupy Pěstování Lesů [Silviculture III. Practical Methods in Silviculture]; Lesnická Práce: Kostelec nad Černými lesy, Czech Republic, 2009; ISBN 978-80-87154-34-2. [Google Scholar]
- Corcuera, L.; Camarero, J.J.; Sisó, S.; Gil-Pelegrín, E. Radial-Growth and Wood-Anatomical Changes in Overaged Quercus Pyrenaica Coppice Stands: Functional Responses in a New Mediterranean Landscape. Trees—Struct. Funct. 2006, 20, 91–98. [Google Scholar] [CrossRef]
- Fabbio, G.; Cutini, A. Il Ceduo Oggi: Quale Gestione Oltre Le Definizioni? Forest 2017, 14, 257–274. [Google Scholar] [CrossRef]
- Cutini, A.; Ferretti, M.; Bertini, G.; Brunialti, G.; Bagella, S.; Chianucci, F.; Fabbio, G.; Fratini, R.; Riccioli, F.; Caddeo, C.; et al. Testing an Expanded Set of Sustainable Forest Management Indicators in Mediterranean Coppice Area. Ecol. Indic. 2021, 130, 108040. [Google Scholar] [CrossRef]
- Camponi, L.; Cardelli, V.; Cocco, S.; Serrani, D.; Salvucci, A.; Cutini, A.; Agnelli, A.; Fabbio, G.; Bertini, G.; Roggero, P.P.; et al. Effect of Coppice Conversion into High Forest on Soil Organic C and Nutrients Stock in a Turkey Oak (Quercus cerris L.) Forest in Italy. J. Environ. Manag. 2022, 312, 114935. [Google Scholar] [CrossRef]
- Bohensky, E.L.; Maru, Y. Indigenous Knowledge, Science, and Resilience: What Have We Learned from a Decade of International Literature on “Integration”? Ecol. Soc. 2011, 16, 19. [Google Scholar] [CrossRef]
- Johnson, J.T.; Howitt, R.; Cajete, G.; Berkes, F.; Louis, R.P.; Kliskey, A. Weaving Indigenous and Sustainability Sciences to Diversify Our Methods. Sustain. Sci. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Scullion, J.J.; Vogt, K.A.; Winkler-Schor, S.; Sienkiewicz, A.; Peña, C.; Hajek, F. Designing Conservation-Development Policies for the Forest Frontier. Sustain. Sci. 2016, 11, 295–306. [Google Scholar] [CrossRef]
- Suchomel, C.; Becker, G.; Pyttel, P. Fully Mechanized Harvesting in Aged Oak Coppice Stands. For. Prod. J. 2011, 61, 290–296. [Google Scholar] [CrossRef]
- Kull, K. Growth Form Parameters of Clonal Herbs. In Consortium Masingii: A Festschrift for Viktor Masing; Aaviksoo, K., Kull, K., Paal, J., Trass, H., Eds.; Tartu University: Tartu, Estonia, 1995; pp. 106–115. [Google Scholar]
- Buckley, P. Coppice Restoration and Conservation: A European Perspective. J. For. Res. 2020, 25, 125–133. [Google Scholar] [CrossRef]
- Cervellini, M.; Fiorini, S.; Cavicchi, A.; Campetella, G.; Simonetti, E.; Chelli, S.; Canullo, R.; Gimona, A. Relationships between Understory Specialist Species and Local Management Practices in Coppiced Forests—Evidence from the Italian Apennines. For. Ecol. Manag. 2017, 385, 35–45. [Google Scholar] [CrossRef]
- Vrška, T.; Janík, D.; Pálková, M.; Adam, D.; Trochta, J. Below-and above-Ground Biomass, Structure and Patterns in Ancient Lowland Coppices. IForest 2017, 10, 23–31. [Google Scholar] [CrossRef]
- Rackham, O. Woodlands; Collins: London, UK, 2006. [Google Scholar]
- Peterken, G.F. Natural Woodland: Ecology and Conservation in Northern Temperate Regions; Cambridge University Press: Cambridge, UK, 1996; ISBN 0521367921. [Google Scholar]
- Verheyen, K.; Bossuyt, B.; Hermy, M.; Tack, G. The Land Use History (1278-1990) of a Mixed Hardwood Forest in Western Belgium and Its Relationship with Chemical Soil Characteristics. J. Biogeogr. 1999, 26, 1115–1128. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Wood Structure and Environment (Springer Series in Wood Science); Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-48299-4. [Google Scholar]
- Campetella, G.; Canullo, R.; Gimona, A.; Garadnai, J.; Chiarucci, A.; Giorgini, D.; Angelini, E.; Cervellini, M.; Chelli, S.; Bartha, S. Scale-Dependent Effects of Coppicing on the Species Pool of Late Successional Beech Forests in the Central Apennines, Italy. Appl. Veg. Sci. 2016, 19, 474–485. [Google Scholar] [CrossRef]
- Bartha, S.; Merolli, A.; Campetella, G.; Canullo, R. Changes of Vascular Plant Diversity along a Chronosequence of Beech Coppice Stands, Central Apennines, Italy. Plant Biosyst. 2008, 142, 572–583. [Google Scholar] [CrossRef]
- Vild, O.; Roleček, J.; Hédl, R.; Kopecký, M.; Utinek, D. Experimental Restoration of Coppice-with-Standards: Response of Understorey Vegetation from the Conservation Perspective. For. Ecol. Manag. 2013, 310, 234–241. [Google Scholar] [CrossRef]
- Vacek, S.; Vacek, Z.; Ulbrichová, I.; Bulušek, D.; Prokupková, A.; Král, J.; Vančura, K. Biodiversity Dynamics of Differently Managed Lowland Forests Left to Spontaneous Development in Central Europe. Austrian J. For. Sci. 2019, 136, 249–282. [Google Scholar]
- Unrau, A.; Becker, G.; Spinelli, R.; Lazdina, D.; Magagnotti, N.; Nicolescu, V.N.; Buckley, P.; Bartlett, D.; Kofman, P.D. Coppice Forests in Europe; Unrau, A., Becker, G., Spinelli, R., Lazdina, D., Magagnotti, N., Nicolescu, V.-N., Buckley, P., Barlett, D., Kofman, P.D., Eds.; Albert Ludwig University Freiburg: Freiburg im Breisgau, Germany, 2018; ISBN 9783981734027. [Google Scholar]
- Matula, R.; Šrámek, M.; Kvasnica, J.; Uherková, B.; Slepička, J.; Matoušková, M.; Kutchartt, E.; Svátek, M. Pre-Disturbance Tree Size, Sprouting Vigour and Competition Drive the Survival and Growth of Resprouting Trees. For. Ecol. Manag. 2019, 446, 71–79. [Google Scholar] [CrossRef]
- Dickmann, D.I. Silviculture and Biology of Short-Rotation Woody Crops in Temperate Regions: Then and Now. Biomass Bioenergy 2006, 30, 696–705. [Google Scholar] [CrossRef]
- Del Tredici, P. Sprouting in Temperate Trees: A Morphological and Ecological Review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Bílek, L.; Král, J.; Ulbrichová, I.; Simon, J.; Bulušek, D. Impact of Applied Silvicultural Systems on Spatial Pattern of Hornbeam-Oak Forests. Cent. Eur. For. J. 2018, 64, 33–45. [Google Scholar] [CrossRef]
- Čížek, L.; Šebek, P.; Bače, R.; Beneš, J.; Doležal, J.; Dvorský, M.; Miklín, J.; Svoboda, M. Metodika Péče o Druhově Bohaté (Světlé) Lesy; Entomologický ústav, Biologické centrum AV ČR, v.v.i.: České Budějovice, Czech Republic, 2016; p. 126. [Google Scholar]
- Müllerová, J.; Hédl, R.; Szabó, P. Coppice Abandonment and Its Implications for Species Diversity in Forest Vegetation. For. Ecol. Manag. 2015, 343, 88–100. [Google Scholar] [CrossRef]
- Volařík, D.; Svátek, M.; Šenfeldr, M.; Kučera, A.; Šrámek, M.; Dreslerová, J.; Matula, R. Variation in Canopy Openness among Main Structural Types of Woody Vegetation in a Traditionally Managed Landscape. Folia Geobot. 2017, 52, 15–32. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.A.; Van Pelt, R.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C. Disturbances and Structural Development of Natural Forest Ecosystems with Silvicultural Implications, Using Douglas-Fir Forests as an Example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Madera, P.; Slach, T.; Úradnícek, L.; Lacina, J.; Cernušáková, L.; Friedl, M.; Repka, R.; Bucek, A. Tree Shape and Form in Ancient Coppice Woodlands. J. Landsc. Ecol. Republic) 2017, 10, 49–62. [Google Scholar] [CrossRef]
- Bouvet, A.; Paillet, Y.; Archaux, F.; Tillon, L.; Denis, P.; Gilg, O.; Gosselin, F. Effects of Forest Structure, Management and Landscape on Bird and Bat Communities. Environ. Conserv. 2016, 43, 148–160. [Google Scholar] [CrossRef]
- UN-ECE; FAO. Forest Resources of Europe, CIS, North America, Australia, Japan and New Zealand (Industrialized Temperate/Boreal Countries): UN-ECE/FAO Contribution to the Global Forest Resources Assessment 2000: Main Report; United Nations: Geneva, Switzerland, 2000; ISBN 9211167353. [Google Scholar]
- Cutini, A.; Brunialti, G.; Amici, V.; Bagella, S.; Bertini, G.; Caddeo, C.; Calderisi, M.; Chianucci, F.; Ciucchi, B.; Corradini, S. Report: Integrated Scientific Synthesis and Evaluation of Project Results–LIFE FutureForCoppiceS–Shaping Future Forestry for Sustainable Coppices in Southern Europe: The Legacy of Past Management Trials (with Synthesis for Resource Managers and Policy Mak. Deliv. LIFE Futur. Proj. Action B 2019, 9, 108. [Google Scholar]
- MPRV. Správa o Lesnom Hospodárstve v Slovenskej Republike Za Rok 2021; Zelená Správa: Bratislava, Slovakia, 2022. [Google Scholar]
- Madĕra, P.; Machala, M.; Slach, T.; Friedl, M.; Černušakova, L.; Volařik, D.; Buček, A. Predicted Occurrence of Ancient Coppice Woodlands in the Czech Republic. IForest 2017, 10, 788–795. [Google Scholar] [CrossRef]
- Kadavý, J.; Kneifl, M.; Servus, M.; Knott, R.; Hurt, V.; Flora, M. Nízký a Střední Les—Plnohodnotná Alternativa Hospodaření Malých a Středních Vlastníků Lesa—Obecná Východiska; Lesnická práce, s. r. o. nakladatelství a vydavatelství: Kostelec nad Černými lesy, Czech Republic, 2011; ISBN 978-80-87154-96-0. [Google Scholar]
- Mairota, P.; Buckley, P.; Suchomel, C.; Heinsoo, K.; Verheyen, K.; Hédl, R.; Terzuolo, P.G.; Sindaco, R.; Carpanelli, A. Integrating Conservation Objectives into Forest Management: Coppice Management and Forest Habitats in Natura 2000 Sites. iForest—Biogeosci. For. 2016, 9, 560–568. [Google Scholar] [CrossRef]
- Spiecker, H.; Hein, S.; Makkonen-Spiecker, K.; Thies, M. (Eds.) Valuable Broadleaved Forests in Europe (European Forest Institute Research Report; 22); Brill: Leiden, The Netherlands, 2009; ISBN 978 90 04 16795 7. [Google Scholar]
- Šplíchalová, M. Aspects of Oak (Quercus Sp.) Management in Spain and Its Application. Coppice Forests: Past, Present and Future. In Proceedings of the Coppice Forests: Past, Present and Future, Brno, Czech Republic, 9–11 April 2015; Vild, O., Ed.; pp. 9–11. [Google Scholar]
- Nicolescu, V.-N.; Carvalho, J.; Hochbichler, E.; Bruckman, V.; Piqué-Nicolau, M.; Hernea, C.; Viana, H.; Štochlová, P.; Ertekin, M.; Tijardovic, M.; et al. Silvicultural Guidelines for European Coppice Forests. COST Action FP1301 Reports; Albert Ludwig University of Freiburg: Freiburg, Germany, 2017. [Google Scholar]
- Coppini, M.; Hermanin, L. Restoration of Selective Beech Coppices: A Case Study in the Apennines (Italy). For. Ecol. Manag. 2007, 249, 18–27. [Google Scholar] [CrossRef]
- Johann, E. Coppice Forests in Austria: The Re-Introduction of Traditional Management Systems in Coppice Forests in Response to the Decline of Species and Landscape and under the Aspect of Climate Change. For. Ecol. Manag. 2021, 490, 119129. [Google Scholar] [CrossRef]
- Valente, A.M.; Pelayo, A.; Figueiredo, A.M.; Fonseca, C.; Torres, R.T. Overabundant Wild Ungulate Populations in Europe: Management with Consideration of Socio-Ecological Consequences. Mamm. Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Carpio, A.J.; Apollonio, M.; Acevedo, P. Wild Ungulate Overabundance in Europe: Contexts, Causes, Monitoring and Management Recommendations. Mamm. Rev. 2021, 51, 95–108. [Google Scholar] [CrossRef]
- Bran, D.; Lobréaux, O.; Maistre, M.; Perret, P.; Romane, F. Germination of Quercus Ilex and Q. Pubescens in a Q. Ilex Coppice—Long-Term Consequences. Vegetatio 1990, 87, 45–50. [Google Scholar] [CrossRef]
- Viscosi, V.; Fortini, P.; D’Imperio, M. A Statistical Approach to Species Identification on Morphological Traits of European White Oaks: Evidence of Morphological Structure in Italian Populations of Quercus Pubescens Sensu Lato. Acta Bot. Gall. 2011, 158, 175–188. [Google Scholar] [CrossRef]
- Gasparini, P. Italian National Forest Inventory—Methods and Results of the Third Survey; Gasparini, P., Di Cosmo, L., Floris, A., De Laurentis, D., Eds.; Springer: Cham, Switzerland, 2022; ISBN 978-3-030-98677-3. [Google Scholar]
- Rossnev, B.; Petkov, P.; Mirchev, P.; Georgiev, G.; Georgieva, M.; Matova, M. System Approach for Determination and Improvement of Quercus cerris L. Forests Status in Bulgaria. In Proceedings of the Integral Protection of Forests; Scientific-Technological Platform: Belgrade, Serbia, 2007; pp. 186–191. [Google Scholar]
- Rossnev, B. Quercus cerris Forests Status in Bulgaria and Measures for Improvement; Bulgarian Academy of Sciences, Forest Research Institute: Sofia, Bulgaria, 2006; p. 120. [Google Scholar]
- Kostić, S.; Levanič, T.; Orlović, S.; Matović, B.; Stojanović, D.B. Turkey Oak (Quercus cerris L.) Is More Drought Tolerant and Better Reflects Climate Variations Compared to Pedunculate Oak (Quercus Robur L.) in Lowland Mixed Forests in Northwestern Serbia: A Stable Carbon Isotope Ratio (Δ13C) and Radial Growth Approach. Ecol. Indic. 2022, 142, 109242. [Google Scholar] [CrossRef]
- Toth, D.; Maitah, M.; Maitah, K.; Jarolínová, V. The Impacts of Calamity Logging on the Development of Spruce Wood Prices in Czech Forestry. Forests 2020, 11, 283. [Google Scholar] [CrossRef]
- Šimůnek, V.; Sharma, R.P.; Vacek, Z.; Vacek, S.; Hůnová, I. Sunspot Area as Unexplored Trend inside Radial Growth of European Beech in Krkonoše Mountains: A Forest Science from Different Perspective. Eur. J. For. Res. 2020, 139, 999–1013. [Google Scholar] [CrossRef]
- Sharma, R.P.; Vacek, Z.; Vacek, S. Individual Tree Crown Width Models for Norway Spruce and European Beech in Czech Republic. For. Ecol. Manag. 2016, 366, 208–220. [Google Scholar] [CrossRef]
- Kraft, G. Beiträge Zur Lehre von Den Durchforstungen, Schlagstellungen Und Lichtungshieben; Klindworth’s Verlag: Hannover, Germany, 1884. [Google Scholar]
- Remeš, J.; Bílek, L.; Novák, J.; Vacek, Z.; Vacek, S.; Putalová, T.; Koubek, L. Diameter Increment of Beech in Relation to Social Position of Trees, Climate Characteristics and Thinning Intensity. J. For. Sci. 2015, 61, 456–464. [Google Scholar] [CrossRef]
- RRinntech. TSAP-WINTM: Time Series Analysis and Presentation for Dendrochronology and Related Applications; Rinntech: Heidelberg, Switzerland, 2010. [Google Scholar]
- Fabrika, M.; Ďurský, J. Algorithms and Software Solution of Thinning Models for SIBYLA Growth Simulator. J. For. Sci. 2005, 51, 431–445. [Google Scholar] [CrossRef]
- Clark, P.J.; Evans, F.C. Distance to Nearest Neighbor as a Measure of Spatial Relationships in Populations. Ecology 1954, 35, 445–453. [Google Scholar] [CrossRef]
- Pretzsch, H. Wissen Nutzbar Machen Für Das Management von Waldökosystemen. Allg. Forstz./Der Wald. 2006, 61, 1158–1159. [Google Scholar]
- Füldner, K. Strukturbeschreibung in Mischbeständen. Forstarchiv 1995, 66, 235–606. [Google Scholar]
- Jaehne, S.; Dohrenbusch, A. A Method to Evaluate Forest Stand Diversity. Forstwiss 1997, 116, 1–6. [Google Scholar]
- Seifert, T.; Schuck, J.; Block, J.; Pretzsch, H. Simulation von Biomasse-Und Nährstoffgehalt von Waldbäumen. In Proceedings of the Deutscher Verband Forstlicher Forschungsanstalten Sektion Ertragskunde: Jahrestagung; Nagel, J., Ed.; Nordwestdeutsche Forstliche Versuchsanstalt, Abteilung Waldwachstum: Göttingen, Germany, 2006; Volume 29, pp. 208–223. [Google Scholar]
- Vacek, Z.; Vacek, S.; Esen, D.; Yildiz, O.; Král, J.; Gallo, J. Effect of Invasive Rhododendron Ponticum l. on Natural Regeneration and Structure of Fagus Orientalis Lipsky Forests in the Black Sea Region. Forests 2020, 11, 603. [Google Scholar] [CrossRef]
- Petráš, R.; Pajtík, J. Sústava Česko-Slovenských Objemových Tabuliek Drevín. Lesn. časopis 1991, 37, 49–56. [Google Scholar]
- Reineke, L.H. Prefecting a Stand-Density Index for Evenaged Forests. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Crookston, N.L.; Stage, A.R. Percent Canopy Cover and Stand Structure Statistics from the Forest Vegetation Simulator; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2014; R Foundation Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bunn, A.G. A Dendrochronology Program Library in R (DplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Bunn, A.G. Statistical and Visual Crossdating in R Using the DplR Library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- van der Maaten-Theunissen, M.; van der Maaten, E.; Bouriaud, O. PointRes: An R Package to Analyze Pointer Years and Components of Resilience. Dendrochronologia 2015, 35, 34–38. [Google Scholar] [CrossRef]
- Bunn, A.; Mikko, K. Chronology Building in Dplr; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Schweingruber, F.H.; Eckstein, D.; Serre-Bachet, F.; Braker, O.U. Identification, Presentation and Interpretation of Event Years and Pointer Years in Dendrochronology. Dendrochronologia 1990, 8, 9–38. [Google Scholar]
- Neuwirth, B.; Schweingruber, F.H.; Winiger, M. Spatial Patterns of Central European Pointer Years from 1901 to 1971. Dendrochronologia 2007, 24, 79–89. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate.; Academic Press Inc.: New York, NY, USA, 1976. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, Arizona, 2010; ISBN 978-0-816-52684-0. [Google Scholar]
- Biondi, F.; Waikul, K. DENDROCLIM2002: A C++ Program for Statistical Calibration of Climate Signals in Tree-Ring Chronologies. Comput. Geosci. 2004, 30, 303–311. [Google Scholar] [CrossRef]
- StatSoft Power Solutions, Inc. Statistica Electronic Manual 2013; StatSoft Power Solutions, Inc.: Tulsa, OK, USA, 2013. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Karamizadeh, S.; Abdullah, S.M.; Manaf, A.A.; Zamani, M.; Hooman, A. An Overview of Principal Component Analysis. J. Signal Inf. Process. 2013, 4, 173–175. [Google Scholar] [CrossRef]
- Notarangelo, M.; La Marca, O.; Moretti, N. Long-Term Effects of Experimental Cutting to Convert an Abandoned Oak Coppice into Transitional High Forest in a Protected Area of the Italian Mediterranean Region. For. Ecol. Manag. 2018, 430, 241–249. [Google Scholar] [CrossRef]
- Di Filippo, A.; Alessandrini, A.; Biondi, F.; Blasi, S.; Portoghesi, L.; Piovesan, G. Climate Change and Oak Growth Decline: Dendroecology and Stand Productivity of a Turkey Oak (Quercus cerris L.) Old Stored Coppice in Central Italy. Ann. For. Sci. 2010, 67, 706. [Google Scholar] [CrossRef]
- Šrámek, M.; Volařík, D.; Ertas, A.; Matula, R. The Effect of Coppice Management on the Structure, Tree Growth and Soil Nutrients in Temperate Turkey. J. For. Sci. 2015, 61, 27–34. [Google Scholar] [CrossRef]
- Vancura, K.; Simkova, M.; Vacek, Z.; Vacek, S.; Gallo, J.; Simunek, V.; Podrazsky, V.; Stefancik, I.; Hájek, V.; Prokupkova, A.; et al. Effects of Environmental Factors and Management on Dynamics of Mixed Calcareous Forests under Climate Change in Central European Lowlands. Dendrobiology 2022, 87, 79–100. [Google Scholar] [CrossRef]
- Longuetaud, F.; Seifert, T.; Leban, J.-M.; Pretzsch, H. Analysis of Long-Term Dynamics of Crowns of Sessile Oaks at the Stand Level by Means of Spatial Statistics. For. Ecol. Manag. 2008, 255, 2007–2019. [Google Scholar] [CrossRef]
- Plauborg, K.U. Analysis of Radial Growth Responses to Changes in Stand Density for Four Tree Species. For. Ecol. Manag. 2004, 188, 65–75. [Google Scholar] [CrossRef]
- Kunstler, G.; Albert, C.H.; Courbaud, B.; Lavergne, S.; Thuiller, W.; Vieilledent, G.; Zimmermann, N.E.; Coomes, D.A. Effects of Competition on Tree Radial-Growth Vary in Importance but Not in Intensity along Climatic Gradients. J. Ecol. 2011, 99, 300–312. [Google Scholar] [CrossRef]
- Bravo-Oviedo, A.; Condés, S.; Del Río, M.; Pretzsch, H.; Ducey, M.J. Maximum Stand Density Strongly Depends on Species-Specific Wood Stability, Shade and Drought Tolerance. Forestry 2018, 91, 459–469. [Google Scholar] [CrossRef]
- Giuggiola, A.; Bugmann, H.; Zingg, A.; Dobbertin, M.; Rigling, A. Reduction of Stand Density Increases Drought Resistance in Xeric Scots Pine Forests. For. Ecol. Manag. 2013, 310, 827–835. [Google Scholar] [CrossRef]
- Sterck, F.; Vos, M.; Hannula, S.E.; de Goede, S.; de Vries, W.; den Ouden, J.; Nabuurs, G.J.; van der Putten, W.; Veen, C. Optimizing Stand Density for Climate-Smart Forestry: A Way Forward towards Resilient Forests with Enhanced Carbon Storage under Extreme Climate Events. Soil Biol. Biochem. 2021, 162, 108396. [Google Scholar] [CrossRef]
- Sohn, J.A.; Hartig, F.; Kohler, M.; Huss, J.; Bauhus, J. Heavy and Frequent Thinning Promotes Drought Adaptation in Pinus Sylvestris Forests. Ecol. Appl. 2016, 26, 2190–2205. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Lexer, M.J. Adaptation Options to Reduce Climate Change Vulnerability of Sustainable Forest Management in the Austrian Alps. Can. J. For. Res. 2011, 41, 694–706. [Google Scholar] [CrossRef]
- Amorini, E.; Biocca, M.; Manetti, M.C.; Motta, E. Dendroecological Study in a Declining Coppice Stand. Ann. Des Sci. For. 1996, 53, 731–742. [Google Scholar] [CrossRef]
- Priwitzer, T.; Pajtík, J.; Ištoňa, J.; Pavlenda, P. Vplyv Zrážok Na Dynamiku Rastu, Fenológiu a Opad Lesných Drevín. In Proceedings of the International Scientific Conference Bioclimatology and Natural Hazards: Proceedings, Zvolen-Polana Nad Detvou, Slovakia, 17–20 September; Střelcová, K., Škvarenina, J., Blažec, M., Eds.; Slovenská Bioklimatologická Spoločnost: Poľana nad Detvou, Slovakia, 2007. [Google Scholar]
- Zafirov, N.; Kostov, G. Main Stress Factors in Coppice Oak Forests in Western Bulgaria. Silva Balc. 2019, 20, 37–52. [Google Scholar] [CrossRef]
- Drobyshev, I.; Niklasson, M.; Eggertsson, O.; Linderson, H.; Sonesson, K. Influence of Annual Weather on Growth of Pedunculate Oak in Southern Sweden. Ann. For. Sci. 2008, 65, 512. [Google Scholar] [CrossRef]
- St. George, S. An Overview of Tree-Ring Width Records across the Northern Hemisphere. Quat. Sci. Rev. 2014, 95, 132–150. [Google Scholar] [CrossRef]
- Deligöz, A.; Bayar, E. Drought Stress Responses of Seedlings of Two Oak Species (Quercus cerris and Quercus Robur). Turkish J. Agric. For. 2018, 42, 114–123. [Google Scholar] [CrossRef]
- Mészáros, I.; Adorján, B.; Nyitrai, B.; Kanalas, P.; Oláh, V.; Levanič, T. Long-Term Radial Growth and Climate-Growth Relationships of Quercus Petraea (Matt.) Liebl. and Quercus cerris L. in a Xeric Low Elevation Site from Hungary. Dendrochronologia 2022, 76, 126014. [Google Scholar] [CrossRef]
- Jajcay, N.; Hlinka, J.; Kravtsov, S.; Tsonis, A.A.; Paluš, M. Time Scales of the European Surface Air Temperature Variability: The Role of the 7—8 Year Cycle. Geophys. Res. Lett. 2016, 43, 902–909. [Google Scholar] [CrossRef]
- Sen, A.K.; Ogrin, D. Analysis of Monthly, Winter, and Annual Temperatures in Zagreb, Croatia, from 1864 to 2010: The 7.7-Year Cycle and the North Atlantic Oscillation. Theor. Appl. Climatol. 2016, 123, 733–739. [Google Scholar] [CrossRef]
- Qian, B.; Xu, H.; Corte-Real, J. Spatial-temporal structures of quasi-periodic oscillations in precipitation over europe. Int. J. Climatol. 2000, 20, 1583–1598. [Google Scholar] [CrossRef]
- Domonkos, P.; Tar, K. Long-Term Changes in Observed Temperature and Precipitation Series 1901–1998 from Hungary and Their Relations to Larger Scale Changes. Theor. Appl. Climatol. 2003, 75, 131–147. [Google Scholar] [CrossRef]
- Ilyés, C.; Turai, E.; Szucs, P. Examination of Rainfall Data for 110 Years Using Spectral and Wavelet Analysis. Cent. Eur. Geol. 2018, 61, 1–15. [Google Scholar] [CrossRef]
- Cutini, A.; Chianucci, F.; Chirichella, R.; Donaggio, E.; Mattioli, L.; Apollonio, M. Mast Seeding in Deciduous Forests of the Northern Apennines (Italy) and Its Influence on Wild Boar Population Dynamics. Ann. For. Sci. 2013, 70, 493–502. [Google Scholar] [CrossRef]
- Bisi, F.; Chirichella, R.; Chianucci, F.; Von Hardenberg, J.; Cutini, A.; Martinoli, A.; Apollonio, M. Climate, Tree Masting and Spatial Behaviour in Wild Boar (Sus Scrofa L.): Insight from a Long-Term Study. Ann. For. Sci. 2018, 75, 9. [Google Scholar] [CrossRef]
- Simeone, M.C.; Zhelev Stojanov, P.; Kandemir, G. EUFORGEN Technical Guidelines for Genetic Conservation and Use of Turkey Oak (Quercus cerris); European Forest Genetic Resources Programme (EUFORGEN), European Forest Institute: Bonn, Germany, 2019; ISBN 978-952-5980-43-1. [Google Scholar]
- Garcia-Barreda, S.; Sangüesa-Barreda, G.; Madrigal-González, J.; Seijo, F.; González de Andrés, E.; Camarero, J.J. Reproductive Phenology Determines the Linkages between Radial Growth, Fruit Production and Climate in Four Mediterranean Tree Species. Agric. For. Meteorol. 2021, 307, 108493. [Google Scholar] [CrossRef]
- Drobyshev, I.; Övergaard, R.; Saygin, I.; Niklasson, M.; Hickler, T.; Karlsson, M.; Sykes, M.T. Masting Behaviour and Dendrochronology of European Beech (Fagus Sylvatica L.) in Southern Sweden. For. Ecol. Manag. 2010, 259, 2160–2171. [Google Scholar] [CrossRef]
- Drobyshev, I.; Niklasson, M.; Mazerolle, M.J.; Bergeron, Y. Agricultural and Forest Meteorology Reconstruction of a 253-Year Long Mast Record of European Beech Reveals Its Association with Large Scale Temperature Variability and No Long-Term Trend in Mast Frequencies. Agric. For. Meteorol. 2014, 192–193, 9–17. [Google Scholar] [CrossRef]
- Bertini, G.; Amoriello, T.; Fabbio, G.; Piovosi, M. Forest Growth and Climate Change: Evidences from the ICP-Forests Intensive Monitoring in Italy. iForest—Biogeosci. For. 2011, 4, 262–267. [Google Scholar] [CrossRef]
- Móricz, N.; Illés, G.; Mészáros, I.; Garamszegi, B.; Berki, I.; Bakacsi, Z.; Kámpel, J.; Szabó, O.; Rasztovits, E.; Cseke, K.; et al. Different Drought Sensitivity Traits of Young Sessile Oak (Quercus Petraea (Matt.) Liebl.) and Turkey Oak (Quercus cerris L.) Stands along a Precipitation Gradient in Hungary. For. Ecol. Manag. 2021, 492, 119165. [Google Scholar] [CrossRef]
- Stafasani, M.; Toromani, E. Growth-Climate Response of Young Turkey Oak (Quercus cerris L.) Coppice Forest Stands along Longitudinal Gradient in Albania. South-East Eur. For. 2015, 6, 25–38. [Google Scholar] [CrossRef]
- Gray, B.M.; Wigley, T.M.L.; Pilcher, J.R. Statistical Significance of Reproducibility of Tree-Ring Response Functions. Tree-Ring Bull. 1981, 41, 21–35. [Google Scholar]
- Gray, B.M.; Pilcher, J.R. Testing the Significance of Summary Response Functions. Tree Ring Bull. 1983, 43, 31–38. [Google Scholar]
- Pérez, A.; Fernández, A. Dendroclimatic Reconstruction of the Last of the XVIII Century in Galicia(Spain) (in Spanish). Inv Agrar. Rec. F 1997, 6, 17–37. [Google Scholar]
- Santini, A.; Bottacci, A.; Gellini, R. Preliminary Dendroecological Survey on Pedunculate Oak (Quercus Robur L.) Stands in Tuscany (Italy). Ann. des Sci. For. 1994, 51, 1–10. [Google Scholar] [CrossRef]
- Tessier, L.; Nola, P.; Serre-Bachet, F. Deciduous Quercus in the Mediterranean Region: Tree-ring/Climate Relationships. New Phytol. 1994, 126, 355–367. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a Severe Drought on Growth and Wood Anatomical Properties of Quercus Faginea. IAWA J. 2004, 25, 185–204. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-Annual Radial Growth and Water Relations of Trees: Implications towards a Growth Mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Fritts, H.C. Growth-Rings of Trees: Their Correlation with Climate. Science 1966, 154, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.; Boswijk, G. Chronology Stripping as a Tool for Enhancing the Statistical Quality of Tree-Ring Chronologies. Tree-Ring Res. 2003, 59, 53–62. [Google Scholar]
- Taeger, S.; Zang, C.; Liesebach, M.; Schneck, V.; Menzel, A. Impact of Climate and Drought Events on the Growth of Scots Pine (Pinus Sylvestris L.) Provenances. For. Ecol. Manag. 2013, 307, 30–42. [Google Scholar] [CrossRef]
- Fulín, M.; Dostál, J.; Čáp, J.; Novotný, P. Evaluation of Silver Fir Provenances at 51 Years of Age in Provenance Trials in the Předhoří Hrubý Jeseník and Nízký Jeseník Mts. Regions, Czech Republic. J. For. Sci. 2023, 69, 44–59. [Google Scholar] [CrossRef]
- Bertolasi, B.; Zago, L.; Gui, L.; Cossu, P.; Vanetti, I.; Rizzi, S.; Cavallini, M.; Lombardo, G.; Binelli, G. Genetic Variability and Admixture Zones in the Italian Populations of Turkey Oak (Quercus cerris L.). Life 2023, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Berg, E.E.; Hamrick, J.L. Fine-scale genetic structure of a turkey oak forest. Evolution (N. Y.). 1995, 49, 110–120. [Google Scholar] [CrossRef]


| Plot Name | Country | Coordinates | Altitude | Exposition | Slope | Geological Bedrock | Soil | Age | DBH | Height | Stand Volume |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (m) | (°) | (cm) | (m) | (m3 ha−1) | |||||||
| IT_1 | Italy | 40°32′53.025′′ N 15°43′38.775′′ E | 1000 | W | 4.3 | limestone, marl | rendzina | 60 | 31 | 22 | 407 |
| BG_2 | Bulgaria | 42°31′13.619′′ N 22°33′4.277′′ E | 1060 | SW | 5.7 | sandstone | cambisol | 50 | 19 | 16 | 141 |
| SK_3 | Slovakia | 48°4′14.677′′ N 18°21′58.449′′ E | 220 | SE | 2.9 | loess clay | cambisol | 60 | 29 | 21 | 275 |
| CZ_4 | Czechia | 48°44′31.877′′ N 16°47′35.951′′ E | 200 | S | 0.0 | marl | cambisol | 60 | 28 | 22 | 342 |
| Plot | Meteo. Station Name | GPS of Meteo. Station | Station Altitude (m a.s.l.) | Distance to Plot (km) | Annual Temperature (°C) | Seasonal Temperature (°C) | Annual Precipitation (mm) | Seasonal Precipitation (mm) |
|---|---|---|---|---|---|---|---|---|
| IT_1 | Abriola | 40°31′8′′ N 15°47′38″ E | 1225 | 6.5 | 13.7 | 26.3 | 1022 | 258 |
| BG_2 | Divlya | 42°28′43′′ N 22°41′34′′ E | 720 | 12.5 | 8.8 | 16.3 | 552 | 268 |
| SK_3 | Hurbanovo | 47°52′00″ N 18°12′00″ E | 115 | 25.8 | 10.4 | 18.5 | 532 | 273 |
| CZ_4 | Lednice | 48°47′35′′ N 16°47′58′′ E | 177 | 5.7 | 9.8 | 17.6 | 497 | 291 |
| Criterion | Quantifiers | Label | Reference | Evaluation |
|---|---|---|---|---|
| Horizontal structure | Aggregation pattern | R (C&Ei) | [72] | mean value R = 1; aggregation R < 1; regularity R > 1 |
| Vertical structure | Arten-profile index | A (Pri) | [73] | range 0–1; balanced vertical structure A < 0.3; selection forest A > 0.9 |
| Vertical diversity | S (J&Di) | [75] | low S < 0.3, medium S = 0.3–0.5, high S = 0.5–0.7, very high S > 0.7 | |
| Structure differentiation | Diameter dif. | TMd (Fi) | [74] | range 0–1; low TM < 0.3; very high differentiation TM > 0.7 |
| Height dif. | TMh (Fi) | |||
| Crown dif. | K (J&Di) | [75] | low K < 1.0, medium K = 1.0–1.5, high K = 1.5–2.0, very high K > 2 | |
| Complex diversity | Stand diversity | B (J&Di) | [75] | monotonous structure B < 4; uniform structure B = 4–5.9; non-uniform structure B = 6–7.9; diverse structure B = 8–8.9; very diverse structure B > 9 |
| PRP | No. Trees | Mean RW (mm) | SD RW (mm) | Mean Min–Max (mm) | Age Min–Max | Ar1 | R-Bar | EPS | SNR |
|---|---|---|---|---|---|---|---|---|---|
| IT_1 | 24 | 2.69 | 1.03 | 1.66–4.65 | 36–66 | 0.60 | 0.34 | 0.90 | 8.71 |
| BG_2 | 25 | 2.09 | 1.11 | 1.46–2.97 | 35–55 | 0.79 | 0.43 | 0.93 | 12.87 |
| SK_3 | 26 | 3.13 | 1.45 | 0.95–5.31 | 33–69 | 0.57 | 0.46 | 0.90 | 8.89 |
| CZ_4 | 29 | 2.23 | 1.06 | 1.60–3.09 | 40–63 | 0.41 | 0.55 | 0.97 | 29.60 |
| PRP | DBH | h | f | v | N | G | V | hd | MAI | CC | SDI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (m) | (m3) | (tree ha−1) | (m2 ha−1) | (m3 ha−1) | (m3 ha−1 year−1) | (%) | ||||
| IT_1 | 30.5 | 22.33 | 0.434 | 0.708 | 575 | 41.6 | 407 | 0.732 | 6.78 | 88.6 | 0.82 |
| BG_2 | 19.0 | 16.22 | 0.395 | 0.182 | 775 | 22.0 | 141 | 0.854 | 2.82 | 77.1 | 0.52 |
| SK_3 | 28.8 | 20.92 | 0.425 | 0.579 | 475 | 30.9 | 275 | 0.726 | 4.58 | 80.8 | 0.62 |
| CZ_4 | 28.2 | 21.80 | 0.402 | 0.547 | 625 | 39.1 | 342 | 0.773 | 5.70 | 87.4 | 0.79 |
| PRP | R (C&Ei) | A (Pri) | S (J&Di) | TMd (Fi) | TMh (Fi) | K (J&Di) | B (J&Di) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IT_1 | 0.896 | 0.266 | ↘↘ | 0.783 | ↗ | 0.314 | ↘ | 0.156 | ↘↘ | 1.219 | → | 4.507 | ↘ |
| BG_2 | 0.676 * | 0.385 | ↘ | 0.594 | → | 0.252 | ↘↘ | 0.147 | ↘↘ | 1.761 | ↗ | 5.075 | ↘ |
| SK_3 | 0.931 | 0.530 | → | 0.758 | ↗ | 0.220 | ↘↘ | 0.188 | ↘↘ | 1.490 | → | 4.693 | ↘ |
| CZ_4 | 1.206 | 0.426 | ↘ | 0.304 | ↘ | 0.163 | ↘↘ | 0.082 | ↘↘ | 0.714 | ↘ | 2.105 | ↘↘ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimková, M.; Vacek, S.; Šimůnek, V.; Vacek, Z.; Cukor, J.; Hájek, V.; Bílek, L.; Prokůpková, A.; Štefančík, I.; Sitková, Z.; et al. Turkey Oak (Quercus cerris L.) Resilience to Climate Change: Insights from Coppice Forests in Southern and Central Europe. Forests 2023, 14, 2403. https://doi.org/10.3390/f14122403
Šimková M, Vacek S, Šimůnek V, Vacek Z, Cukor J, Hájek V, Bílek L, Prokůpková A, Štefančík I, Sitková Z, et al. Turkey Oak (Quercus cerris L.) Resilience to Climate Change: Insights from Coppice Forests in Southern and Central Europe. Forests. 2023; 14(12):2403. https://doi.org/10.3390/f14122403
Chicago/Turabian StyleŠimková, Michaela, Stanislav Vacek, Václav Šimůnek, Zdeněk Vacek, Jan Cukor, Vojtěch Hájek, Lukáš Bílek, Anna Prokůpková, Igor Štefančík, Zuzana Sitková, and et al. 2023. "Turkey Oak (Quercus cerris L.) Resilience to Climate Change: Insights from Coppice Forests in Southern and Central Europe" Forests 14, no. 12: 2403. https://doi.org/10.3390/f14122403





