Agroforestry Systems of Cocoa (Theobroma cacao L.) in the Ecuadorian Amazon
Abstract
1. Introduction
2. Materials and Methods
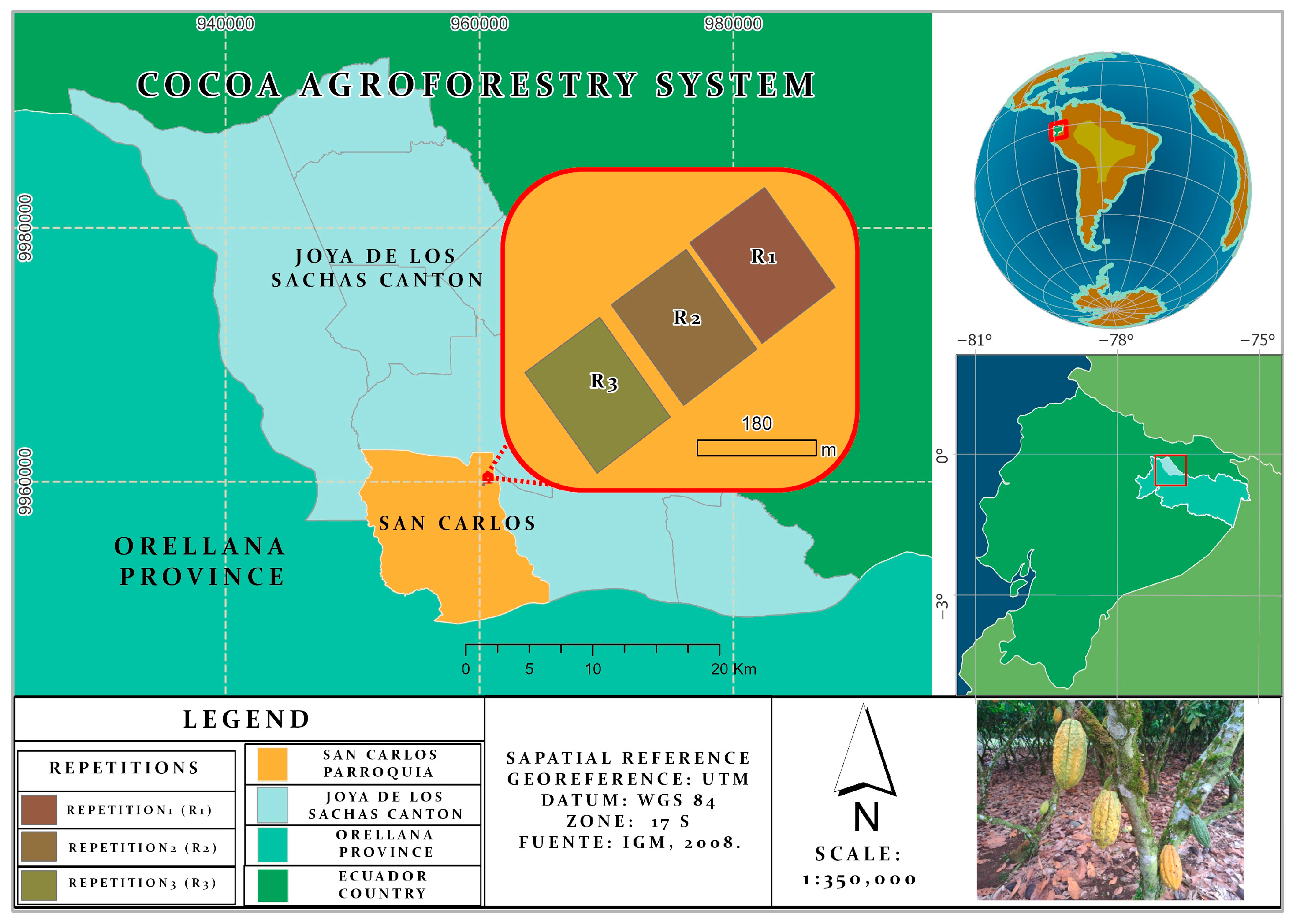
2.1. Experimental Site
2.2. Experimental Treatments
2.3. Crop Management
2.4. Study Variables
2.4.1. Cocoa Yield
2.4.2. Concentration of Nutrients in Leaf Biomass
2.4.3. Soil Nutrient Concentration
2.4.4. Estimation of Carbon Storage
2.4.5. Number of Earthworms
2.5. Data Analysis
3. Results
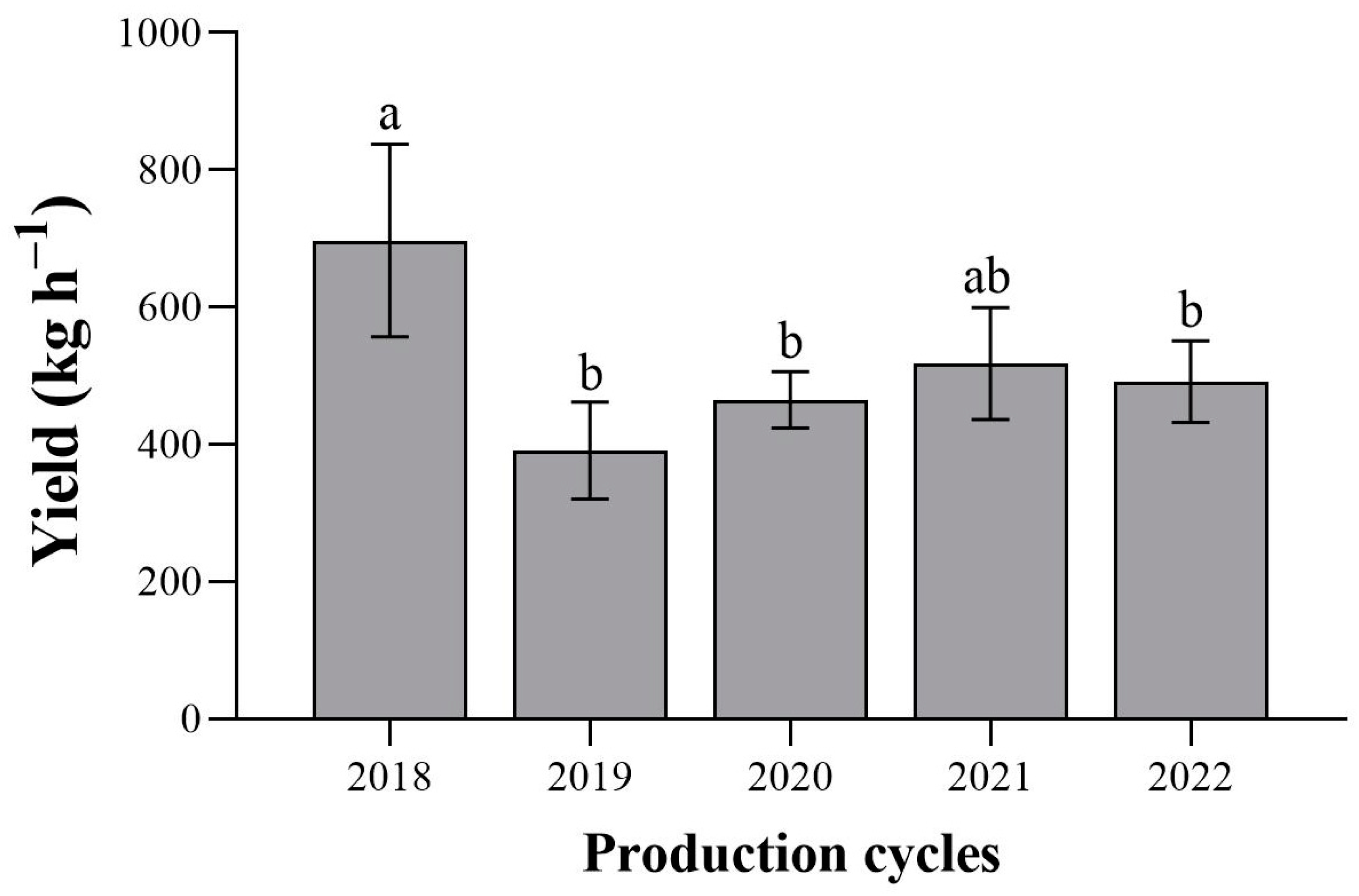
3.1. Cocoa Yield
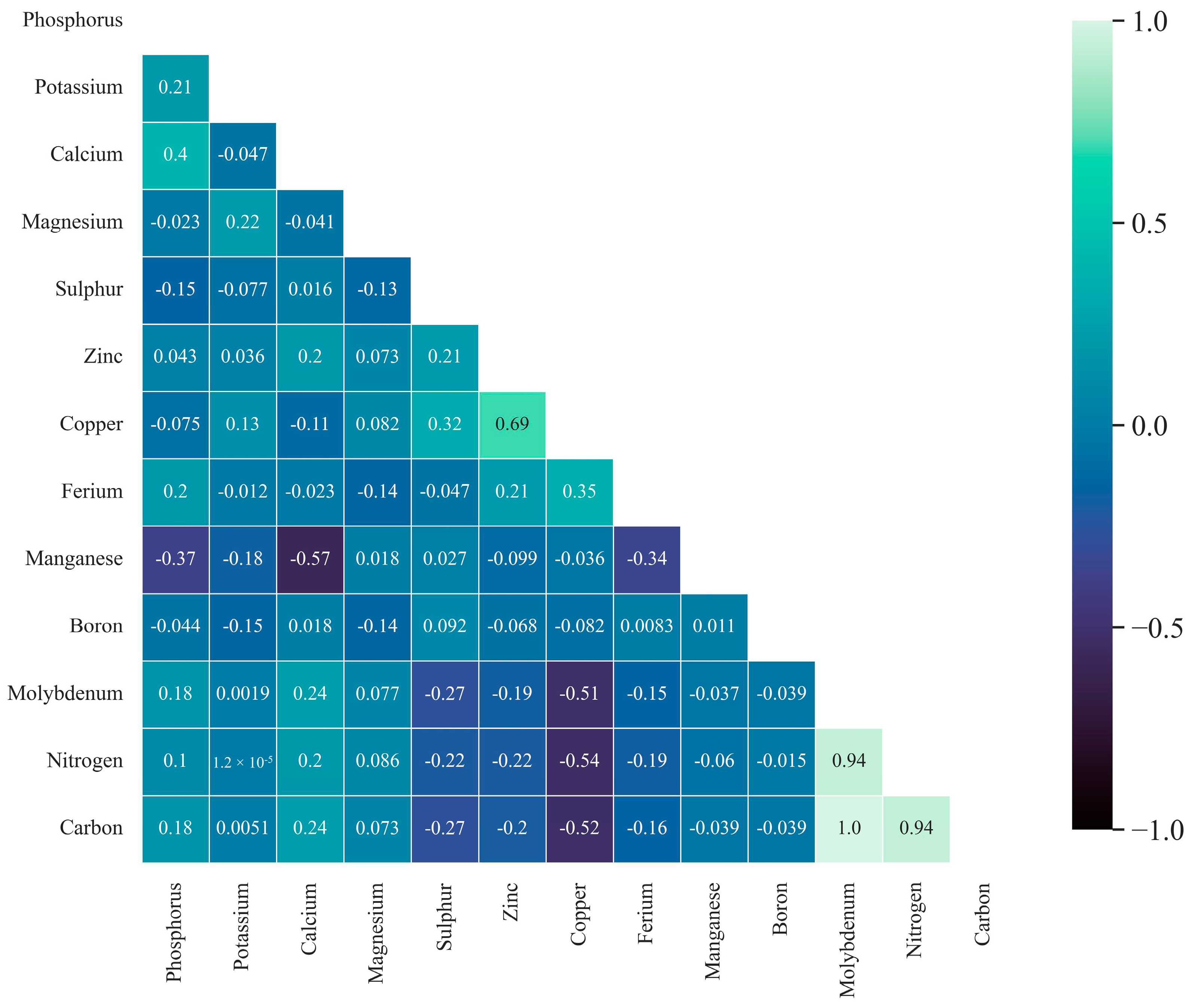
3.2. Nutrients Influencing Cocoa Yield
3.3. Total Carbon Stored
3.4. Abundance of Earthworms
3.5. Nutrients Influencing Earthworm Abundance
4. Discussion
4.1. Cacao Yield
4.2. Nutrients Influencing Cocoa Yield
4.3. Total Carbon Stored
4.4. Abundance of Earthworms
4.5. Nutrients Influencing Earthworm Abundance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Pimentel, M.H. Food, Energy, and Society, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-4668-7. [Google Scholar]
- Mutengwa, C.S.; Mnkeni, P.; Kondwakwenda, A. Climate-smart agriculture and food security in Southern Africa: A Review of the Vulnerability of Smallholder Agriculture and Food Security to Climate Change. Sustainability 2023, 15, 2882. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Is there a need for a more sustainable agriculture? Crit. Rev. Plant Sci. 2011, 30, 6–23. [Google Scholar] [CrossRef]
- Arizpe, N.; Giampietro, M.; Ramos-Martin, J. Food security and fossil energy dependence: An international comparison of the use of fossil energy in agriculture (1991–2003). Crit. Rev. Plant Sci. 2011, 30, 45–63. [Google Scholar]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Vergara-Camus, L.; Kay, C. The agrarian political economy of left-wing governments in latin america: Agribusiness, peasants, and the limits of neo-developmentalism. J. Agrar. Chang. 2017, 17, 415–437. [Google Scholar] [CrossRef]
- Toledo, L.; Salmoral, G.; Viteri-Salazar, O. Rethinking agricultural policy in Ecuador (1960–2020): Analysis Based on the Water–Energy–Food Security Nexus. Sustainability 2023, 15, 12850. [Google Scholar]
- Von Bennewitz, E. Land tenure in Latin America: From land reforms to counter-movement to Neoliberalism. Acta Univ. Agric. Silvic. Mendel. Brun. 2017, 65, 1793–1798. [Google Scholar]
- Caicedo Vargas, C.E. Sistemas Agroforestales con cacao (Theobroma cacao L.), en la Amazonía Ecuatoriana: Un Enfoque Agroecológico. Master’s Thesis, Universidad Internacional de Andalucía, Seville, Spain, 2019. [Google Scholar]
- Larrea Maldonado, C.A.; Arroyo, L.M. ¿Está Agotado el Periodo Petrolero en Ecuador? Alternativas Hacia una Sociedad más Sustentable y Equitativa: Un Estudio Multicriterio; La Tierra. Universidad Andina Simón Bolívar, Sede Ecuador: Quito, Ecuador, 2017; ISBN 978-9942-75-101-0. [Google Scholar]
- Caicedo, C. Agroforestería: Una alternativa de agricultura sostenible en la Amazonía Ecuatoriana. Ecuad. Es Calid. 2020, 7, 17–20. [Google Scholar]
- Viera, W.; Díaz, A.; Caicedo, C.; Suárez, A.; Vargas, Y. Key Agronomic fertilization practices that influence yield of naranjilla (Solanum quitoense Lam.) in the Ecuadorian Amazon. Agronomy 2021, 11, 310. [Google Scholar] [CrossRef]
- Diéguez-Santana, K.; Sarduy-Pereira, L.B.; Sablón-Cossío, N.; Bautista-Santos, H.; Sánchez-Galván, F.; Ruíz Cedeño, S. del M. Evaluation of the circular economy in a pitahaya agri-food Chain. Sustainability 2022, 14, 2950. [Google Scholar] [CrossRef]
- Yasin, G.; Nawaz, M.; Siddiqui, M.T.; Niazi, N. Biomass, carbon stocks and CO2 sequestration in three different aged irrigated Populus deltoides Bartr. Ex Marsh. bund planting agroforestry systems. Appl. Ecol. Environ. Res. 2018, 16, 6239–6252. [Google Scholar]
- Vargas-Tierras, Y.; Díaz, A.; Caicedo, C.; Macas, J.; Suárez-Tapia, A.; Viera, W. Benefits of Legume Species in an Agroforestry Production System of Yellow Pitahaya in the Ecuadorian Amazon. Sustainability 2021, 13, 9261. [Google Scholar] [CrossRef]
- Mendoza-Meneses, C.J.; Feregrino-Pérez, A.A.; Guevara-González, R.G.; García-Trejo, J.F. Implementation of pre-harvest techniques in emerging agroforestry systems to increase the yield of cocoa tree (Theobroma cacao L.). Heliyon 2023, 9, e14542. [Google Scholar] [CrossRef] [PubMed]
- De Melo Virginio Filho, E.; Vargas, C.; Astorga, C.; Bastidas, F.; Caicedo Albán, W.; Criollo, N.; Congo Yépez, C.; Chávez, J.; Díaz, A.; Fernández, F.; et al. Agroforestería Sostenible en la Amazonía Ecuatoriana; 398; CATIE: Turrialba, Costa Rica, 2014; ISBN 978-9977-57-623-7. [Google Scholar]
- Vargas, Y.; Viera, W.; Díaz, A.; Tinoco, L.; Macas, J.; Caicedo, C.; Almeida, M.; Vásquez-Castillo, W. Contribution of agroforestry systems in the cultivation of naranjilla (Solanum quitoense) grown in the Amazon Region of Ecuador. Appl. Sci. 2022, 12, 10637. [Google Scholar] [CrossRef]
- Salazar, O.V.; Latorre, S.; Godoy, M.Z.; Quelal-Vásconez, M.A. The challenges of a sustainable cocoa value chain: A study of traditional and “Fine or Flavour” cocoa produced by the kichwas in the Ecuadorian Amazon Region. J. Rural Stud. 2023, 98, 92–100. [Google Scholar] [CrossRef]
- Banco Central del Ecuador [BCE]. Evolución de la Balanza Comercial por Productos Enero–Diciembre 2022. Available online: https://www.bce.fin.ec/informacioneconomica/sector-externo (accessed on 23 October 2023).
- Corporación Financiera Nacional [CFN]. Ficha Sectorial: Cacao y Chocolate. Available online: https://www.cfn.fin.ec/ (accessed on 17 January 2019).
- Instituto Nacional de Estadísticas y Censos Estadísticas Agropecuarias. Available online: https://www.ecuadorencifras.gob.ec/estadisticas-agropecuarias-2/ (accessed on 23 October 2023).
- Caicedo-Vargas, C.; Pérez-Neira, D.; Abad-González, J.; Gallar, D. Assessment of the environmental impact and economic performance of cacao agroforestry systems in the Ecuadorian Amazon Region: An LCA Approach. Sci. Total Environ. 2022, 849, 157795. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, L.A.; Vargas Tierras, Y.B. Sistemas Agroforestales de Cacao: Revisión de Literatura Sobre el Efecto de la Sombra en la Producción de Theobroma cacao L. In Proceedings of the 1st Congreso Internacional Alternativas Tecnológicas para la Producción Agropecuaria Sostenible en la Amazonía Ecuatoriana, Orellana, Ecuador, 21–23 November 2018; INIAP: Joya de los Sachas, Ecuador, 2018; pp. 1–7. [Google Scholar]
- Kaba, J.S.; Yamoah, F.A.; Acquaye, A. towards sustainable agroforestry management: Harnessing the nutritional soil value through cocoa mix waste. Waste Manag. 2021, 124, 264–272. [Google Scholar] [CrossRef]
- Basha, A.M.; Yasovardhan, N.; Satyanarayana, S.V.; Reddy, G.V.S.; Vinod Kumar, A. trace metals in vegetables and fruits cultivated around the surroundings of tummalapalle uranium mining site, Andhra Pradesh, India. Toxicol. Rep. 2014, 1, 505–512. [Google Scholar]
- Jadán, O.; Torres, B.; Günter, S. Influencia del uso de la tierra sobre almacenamiento de carbono en sistemas productivos y bosque primario en Napo, Reserva de Biosfera Sumaco, Ecuador. Rev. Amaz. Cienc. Tecnol. 2012, 1, 173–184. [Google Scholar] [CrossRef]
- Grefa Grefa, I.K.; Aguinda Licuy, E.G. Estimación de carbono en plantaciones de Cedrelinga catenaeformis D. Duke, Cordia alliodora (Ruíz/Pav.) Oken y Tabebuia Donnell—Smithii Rose en La Hacienda Los Laureles, Cantón Archidona. Bachelor’s Thesis, Universidad Estatal Amazónica, Puyo, Ecuador, 2020. [Google Scholar]
- Del Águila Martínez, C. Secuestro de CO2 y Almacenamiento de Carbono en Plantaciones de Cedrelinga cateniformis Ducke “tornillo” en tres Edades Diferentes en el CIEFOR—Puerto Almendra, río Nanay, Iquitos–Perú. Bachelor’s Thesis, Escuela de Formacion Profesional de Ingenieria en Ecologia de Bosques Tropicales, Iquitos, Peru, 2014. [Google Scholar]
- Somarriba, E.; Cerda, R.; Orozco, L.; Cifuentes, M.; Dávila, H.; Espin, T.; Mavisoy, H.; Ávila, G.; Alvarado, E.; Poveda, V.; et al. Carbon stocks and cocoa yields in agroforestry systems of Central America. Agric. Ecosyst. Environ. 2013, 173, 46–57. [Google Scholar]
- Afele, J.T.; Dawoe, E.; Abunyewa, A.A.; Afari-Sefa, V.; Asare, R. Carbon storage in cocoa growing systems across different agroecological zones in Ghana. Pelita Perkeb. 2021, 37. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Cardinael, R.; Hoeffner, K.; Chenu, C.; Chevallier, T.; Camille, B.; Dewisme, A.; Cluzeau, D. Spatial Variation of Earthworm communities and soil organic carbon in temperate agroforestry. Biol. Fertil. Soils 2019, 55, 171–183. [Google Scholar]
- Zhang, H.; Schrader, S. Earthworm effects on selected physical and chemical properties of soil aggregates. Biol. Fertil. Soils 1993, 15, 229–234. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.E.; et al. a review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar]
- Lavelle, P.; Barois, I.; Cruz, I.; Fragoso, C.; Hernandez, A.; Pineda, A.; Rangel, P. Adaptive strategies of Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta), a Peregrine Geophagous earthworm of the humid tropics. Biol. Fertil. Soils 1987, 5, 188–194. [Google Scholar] [CrossRef]
- Juárez-Ramón, D.; Fragoso, C. Comunidades de lombrices de tierra en sistemas agroforestales intercalados, en dos regiones del centro de México. Acta Zool. Mex. 2014, 30, 637–654. [Google Scholar] [CrossRef]
- Tao, Y.; Gu, W.; Chen, J.; Tao, J.; Yj, X.; Zhang, H. The influence of land use practices on earthworm communities in saline agriculture soils of the west Coast Region of China’s Bohai Bay. Plant Soil Environ. 2012, 59, 8–13. [Google Scholar]
- Vargas Tierras, Y.B.; Pico, J.T.; Díaz, A.; Sotomayor Akopyan, D.A.; Burbano, A.; Caicedo, C.; Paredes Andrade, N.; Congo, C.; Tinoco, L.A.; Bastidas, S.; et al. Manual del Cultivo de Pitahaya para la Amazonía Ecuatoriana; INIAP, Estación Experimental Central de la Amazonía, Programa Nacional de Fruticultura: La Joya de los Sachas, Ecuador, 2020; ISBN 978-9942-22-489-7. [Google Scholar]
- Climate Data Clima Provincia de Orellana: Temperatura, Gráfico Climático, Tabla Climática de Provincia de Orellana. Available online: https://en.climate-data.org/south-america/ecuador/provincia-de-orellana-63/ (accessed on 24 October 2023).
- Ministerio del Ambiente. Sistema de Clasificación de los Ecosistemas del Ecuador Continental; Ministerio del Ambiente: Quito, Ecuador, 2012. [Google Scholar]
- Piato, K.; Subía, C.; Pico, J.; Calderón, D.; Norgrove, L.; Lefort, F. Organic farming practices and shade trees reduce pest infestations in robusta coffee systems in Amazonia. Life 2021, 11, 413. [Google Scholar]
- Caicedo Albán, W.J. Evaluación de Sistemas Silvopastoriles Como Alternativa para la Sostenibilidad de los Recursos Naturales, en la Estación Experimental Central de la Amazonia, del INIAP. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador, 2013. [Google Scholar]
- Niether, W.; Armengot, L.; Andres, C.; Schneider, M.; Gerold, G. Shade Trees and tree pruning alter throughfall and microclimate in cocoa (Theobroma cacao L.) production systems. Ann. For. Sci. 2018, 75, 38. [Google Scholar] [CrossRef]
- Di Liberto Porles, S. Selección de Árboles Semilleros de Cedrelinga Cateniformis, Simarouba amara y Guatteria elata en Plantaciones Forestales, Mediante el uso de RPAS; Universidad Agraria La Molina: Lima, Peru, 2022. [Google Scholar]
- Sánchez-Chacón, E.; Alvarado-Rodríguez, O.; Rodríguez-Arrieta, A.; Gómez-Alpízar, L. Micromorfología de los foliolos de pejibaye Bactris gasipaes (Arecaceae) var. Diamantes-10. Rev. Biol. Trop. 2016, 64, 1273–1285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lozano, E.C.; Zapater, M.A. El Género Erythrina (Leguminosae) En Argentina. Darwiniana Nueva Ser. 2010, 48, 179–200. [Google Scholar]
- Russo Andrade, R.O. Efecto de la Poda de Erythrina Poeppigiana (Walpers) O.F. Cook (Poró), Sobre la Nodulación, Producción de Biomasa y Contenido de Nitrógeno en el suelo en un Sistema Agroforestal “café-poró”; CATIE: Turrialba, Costa Rica, 1983. [Google Scholar]
- Bateman, R.; Crozier, J. Pesticide Use in Cocoa—Practical Manual, 4th ed.; Organisation Internationale du Cacao: Yamuzukro, Côte d'Ivoire, 2023. [Google Scholar]
- Sánchez-Mora, F.D.S.; Garcés, F.R.G.; Vásconez, G.H.V.; Vera, J.F.V.; Zambrano, J.Z.; Ramos, R.R. Productividad de clones de cacao tipo nacional en una zona del bosque húmedo tropical de la provincia de Los Ríos, Ecuador. Cienc. Tecnol. 2014, 7, 33–41. [Google Scholar] [CrossRef]
- Díaz, A. Caracterización de los Suelos de la Amazonía Ecuatoriana; INIAP: Joya de los Sachas, Ecuador, 2018. [Google Scholar]
- Sáez-Plaza, P.; Navas, M.; Wybraniec, S.; Michałowski, T.; Garcia Asuero, A. An Overview of the Kjeldahl Method of nitrogen determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar]
- Jiménez, A.; Farfán, F.; Morales-Londoño, C. Biomasa seca y contenido de nutrientes de Cajanus cajan, Crotalaria juncea y Tephrosia candida empleadas como abonos verdes en cafetales. Cenicafé 2005, 56, 93–109. [Google Scholar]
- Montenegro, E. Efecto del Aporte de Nutrientes de la Biomasa de Tres Tipos de Árboles de Sombra en Sistemas de Manejo de Café orgánico y Convencional; Posgrado, Centro Agronómico Tropical de Investigación y Enseñanza CATIE: Turrialba, Costa Rica, 2005. [Google Scholar]
- Carvajal-Agudelo, B.N.; Andrade, H.J. Captura de carbono en biomasa de sistemas de uso del suelo, municipio de Yopal, Casanare, Colombia. Orinoquia 2020, 24, 13–22. [Google Scholar]
- Poveda, V.; Orozco-Aguilar, L.; Medina, C.; Cerda, R.; López Sampson, A. Almacenamiento de carbono en sistemas agroforestales de cacao en Waslala, Nicaragua. Desarro. Rural Américas 2013, 49, 42–50. [Google Scholar]
- Solis, R.; Vallejos-Torres, G.; Arévalo, L.; Marín-Díaz, J.; Ñique-Alvarez, M.; Engedal, T.; Bruun, T.B. Carbon stocks and the use of shade trees in different coffee growing systems in the Peruvian Amazon. J. Agric. Sci. 2020, 158, 450–460. [Google Scholar] [CrossRef]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a Worldwide Wood Economics Spectrum; Dryad: Davis, CA, USA, 2009. [Google Scholar]
- Scudder, M.; Wampe, N.; Waviki, Z.; Applegate, G.; Herbohn, J. Smallholder cocoa agroforestry systems; is increased yield worth the labour and capital inputs? Agric. Syst. 2022, 196, 103350. [Google Scholar]
- Curry, G.; Koczberski, G.; Lemerle, C. Mejorar la Productividad y la Participación de los Jóvenes y las Mujeres en las Industrias del Cacao, el Coco y la Palma Aceitera de Papua Nueva Guinea. Available online: https://catalog.ihsn.org/citations/89411 (accessed on 13 November 2023).
- Asante, P.A.; Rozendaal, D.M.A.; Rahn, E.; Zuidema, P.A.; Quaye, A.K.; Asare, R.; Läderach, P.; Anten, N.P.R. Unravelling drivers of high variability of on-farm cocoa yields across environmental gradients in Ghana. Agric. Syst. 2021, 193, 103214. [Google Scholar] [CrossRef]
- Quiroga Gómez, V. Patrón de Variabilidad de la Producción de Cacao en la zona Atlántica de Costa Rica; IICA: San José, Costa Rica, 1972. [Google Scholar]
- Kouassi, J.-L.; Diby, L.; Konan, D.; Kouassi, A.; Bene, Y.; Kouamé, C. Drivers of cocoa agroforestry adoption by smallholder farmers around the taï national park in southwestern Côte d’Ivoire. Sci. Rep. 2023, 13, 14309. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Andres, C.; Trujillo, G.; Alcon, F.; Amurrio, P.; Perez, E.; Weibel, F.; Milz, J. Cocoa and total system yields of organic and conventional agroforestry vs. monoculture systems in a long-term field trial in Bolivia. Exp. Agric. 2017, 53, 351–374. [Google Scholar]
- Mensah, E.O.; Ræbild, A.; Asare, R.; Amoatey, C.A.; Markussen, B.; Owusu, K.; Asitoakor, B.K.; Vaast, P. Combined effects of shade and drought on physiology, growth, and yield of mature cocoa trees. Sci. Total Environ. 2023, 899, 165657. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Argueta, O.; Orozco-Aguilar, L.; Dubón, A.D.; Díaz, F.J.; Sánchez, J.; Casanoves, F. Timber growth, cacao yields, and financial revenues in a long-term experiment of cacao agroforestry systems in Northern Honduras. Front. Sustain. Food Syst. 2022, 6, 941743. [Google Scholar] [CrossRef]
- Infocacao. Evaluando la Producción de Cacao bajo Sombra de Cinco Especies Forestales. Available online: http://www.fhia.org.hn/descargas/proyecto_procacao/infocacao/InfoCacao_No4_Nov_2015.pdf (accessed on 25 August 2023).
- Heuveldop, J.; Fassbender, H.W.; Alpízar, L.; Enríquez, G.; Fölster, H. Modelling agroforestry systems of cacao (Theobroma cacao) with laurel (Cordia alliodora) and poro (Erythrina poeppigiana) in Costa Rica II. Cacao and Wood Production, Litter Production and Decomposition. Agrofor. Syst. 1988, 6, 37–48. [Google Scholar] [CrossRef]
- Paredes Andrade, N.; Monteros Altamirano, Á.; Lima Tandazo, L.; Caicedo Vargas, C.; Tinoco, L.A.; Fernández, F.; Vargas Tierras, Y.B.; Pico, J.T.; Subía, C.; Burbano Cachiguango, A.; et al. Manual del Cultivo de Cacao Sostenible para la Amazonía Ecuatoriana; INIAP: Joya de los Sachas, Ecuador, 2022. [Google Scholar]
- Blaser-Hart, W.J.; Hart, S.P.; Oppong, J.; Kyereh, D.; Yeboah, E.; Six, J. The effectiveness of cocoa agroforests depends on shade-tree canopy height. Agric. Ecosyst. Environ. 2021, 322, 107676. [Google Scholar] [CrossRef]
- Bai, S.H.; Gallart, M.; Singh, K.; Hannet, G.; Komolong, B.; Yinil, D.; Field, D.J.; Muqaddas, B.; Wallace, H.M. Leaf Litter Species Affects Decomposition rate and nutrient release in a cocoa plantation. Agric. Ecosyst. Environ. 2022, 324, 107705. [Google Scholar] [CrossRef]
- Carmona-Rojas, L.M.; Gutiérrez-Rodríguez, E.A.; Henao-Ramírez, A.M.; Urrea-Trujillo, A.I. Nutrition in cacao (Theobroma cacao L.) crops: What determining factors should be considered? Rev. Fac. Agron. 2022, 121, 101. [Google Scholar] [CrossRef]
- Kouadio, S.; Tienebo, E.-O.; Kouadio, K.; Kouamé, K.; Koko, L.; Abo, K. Foliar Application of boron during flowering promotes tolerance to cocoa (Theobroma cacao L.) swollen shoot viral disease. Eur. Sci. J. 2017, 13, 387–406. [Google Scholar] [CrossRef]
- van Vliet, J.A.; Giller, K.E. Mineral nutrition of cocoa. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2017; Volume 141, pp. 185–270. ISBN 978-0-12-812423-9. [Google Scholar]
- Appa, F.E.; Rombe, Y.P.; Lidiawati, D. Concentration of micronutrient (Fe, Cu, Mn) in cocoa plantation land in transmigration area, East luwu regency. J-HEST J. Health Educ. Econ. Sci. Technol. 2022, 5, 143–147. [Google Scholar]
- Nadège, M.T.; Louis, Z.; Cédric, C.D.; Louis-Paul, K.B.; Funwi, F.P.; Ingrid, T.T.; Clotex, T.V.; Flore, N.Y.A.; Bruno, T.M.R.; Julliete Mancho, N. Carbon storage potential of cacao agroforestry systems of different age and management intensity. Clim. Dev. 2019, 11, 543–554. [Google Scholar]
- Asigbaase, M.; Dawoe, E.; Lomax, B.H.; Sjogersten, S. Temporal changes in litterfall and potential nutrient return in cocoa agroforestry systems under organic and conventional management, Ghana. Heliyon 2021, 7, e08051. [Google Scholar] [CrossRef]
- Hartemink, A.E. Nutrient stocks, nutrient cycling, and soil changes in cocoa ecosystems: A Review. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 86, pp. 227–253. ISBN 978-0-12-000784-4. [Google Scholar]
- Berhongaray, G.; Cotrufo, F.M.; Janssens, I.A.; Ceulemans, R. Below-ground carbon inputs contribute more than above-ground inputs to soil carbon accrual in a bioenergy poplar plantation. Plant Soil 2019, 434, 363–378. [Google Scholar] [CrossRef]
- Goñas, M.; Rojas-Briceño, N.B.; Culqui-Gaslac, C.; Arce-Inga, M.; Marlo, G.; Pariente-Mondragón, E.; Oliva-Cruz, M. Carbon sequestration in fine aroma cocoa agroforestry systems in Amazonas, Peru. Sustainability 2022, 14, 9739. [Google Scholar] [CrossRef]
- Alcudia-Aguilar, A.; Martínez-Zurimendi, P.; van der Wal, H.; Castillo-Uzcanga, M.M.; Suárez-Sánchez, J. Allometric estimation of the biomass of Musa spp. in Homegardens of Tabasco, Mexico. Trop. Subtrop. Agroecosyst. 2019, 22. [Google Scholar] [CrossRef]
- Montagnini, F.; Nair, P.K.R. Carbon sequestration: An underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 2004, 61, 281–295. [Google Scholar]
- Isinkaralar, O. Discovery of spatial climate parameters and bioclimatic comfort change simulation in Türkiye under socioeconomic pathway scenarios: A basin-scale case study for urban environments. Nat. Hazards 2023. [Google Scholar] [CrossRef]
- Isinkaralar, O.; Isinkaralar, K. Projection of bioclimatic patterns via cmip6 in the southeast region of Türkiye: A guidance for adaptation strategies for climate policy. Environ. Monit. Assess. 2023, 195, 1448. [Google Scholar]
- Vršič, S. Soil Erosion and earthworm population responses to soil management systems in steep-slope vineyards. Plant Soil Environ. 2011, 57, 258–263. [Google Scholar] [CrossRef]
- Hodson, M.E.; Brailey-Jones, P.; Burn, W.L.; Harper, A.L.; Hartley, S.E.; Helgason, T.; Walker, H.F. Enhanced plant growth in the presence of earthworms correlates with changes in soil microbiota but not nutrient availability. Geoderma 2023, 433, 116426. [Google Scholar]
- Dekemati, I.; Simon, B.; Vinogradov, S.; Birkás, M. The effects of various tillage treatments on soil physical properties, earthworm abundance and crop yield in Hungary. Soil Tillage Res. 2019, 194, 104334. [Google Scholar] [CrossRef]
- Mulia, R.; Hoang, S.V.; Dinh, V.M.; Duong, N.B.T.; Nguyen, A.D.; Lam, D.H.; Thi Hoang, D.T.; van Noordwijk, M. Earthworm diversity, forest conversion and agroforestry in Quang Nam Province, Vietnam. Land 2021, 10, 36. [Google Scholar] [CrossRef]
- Muvahhid, K.; Coşkan, A.; İbrahim, E. Earthworm avoidance as an ecotoxicological test to different sources of boron. Sci. Educ. Cult. 2022, 1, 354–358. [Google Scholar]
- Kanchilakshmi, M. Earthworm: A Potential and sustainable source for soil fertility—An altitude based biophysical study. IJEPP 2016, 4, 77. [Google Scholar] [CrossRef]
- Dos Santos, J.B.; Ramos, A.C.; Azevedo Júnior, R.; de Oliveira Filho, L.C.I.; Baretta, D.; Cardoso, E.J.B.N. Soil macrofauna in organic and conventional coffee plantations in Brazil. Biota Neotrop. 2018, 18, e20180515. [Google Scholar]
- Barreto, A.; Santos, J.; Amorim, M.J.B.; Maria, V.L. Environmental hazards of boron and vanadium nanoparticles in the terrestrial ecosystem—A case study with enchytraeus crypticus. Nanomaterials 2021, 11, 1937. [Google Scholar] [CrossRef]
- Morgan, J.E.; Morgan, A.J. Earthworms as biological monitors of cadmium, copper, lead and zinc in metalliferous soils. Environ. Pollut. 1988, 54, 123–138. [Google Scholar] [CrossRef]
- Tőzsér, D.; Mizser, S.; Karaffa, K.; Málik-Roffa, H.; Magura, T. A Meta-Analysis-based evaluation of metallic element accumulation in earthworms. Environ. Int. 2022, 169, 107546. [Google Scholar] [CrossRef]
- Bart, S.; Pelosi, C.; Barraud, A.; Péry, A.R.R.; Cheviron, N.; Grondin, V.; Mougin, C.; Crouzet, O. Earthworms mitigate pesticide effects on soil microbial activities. Front. Microbiol. 2019, 10, 1535. [Google Scholar] [CrossRef]
| Species | Use | Sowing Distance | Cup Shape |
|---|---|---|---|
| T. cacao | Fruit tree | 3 m × 3 m | Ellipsoidal [44] |
| C. cateniformis | Forestry | 12 m × 12 m | Rounded [45] |
| B. gasipaes | Fruit tree | 12 m × 12 m | Palemiform [46] |
| E. poeppigiana | Service | 6 m × 6 m | Oval [47] |
| Species | Equation |
|---|---|
| B. gasipaes | [55] |
| C. cateniformis | [57] |
| Root biomass | [58] |
| Factors | Yield (kg ha−1) |
|---|---|
| Production cycles | ** |
| Agroforestry systems | * |
| Production cycles × agroforestry systems | NS |
| Agroforestry System | Yield (kg ha−1) |
|---|---|
| E. poeppigiana | 575.3 ab |
| C. cateniformis + E. poeppigiana | 603.9 a |
| C. cateniformis | 510.4 b |
| B. gasipaes | 438.3 b |
| Monoculture | 435.4 c |
| Nutrient | Estimate | Probability |
|---|---|---|
| S | −3.6 | 0.5 NS |
| Mg | 33.7 | 0.7 NS |
| Fe | −0.9 | 0.1 * |
| B | 119.7 | 0.1 NS |
| K | −188.0 | 0.03 * |
| Ca | −20.0 | 0.04 * |
| Factor | Total Stored C |
|---|---|
| Production cycle | * |
| Agroforestry system | * |
| Production cycles × agroforestry systems | NS |
| Production Cycle | Stored C Biomass (Aboveground and Roots) (t ha−1) | Stored C in the Soil (t ha−1) | Total Stored C (t ha−1) |
|---|---|---|---|
| 2022 | 2.2 b | 43.9 c | 47.2 a |
| 2021 | 2.1 b | 40.0 bc | 44.8 a |
| 2020 | 2.1 b | 32.0 a | 36.5 b |
| 2019 | 1.6 a | 28.4 a | 33.1 b |
| 2018 | 1.6 a | 34.0 ab | 36.3 b |
| Agroforestry System | Stored C Biomass (Aboveground and Roots) (t ha−1) | Stored C in the Soil (t ha−1) | Total Stored C (t ha−1) |
|---|---|---|---|
| E. poeppigiana | 3.6 c | 38.3 a | 42.0 b |
| C. cateniformis + E. poeppigiana | 1.9 b | 37.1 a | 39.1 ab |
| C. cateniformis | 1.7 b | 32.4 a | 34.1 a |
| B. gasipaes | 1.2 a | 31.7 a | 32.9 a |
| Monoculture | 1.1 a | 38.4 a | 39.6 ab |
| Factor | Earthworm Abundance | Season |
|---|---|---|
| Production cycle | NS | Rainy season |
| Agroforestry system | * | |
| Production cycles × agroforestry systems | NS | |
| Production cycle | NS | Dry season |
| Agroforestry system | * | |
| Production cycles × agroforestry system | NS |
| Agroforestry System | Season | Earthworm Abundance |
|---|---|---|
| E. poeppigiana | Rainy season | 14.07 b |
| C. cateniformis + E. poeppigiana | 28.17 a | |
| C. cateniformis | 22.26 ab | |
| B. gasipaes | 23.93 ab | |
| Monoculture | 11.73 b | |
| E. poeppigiana | Dry season | 17.13 b |
| C. cateniformis + E. poeppigiana | 24.87 a | |
| C. cateniformis | 15.13 b | |
| B. gasipaes | 27.13 a | |
| Monoculture | 16.21 b |
| Season | Nutrient | Estimate | Probability |
|---|---|---|---|
| Rainy season | Cu | 0.65 | 0.4730 NS |
| B | −7.84 | 0.353 NS | |
| Ca | 0.46 | 0.588 NS | |
| Zn | −1.15 | 0.031 * | |
| N | −53.63 | 0.147 NS | |
| Dry season | Cu | −0.58 | 0.521 NS |
| B | −7.57 | 0.369 NS | |
| Ca | 2.02 | 0.020 * | |
| Zn | 0.42 | 0.424 NS | |
| N | 12.47 | 0.733 NS |
| Nutrient | Season | Years | ||||
|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2022 | ||
| Earthworms vs. Ca | Rainy | −0.69 | −0.39 | −0.38 | 0.52 | 0.18 |
| Earthworms vs. Mg | −0.05 | −0.31 | −0.42 | 0.33 | 0.23 | |
| Earthworms vs. Zn | −0.41 | −0.45 | −0.37 | 0.19 | 0.28 | |
| Earthworms vs. B | −0.57 | 0.28 | −0.24 | 0.16 | 0.13 | |
| Earthworms vs. Ca | Dry | 0.20 | 0.07 | 0.24 | 0.31 | 0.69 |
| Earthworms vs. Mg | 0.16 | 0.32 | 0.29 | 0.33 | −0.60 | |
| Earthworms vs. Zn | 0.30 | −0.07 | 0.54 | 0.75 | −0.24 | |
| Earthworms vs. B | 0.73 | −0.24 | −0.61 | 0.60 | 0.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinoco-Jaramillo, L.; Vargas-Tierras, Y.; Habibi, N.; Caicedo, C.; Chanaluisa, A.; Paredes-Arcos, F.; Viera, W.; Almeida, M.; Vásquez-Castillo, W. Agroforestry Systems of Cocoa (Theobroma cacao L.) in the Ecuadorian Amazon. Forests 2024, 15, 195. https://doi.org/10.3390/f15010195
Tinoco-Jaramillo L, Vargas-Tierras Y, Habibi N, Caicedo C, Chanaluisa A, Paredes-Arcos F, Viera W, Almeida M, Vásquez-Castillo W. Agroforestry Systems of Cocoa (Theobroma cacao L.) in the Ecuadorian Amazon. Forests. 2024; 15(1):195. https://doi.org/10.3390/f15010195
Chicago/Turabian StyleTinoco-Jaramillo, Leider, Yadira Vargas-Tierras, Nasratullah Habibi, Carlos Caicedo, Alexandra Chanaluisa, Fernando Paredes-Arcos, William Viera, Marcelo Almeida, and Wilson Vásquez-Castillo. 2024. "Agroforestry Systems of Cocoa (Theobroma cacao L.) in the Ecuadorian Amazon" Forests 15, no. 1: 195. https://doi.org/10.3390/f15010195
APA StyleTinoco-Jaramillo, L., Vargas-Tierras, Y., Habibi, N., Caicedo, C., Chanaluisa, A., Paredes-Arcos, F., Viera, W., Almeida, M., & Vásquez-Castillo, W. (2024). Agroforestry Systems of Cocoa (Theobroma cacao L.) in the Ecuadorian Amazon. Forests, 15(1), 195. https://doi.org/10.3390/f15010195










