Variations in Physiological and Biochemical Characteristics of Kalidium foliatum Leaves and Roots in Two Saline Habitats in Desert Region
Abstract
:1. Introduction
2. Materials and Methods
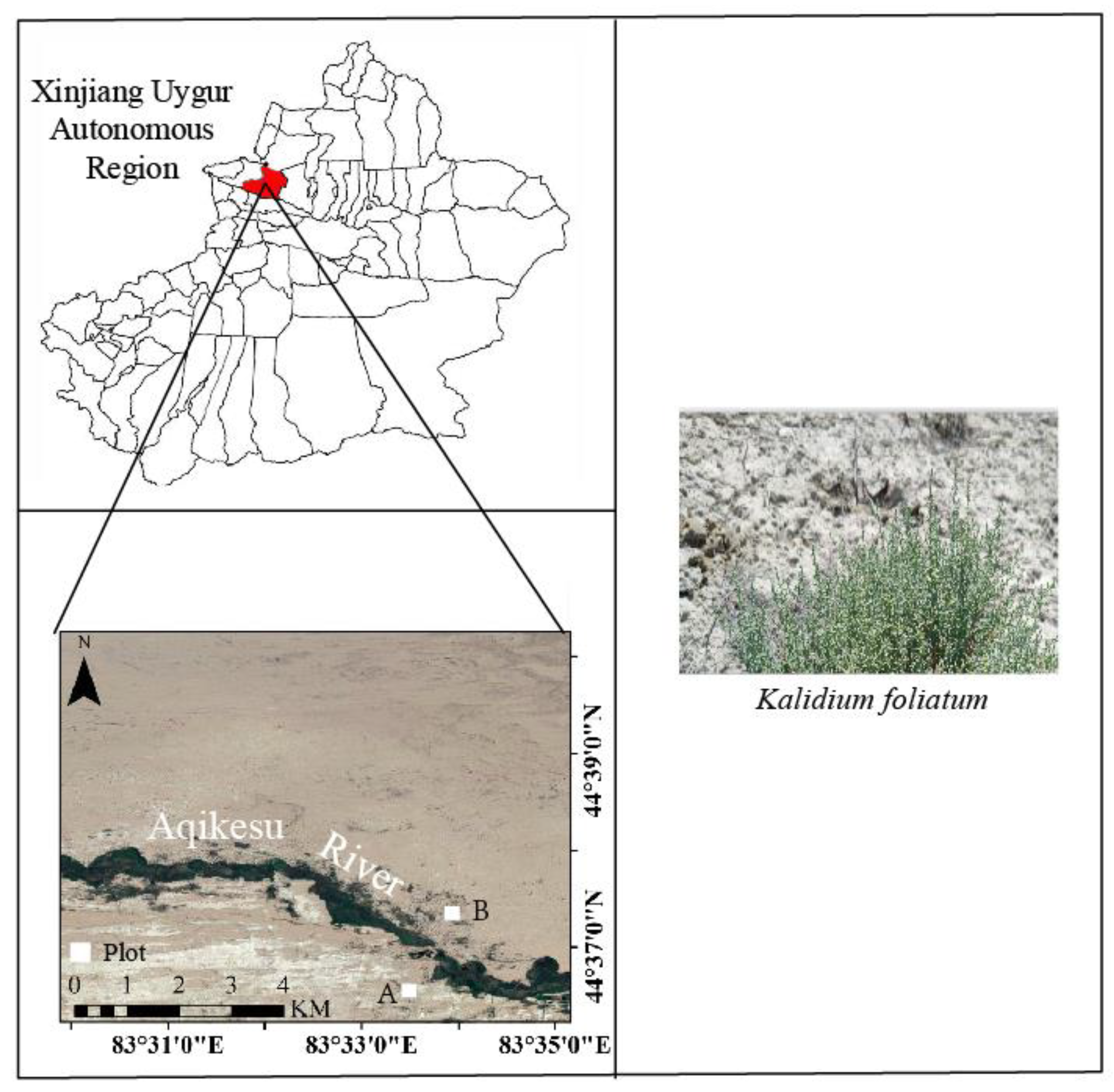
2.1. Overview of the Research Area
2.2. Sample Site Layout and Sample Collection
2.2.1. Layout of Sample Plots
2.2.2. Sample Collection
2.2.3. Sample Determination
2.3. Data Analysis Methods
3. Results and Analysis
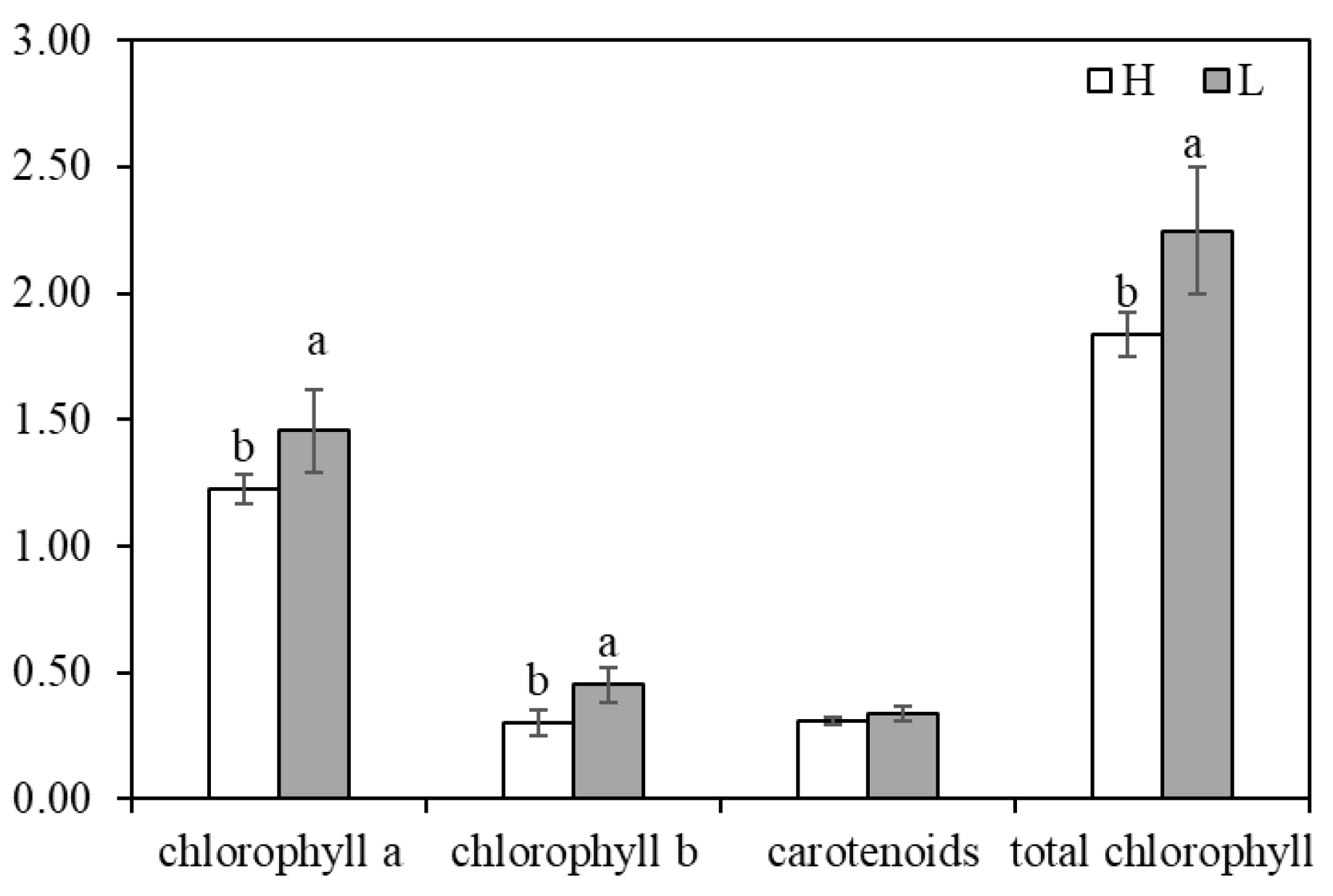
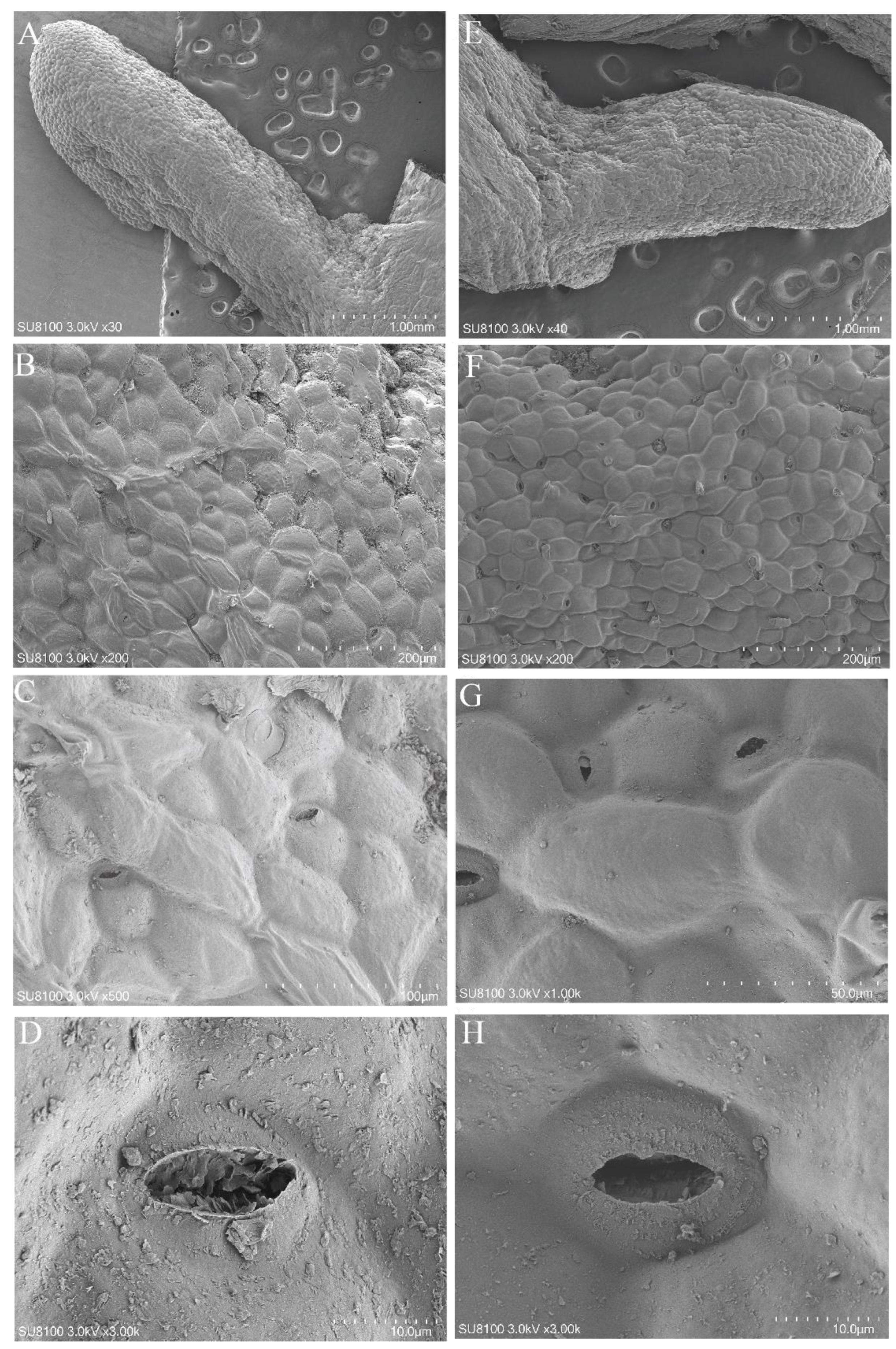
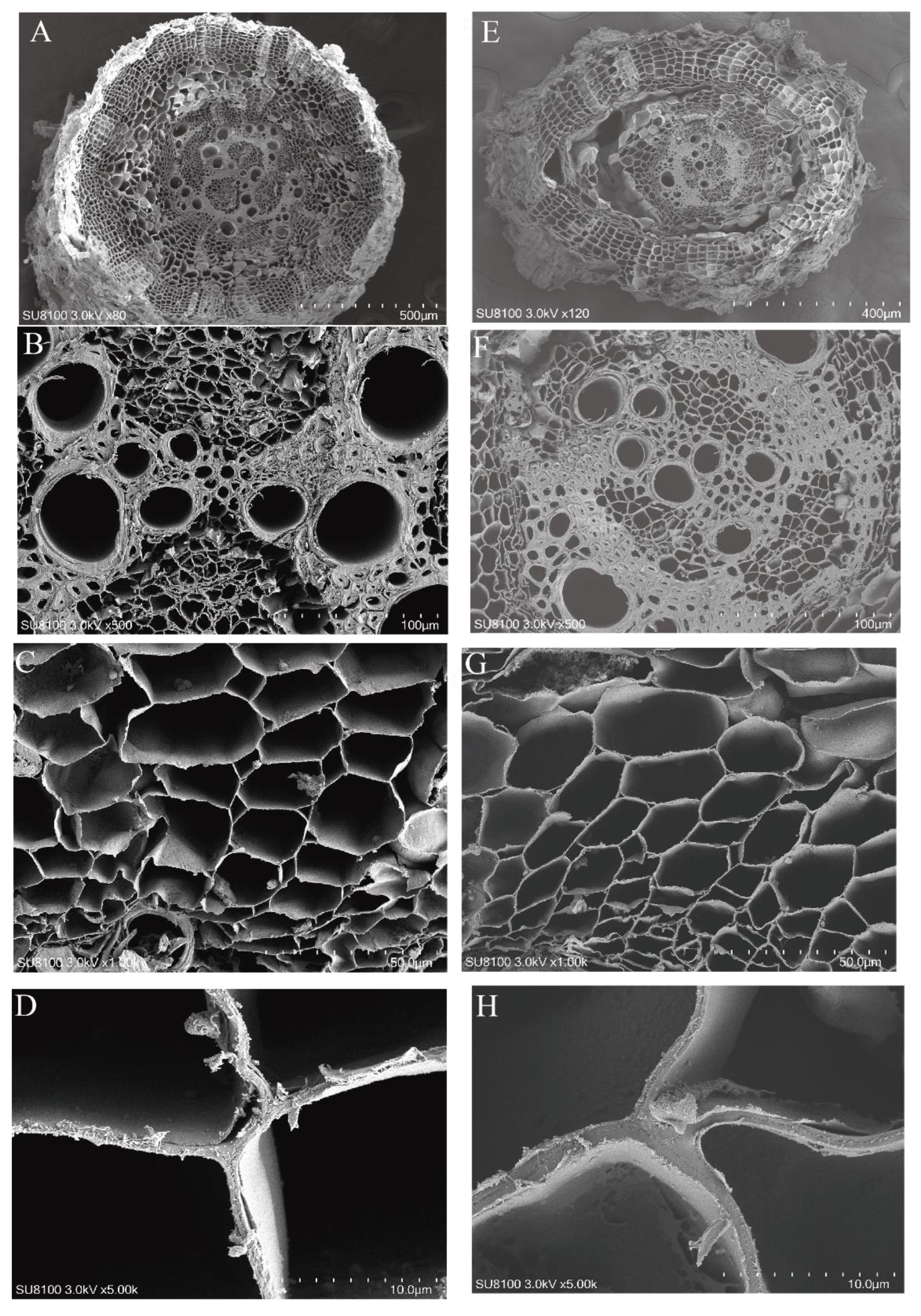
3.1. Differences in Chlorophyll, Leaf, and Root Anatomical Structures of K. foliatum under Two Saline Habitats
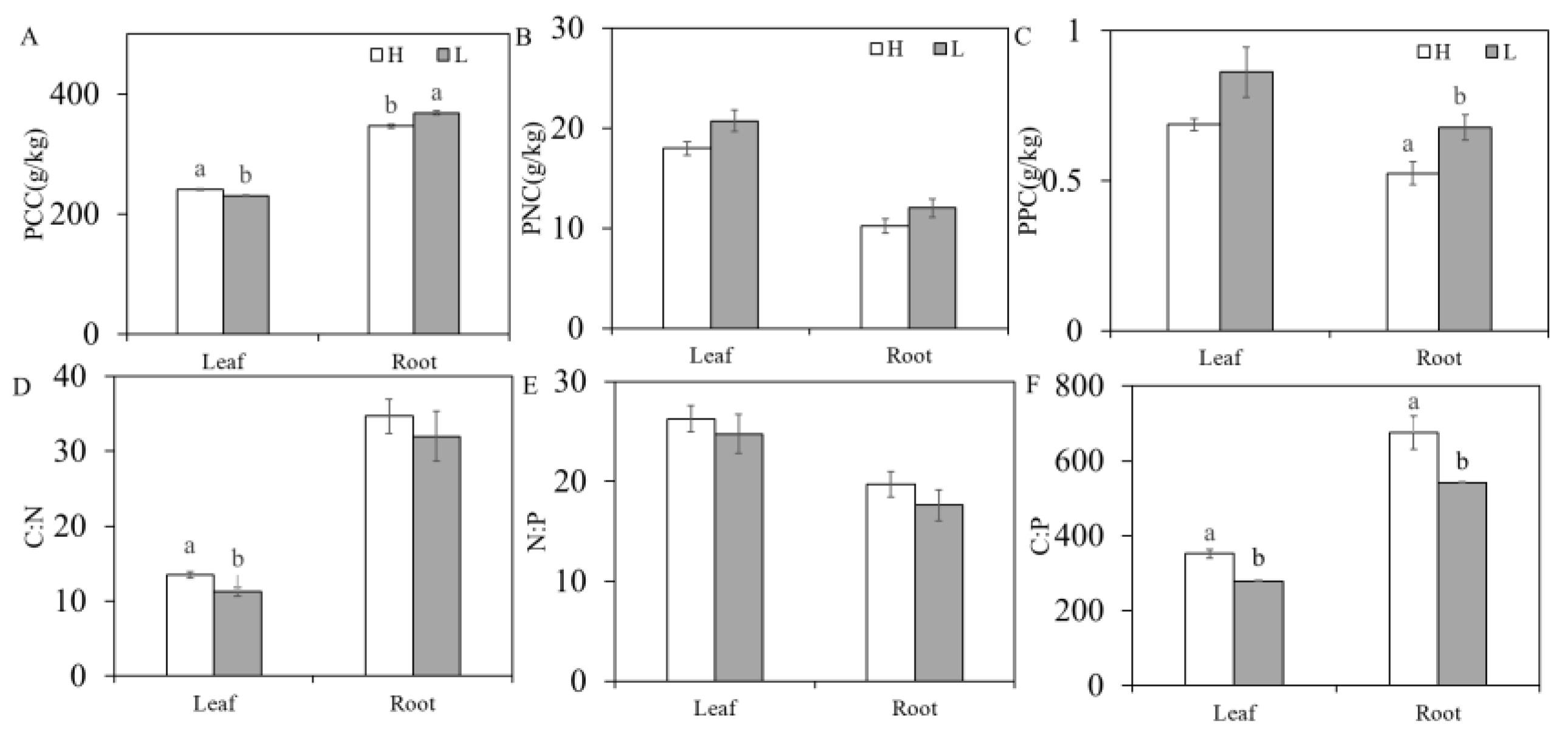
3.2. Differences in Nutrient Contents and Stoichiometric Ratios of K. foliatum Leaves and Roots in Two Saline Habitats
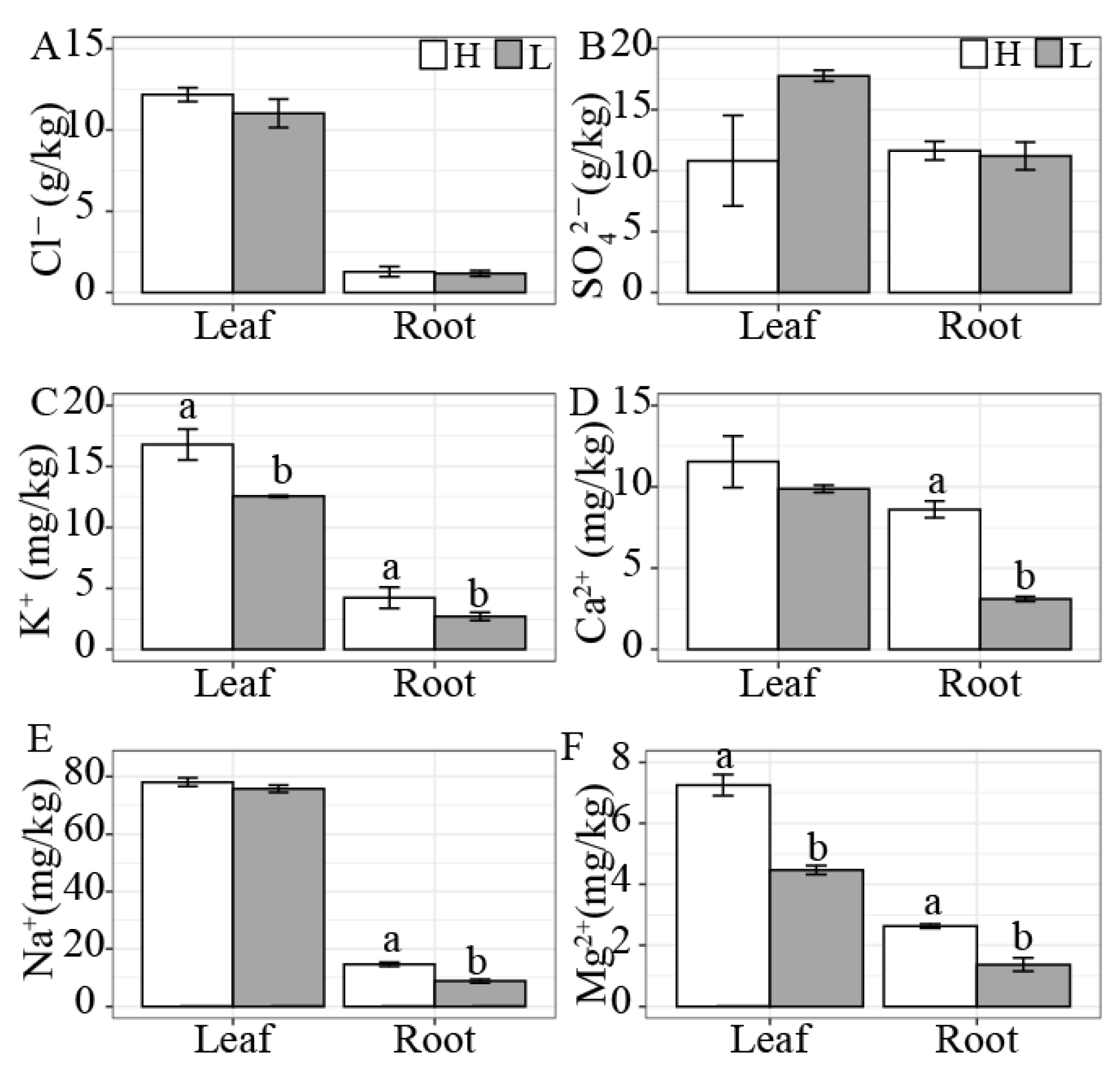
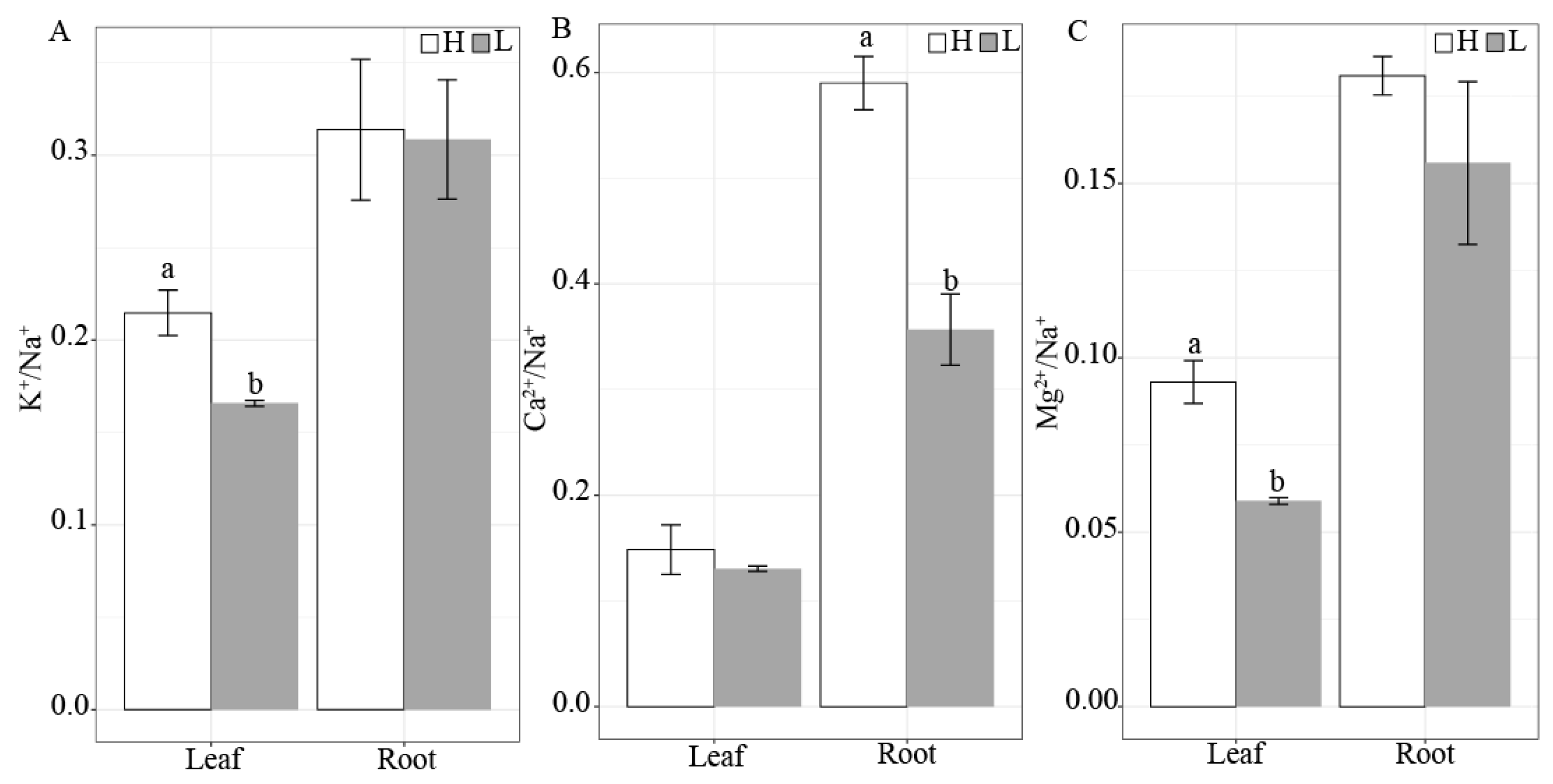
3.3. Differences in Inorganic Ion Contents and Stoichiometric Ratios between K. foliatum Leaves and Roots under Two Different Salt Habitats
3.4. Differences in Osmoregulation Substances in K. foliatum Leaves and Roots under Two Different Salt Habitats
3.5. Differences in Antioxidant and Membrane Lipid Peroxidation Substances in K. foliatum Leaves and Roots under Two Different Salt Habitats
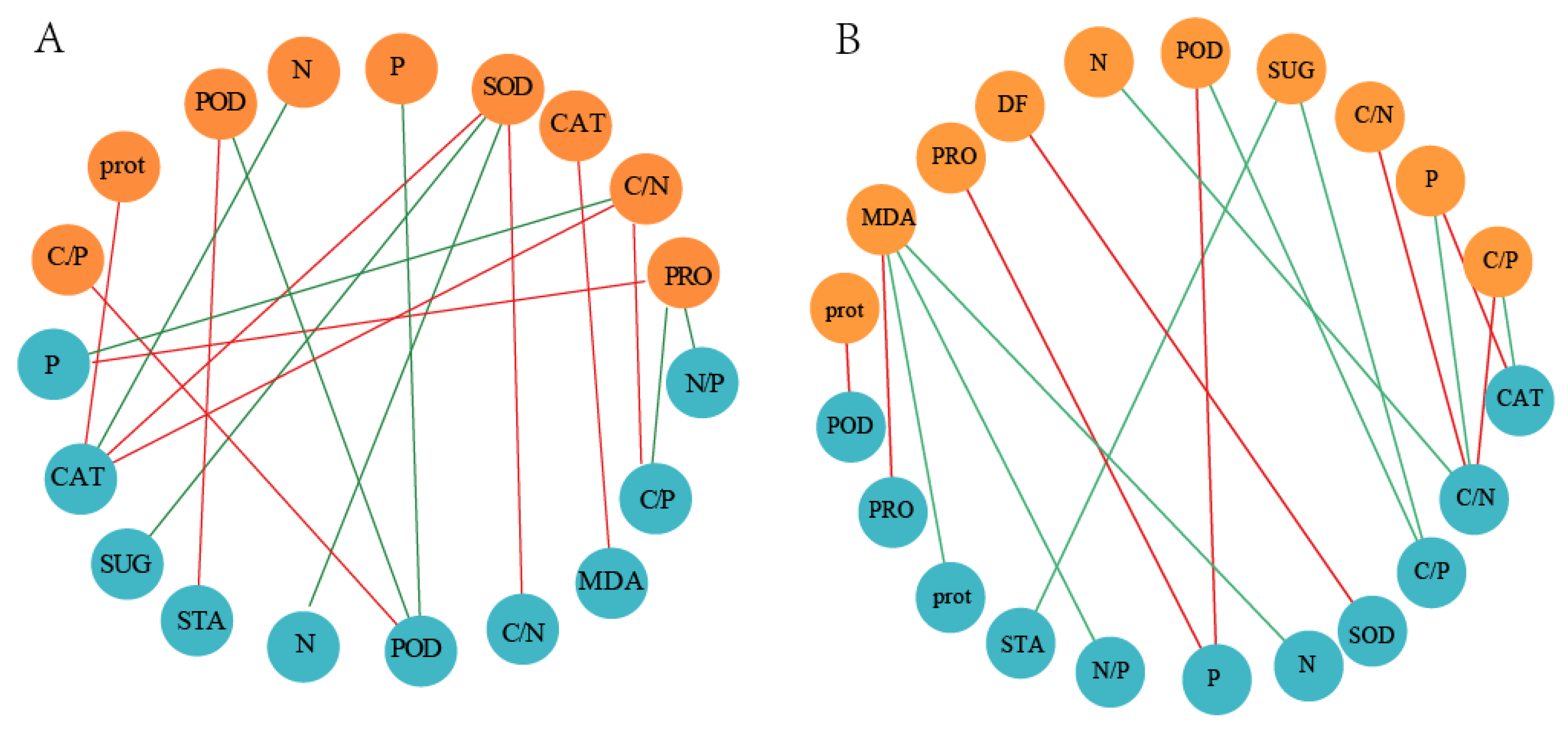
3.6. Network Association of Physiological Traits of K. foliatum Leaves and Roots in Two Salt Habitats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of plant saline-alkaline tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Ghazali, G.E. Suaeda vermiculata Forssk. ex J.F. Gmel.: Structural characteristics and adaptations to salinity and drought: A review. Int. J. Sci. 2020, 9, 28–33. [Google Scholar]
- Neel, J.P.S.; Alloush, G.A.; Belesky, D.P.; Clapham, W.M. Influence of rhizosphere ionic strength on mineral composition, dry matter yield and nutritive value of forage chicory. J. Agron. Crop Sci. 2002, 188, 398–407. [Google Scholar] [CrossRef]
- Teng, X.X.; Cao, W.L.; Lan, H.X.; Tang, H.J.; Bao, Y.M.; Zhang, H.S. OsNHX2, an Na+/H+ antiporter gene, can enhance salt tolerance in rice plants through more effective accumulation of toxic Na in leaf mesophyll and bundle sheath cells. Acta Physiol. Plant. 2017, 39, 113. [Google Scholar] [CrossRef]
- Liu, J.; Gao, H.; Wang, X.; Zheng, Q.; Wang, C.; Wang, X.; Wang, Q. Effects of 24-epibrassinolide on plant growth, osmotic regulation and ion homeostasis of salt-stressed canola. Plant Biol. 2014, 16, 440–450. [Google Scholar] [CrossRef]
- Rong, Q.; Liu, J.; Cai, Y.; Lu, Z.; Zhao, Z.; Yue, W.; Xia, J. Leaf carbon, nitrogen and phosphorus stoichiometry of Tamarix chinensis Lour. in the Laizhou Bay coastal wetland, China. Ecol. Eng. J. Ecotechnol. 2015, 76, 57–65. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Seki, M.; Ishida, J.; Narusaka, M.; Fujita, M.; Nanjo, T.; Umezawa, T.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T. Monitoring the expression pattern of around 7000 Arabidopsis genes under aba treatments using a full-length cDNA microarray. Funct. Integr. Genom. 2002, 2, 282–291. [Google Scholar] [CrossRef]
- Theerawitaya, C.; Boriboonkaset, T.; Cha-Um, S.; Supaibulwatana, K.; Kirdmanee, C. Transcriptional regulations of the genes of starch metabolism and physiological changes in response to salt stress rice (Oryza sativa L.) seedlings. Physiol. Mol. Biol. Plants 2012, 18, 197–208. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Henry, C.; Bledsoe, S.W.; Griffiths, C.A.; Paul, M.J.; Kollman, A.; SAKR, S.; Lagrimini, M. Differential role for trehalose metabolism in salt-stressed maize. Plant Physiol. 2015, 169, 1072–1089. [Google Scholar] [CrossRef]
- Oury, V.; Caldeira, C.F.; Prodhomme, D.; Pichon, J.-P.; Gibon, Y.; Tardieu, F.; Turc, O. Is change in ovary carbon status a cause or a consequence of maize ovary abortion in water deficit during flowering? Plant Physiol. 2016, 171, 997–1008. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Zhang, Q.; Dai, W. Plant Response to Salinity Stress. In Stress Physiology of Woody Plants; Dai, W., Ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Sonmez, O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res. Commun. 2018, 46, 67–78. [Google Scholar] [CrossRef]
- Grebosz, J.; Badowiec, A.; Weidner, S. Changes in the root proteome of Triticosecale grains germinating under osmotic stress. Acta Physiol. Plant. 2014, 36, 825–835. [Google Scholar] [CrossRef]
- Wang, G.; Jin, J. Effects of NaCl on physiology and leaf ultrastructure in the halophyte Kalidium foliatum. Nord. J. Bot. 2015, 33, 232–238. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Gu, M.Y.; Tang, Q.Y.; Chu, M.; Zhu, J.; Sun, J.; Yang, R.; Xu, W.L. Screening of Salt-tolerant and Growth-promoting Bacteria in the rhizosphere of Kalidium foliatum and the Functional Identification in Pot Experiments. J. Agric. Sci. Technol. 2021, 23, 186–192. [Google Scholar]
- Yang, X.-D.; Anwar, E.; Zhou, J.; He, D.; Gao, Y.-C.; Lv, G.-H.; Cao, Y.-E. Higher association and integration among functional traits in small tree than shrub in resisting drought stress in an arid desert. Environ. Exp. Bot. 2022, 201, 104993. [Google Scholar] [CrossRef]
- Gong, Y.; Ling, H.; Lv, G.; Chen, Y.; Guo, Z.; Cao, J. Disentangling the influence of aridity and salinity on community functional and phylogenetic diversity in local dryland vegetation. Sci. Total. Environ. 2019, 653, 409–422. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, D.; Wang, H.F.; Lv, G. Discriminating ecological processes affecting different dimensions of α- and β-diversity in desert plant communities. Ecol. Evol. 2022, 12, e8710. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agrochemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Harmut, A.J.M.E. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–383. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Khelil, A.; Menu, T.; Ricard, B. Adaptive response to salt involving carbohydrate metabolism in leaves of a salt-sensitive tomato cultivar. Plant Physiol. Biochem. 2007, 45, 551–559. [Google Scholar] [CrossRef]
- Lepesant, J.A.; Kunst, F.; Lepesant-Kejzlarová, J.; Dedonder, R. Chromosomal location of mutations affecting sucrose metabolism in Bacillus subtilis Marburg. Mol. Gen. Genet. 1972, 118, 135–160. [Google Scholar] [CrossRef]
- Gomes, H.T.; Bartos, P.M.C.; Silva, C.O.; Amaral, L.I.V.D.; Scherwinskipereira, J.E. Comparative biochemical profiling during the stages of acquisition and development of somatic embryogenesis in African oil palm (Elaeis guineensis Jacq.). Plant Growth Regul. 2014, 74, 199–208. [Google Scholar] [CrossRef]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Panda, A.; Rangani, J.; Kumari, A.; Parida, A.K. Efficient regulation of arsenic translocation to shoot tissue and modulation of phytochelatin levels and antioxidative defense system confers salinity and arsenic tolerance in the Halophyte Suaeda maritima. Environ. Exp. Bot. 2017, 143, 49–171. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Yang, J.F.; Zhang, X.N.; Song, D.H.; Tian, J.Y. Interspecific Integration of Chemical Traits in Desert Plant Leaves with Variations in Soil Water and Salinity Habitats. Forest 2023, 14, 1963. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Sack, L.; Xu, L.I.; Li, M.; Zhang, J.; He, N.; Penuelas, J. Leaf trait network architecture shifts with species-richness and climate across forests at continental scale. Ecol. Lett. 2022, 25, 1442–1457. [Google Scholar] [CrossRef]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martinez-Vilalta, J. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef]
- Kiani-Pouya, A.; Rasouli, F. The potential of leaf chlorophyll content to screen bread-wheat genotypes in saline condition. Photosynthetica 2014, 52, 288–300. [Google Scholar] [CrossRef]
- Wu, S.; Wang, J.; Yan, Z.; Wu, J. Monitoring tree-crown scale autumn leaf phenology in a temperate forest with an integration of Planet Scope and drone remote sensing observations. ISPRS J. Photogramm. Remote Sens. 2021, 171, 36–48. [Google Scholar] [CrossRef]
- Larcher, W. Photosynthesis as a tool for indicating temperature stress events. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Mohsin, S.M.; Fujita, M. Comparative physiological and biochemical changes in tomato (Solanum lycopersicum L.) under salt stress and recovery: Role of antioxidant defense and glyoxalase systems. Antioxidants 2019, 8, 350. [Google Scholar] [CrossRef]
- Gong, D.H.; Wang, G.Z.; Si, W.T.; Zhou, Y.; Liu, Y.; Jia, J. Effects of salt stress on photosynthetic pigments and activity of ribulose-1,5-bisphosphate carboxylase/oxygenase in Kalidium foliatum. Russ. J. Plant Physiol. 2018, 65, 98–103. [Google Scholar] [CrossRef]
- Rao, G.C.; Rao, G.R. Pigment composition and chlorophyllase activity in pigeon pea (Cajanus indicus Spreng) and Gingelley (Sesamum indicum L.) under NaCl salinity. Indian J. Exp. Biol. 1981, 19, 768–770. [Google Scholar]
- Kacharava, N.; Chanishvili, S.; Badridze, G.; Chkhubianishvili, E.; Janukashvili, N. Effect of seed irradiation on the content of antioxidants in leaves of Kidney bean, Cabbage and Beet cultivars. Aust. J. Crop Sci. 2009, 3, 137. [Google Scholar]
- Ashraf, M.; Ozturk, M.; Ahmad, M.S.A. Plant Adaptation and Phytoremediation: Structural and Functional Adaptations in Plants for Salinity Tolerance; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Luo, Y.; Peng, Q.; Li, K.; Gong, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- He, M.Z.; Zhang, K.; Tan, H.J.; Hu, R.; Su, J.Q.; Wang, J.; Huang, L.; Zhang, Y.F.; Li, X.R. Nutrient levels within leaves, stems, and roots of the xeric species Reaumuria soongoricain relation to geographical, climatic, and soil conditions. Ecol. Evol. 2015, 5, 1494–1503. [Google Scholar] [CrossRef]
- Thompson, K.E.N.; Parkinson, J.A.; Band, S.R.; Spencer, R.E. A comparative study of leaf nutrient concentrations in a regional herbaceous flora. New Phytol. 1997, 36, 679–689. [Google Scholar] [CrossRef]
- Sun, X.; Gao, Y.; Wang, D.; Chen, J.; Zhang, F.; Zhou, J.; Yan, X.; Li, Y.; Luo, Z.B. Stoichiometric variation of halophytes in response to changes in soil salinity. Plant Biol. 2017, 19, 360–367. [Google Scholar] [CrossRef]
- Balba, M.A. Management of Problem Soils in Arid Ecosystems; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Joshi, S.S. Effect of salinity stress on organic and mineral constituents in the leaves of pigeonpea (Cajanus cajan L. var C-11). Plant Soil 1984, 82, 69–76. [Google Scholar] [CrossRef]
- Ramoliya, P.J.; Patel, H.M.; Joshi, J.B.; Pandey, A.N. Effect of salinization of soil on growth and nutrient accumulation in seedlings of Prosopis cineraria. J. Plant Nutr. 2006, 29, 283–303. [Google Scholar] [CrossRef]
- Azzeme, A.M.; Abdullah, S.; Aziz, M.A.; Wahab, P.E.M. Oil palm leaves and roots differ in physiological response, antioxidant enzyme activities and expression of stress-responsive genes upon exposure to drought stress. Acta Physiol. Plant. 2016, 38, 52. [Google Scholar] [CrossRef]
- Barragan, V.; Leidi, E.O.; Andrés, Z.; LRubio Luca, A.D.; Fernandez, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F. Plasma membrane permeability as an indicator of salt tolerance in plants. Biol. Plant. 2012, 57, 1–10. [Google Scholar] [CrossRef]
- Patade, V.Y.; Bhargava, S.; Suprasanna, P. Effects of NaCl and iso-osmotic peg stress on growth, osmolytes accumulation and antioxidant defense in cultured sugarcane cells. Plant Cell Tissue Organ. Cult. 2012, 108, 279–286. [Google Scholar] [CrossRef]
- Wang, X.J.; Yang, D.; Yi, F.Y.; Zhang, Y.Y.; Saixiyala, B.S.; Sun, H.L. Research Progress on the Halophyte Kalidium foliatum and Its Resources Characteristics. Anim. Husb. Feed. Sci. 2015, 36, 64–67. [Google Scholar]
- Luo, D.; Wu, Z.B.; Shi, Y.J. Effects of salt stress on leaf analomical structure and ion absorption, transportation and distribution of three Ping’ ou hybrid hazelnut seedlings. Acta Ecol. Sin. 2022, 42, 1876–1888. [Google Scholar]
- Tang, X.Q.; Li, H.Y.; Yang, X.Y.; Liu, Z.X.; Zhang, H.X. Effects of short-time salt stress on distribution and balance of Na+ and K+ in Nitraria sibirica Pall. seedlings. For. Res. 2017, 30, 1022–1027. [Google Scholar]
- Lekklar, C.; Chadchawan, S.; Boon-Long, P.; Pfeiffer, W.; Chaidee, A. Salt stress in rice: Multivariate analysis separates four components of beneficial silicon action. Protoplasma 2019, 256, 331–347. [Google Scholar] [CrossRef]
- Ehsan, T.; Pichu, R.; Mcdonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 1364, 4159–4168. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388. [Google Scholar] [CrossRef]
- Wang, Y.; Stevanato, P.; Yu, L.; Zhao, H.J.; Sun, X.W.; Sun, F.; Li, J.; Geng, G. The physiological and metabolic changes in sugar beet seedlings under different levels of salt stress. J. Plant Res. 2017, 1306, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bi, S.T.; Liu, C.Y.; Li, M.; He, M. Arabidopsis ADF7 inhibits VLN1 to regulate actin filament dynamics and ROS accumulation in root hair development responses to osmotic stress. Authorea 2021, 2. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.Z.; Li, F.; Yan, C.; Zhong, X.; Qi, L.; Xu, X.; Li, H.; Long, Z. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Afefe, A.A.; Khedr, A.; Abbas, M.S.; Soliman, A. Responses and tolerance mechanisms of mangrove trees to the ambient salinity along the Egyptian red sea coast. Limnol. Rev. 2021, 21, 3–13. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bhowmik, S.D.; Long, H.; Cheng, Y.; Mundree, S.; Mundree, S.; Hoang, L.T.M. Rapid accumulation of proline enhances salinity tolerance in Australian wild rice Oryza australiensis domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Kumar, A.; Yadav, R.K.; Yadav, T.; Kushwaha, M. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J. Biol. Sci. 2020, 27, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Chen, T.S.; Fei, G.Q.; Zhang, M.X.; An, L.Z. Effects of Salinity on the Contents of Osmotica of Three Desert Plants. J. Desert Res. 2007, 27, 787–790. [Google Scholar]
- Watanabe, S.; Kojima, K.; Ide, Y.; Sasaki, S. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ. Cult. 2000, 63, 199–206. [Google Scholar] [CrossRef]
- Liu, S.G.; Zhu, D.Z.; Chen, G.H.; Gao, X.Q.; Zhang, X.S. Disrupted actin dynamics trigger an increment in the reactive oxygen species levels in the Arabidopsis root under salt stress. Plant Cell Rep. 2012, 31, 1219–1226. [Google Scholar] [CrossRef]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; Van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wen, Z.N.; Cong, S.H. Comparisons of relationships between leaf and fine root traits in hilly are of the loess Plateau, Yanne Biver basin. Acta Ecol. Sin. 2011, 31, 6805–6814. [Google Scholar]
- Wang, Q.; Wu, C.; Xie, B.; Liu, Y.; Cui, J.; Chen, G.; Zhang, Y. Model analysing the antioxidant responses of leaves and roots of switchgrass to NaCl-salinity stress. Plant Physiol. Biochem. 2012, 58, 288–296. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Compari-sons of structure and life span in roots and leaves amongtemperate trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Flores-Moreno, H.; Fazayeli, F.; Banerjee, A.; Datta, A.; Kattge, J.; Butler, E.E.; Atkin, O.K.; Wythers, K.; Chen, M.; Anand, M.; et al. Robustness of trait connections across environmental gradients and growth forms. Glob. Ecol. Biogeogr. 2019, 28, 1806–1826. [Google Scholar] [CrossRef]
- Liu, C.C.; Li, Y.; Xu, L.; Chen, Z.; He, N.P. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef]
- Laughlin D, C. The intrinsic dimensionality of plant traits and its relevance to community assembly. J. Ecol. 2014, 102, 186–193. [Google Scholar] [CrossRef]


| H | L | |
|---|---|---|
| Soil water content (%) | 15.25 ± 0.61 | 12.05 ± 0.18 |
| Soil Organic carbon content (g/kg) | 3.93 ± 0.19 | 2.84 ± 0.13 |
| Soil total nitrogen content (g/kg) | 0.64 ± 0.16 | 0.48 ± 0.02 |
| Soil total phosphorus content (g/kg) | 0.53 ± 0.01 | 0.51 ± 0.01 |
| pH | 8.33 ± 0.04 | 8.00 ± 0.03 |
| Soil ammonium nitrogen content (mg/kg) | 4.47 ± 0.14 | 5.03 ± 0.17 |
| Soil nitrate nitrogen content (mg/kg) | 28.33 ± 0.80 | 34.24 ± 0.63 |
| Soil available phosphorus content (mg/kg) | 23.96 ± 0.86 | 22.57 ± 1.04 |
| Index | High-Salt Habitat | Low-Salt Habitat |
|---|---|---|
| Leaf thickness (μm) | 1325.82 ± 101.53 a | 1262.14 ± 23.87 a |
| Upper-epidermis thickness (μm) | 44.53 ± 2.38 a | 33.48 ± 1.69 b |
| Lower-epidermis thickness (μm) | 46.42 ± 1.59 a | 35.44 ± 2.1 b |
| Length of upper epidermis (μm) | 44.34 ± 3.79 a | 38.31 ± 3.29 a |
| Length of lower epidermis (μm) | 53.87 ± 4.90 a | 41.36 ± 3.17 b |
| Palisade tissue thickness (μm) | 23.40 ± 1.24 a | 17.11 ± 1.20 b |
| Vascular bundle aperture (μm) | 7.71 ± 0.40 a | 5.97 ± 0.31 b |
| Thickness ratio of palisade (%) | 3.60 ± 0.15 a | 2.83 ± 0.24 b |
| Name | Type | Degree | Weight Degree |
|---|---|---|---|
| C/N | leaf | 3 | 2.600 |
| C/P | leaf | 1 | 0.829 |
| CAT | leaf | 1 | 0.886 |
| prot | leaf | 1 | 1.000 |
| N | leaf | 1 | 0.829 |
| P | leaf | 1 | 0.829 |
| POD | leaf | 2 | 1.771 |
| PRO | leaf | 3 | 2.657 |
| SOD | leaf | 4 | 3.543 |
| CAT | root | 4 | 3.600 |
| C/N | root | 1 | 0.886 |
| C/P | root | 2 | 1.714 |
| STA | root | 1 | 0.943 |
| MDA | root | 1 | 0.886 |
| N | root | 1 | 0.886 |
| N/P | root | 1 | 0.886 |
| P | root | 2 | 1.710 |
| POD | root | 3 | 2.486 |
| SUG | root | 1 | 0.943 |
| Name | Type | Degree | Weight Degree |
|---|---|---|---|
| C/N | leaf | 1 | 0.829 |
| C/P | leaf | 2 | 1.714 |
| prot | leaf | 1 | 0.829 |
| STA | leaf | 1 | 0.829 |
| MDA | leaf | 4 | 3.599 |
| N | leaf | 1 | 0.829 |
| P | leaf | 2 | 1.714 |
| POD | leaf | 2 | 1.771 |
| PRO | leaf | 1 | 0.886 |
| SUG | leaf | 2 | 1.829 |
| CAT | root | 2 | 1.771 |
| C/N | root | 4 | 3.314 |
| CP | root | 2 | 1.771 |
| protein | root | 1 | 0.942 |
| STA | root | 1 | 0.886 |
| N | root | 1 | 0.886 |
| N/P | root | 1 | 0.829 |
| P | root | 2 | 1.829 |
| POD | root | 1 | 0.829 |
| PRO | root | 1 | 0.943 |
| SOD | root | 1 | 0.829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wu, D.; Li, W.; Liu, Y.; Li, E.; Li, X.; Yang, G.; He, X. Variations in Physiological and Biochemical Characteristics of Kalidium foliatum Leaves and Roots in Two Saline Habitats in Desert Region. Forests 2024, 15, 148. https://doi.org/10.3390/f15010148
Jiang L, Wu D, Li W, Liu Y, Li E, Li X, Yang G, He X. Variations in Physiological and Biochemical Characteristics of Kalidium foliatum Leaves and Roots in Two Saline Habitats in Desert Region. Forests. 2024; 15(1):148. https://doi.org/10.3390/f15010148
Chicago/Turabian StyleJiang, Lamei, Deyan Wu, Wenjing Li, Yuehan Liu, Eryang Li, Xiaotong Li, Guang Yang, and Xuemin He. 2024. "Variations in Physiological and Biochemical Characteristics of Kalidium foliatum Leaves and Roots in Two Saline Habitats in Desert Region" Forests 15, no. 1: 148. https://doi.org/10.3390/f15010148
APA StyleJiang, L., Wu, D., Li, W., Liu, Y., Li, E., Li, X., Yang, G., & He, X. (2024). Variations in Physiological and Biochemical Characteristics of Kalidium foliatum Leaves and Roots in Two Saline Habitats in Desert Region. Forests, 15(1), 148. https://doi.org/10.3390/f15010148





