The Effects of Shading on the Photosynthetic Performance of Endangered Plant Horsfieldia hainanensis Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Materials
2.3. Experiment Design
2.4. Measurements
2.4.1. Photosynthetic Gas Exchange Parameters
2.4.2. Determination of OJIP Curve and 820 nm Light Absorption Curve
2.4.3. Measurements of Net Seedling Growth
2.4.4. Determination of Physiological and Biochemical Indicators Such as Soluble Sugar, Soluble Protein, Proline, and Malondialdehyde Content
2.5. Data Analysis
3. Results
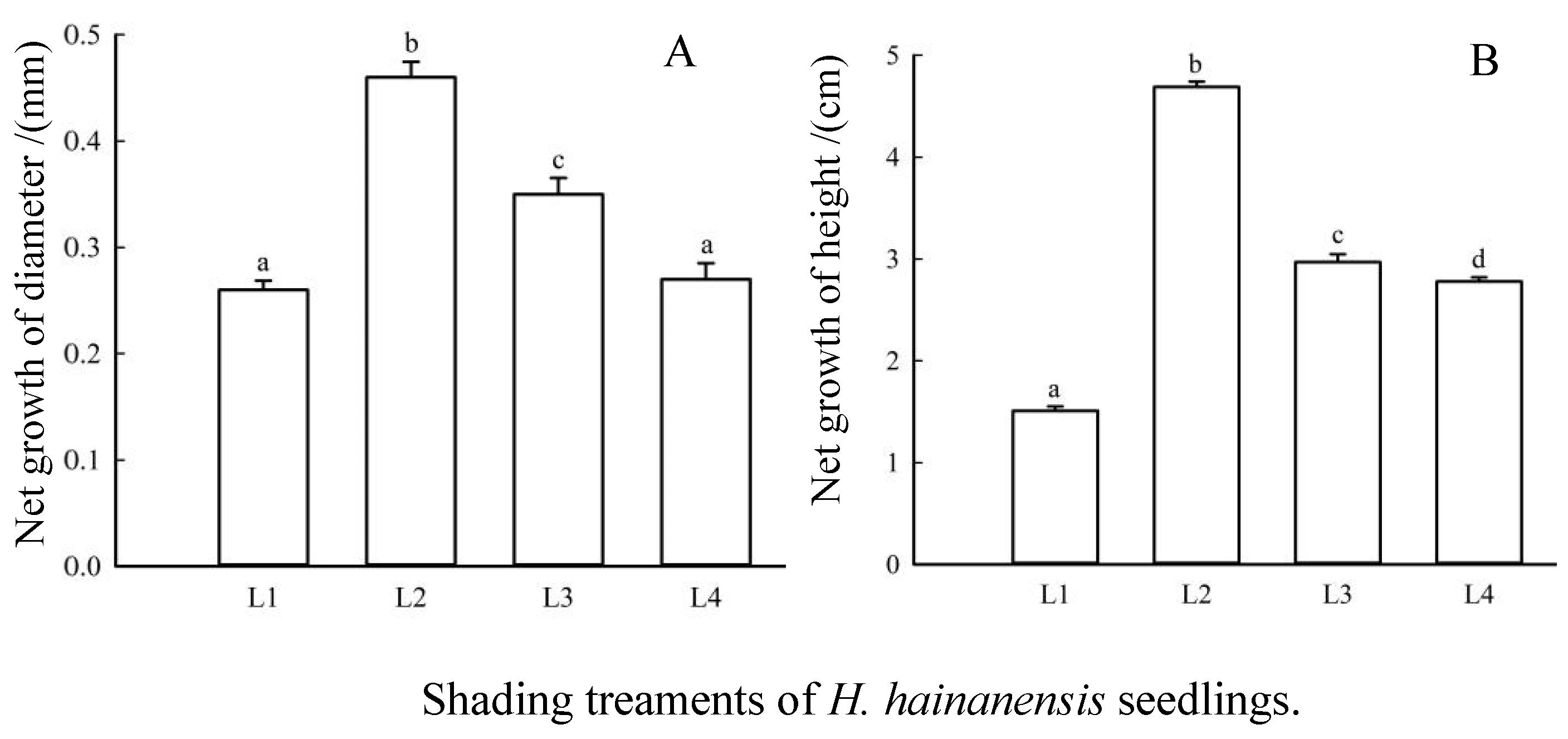
3.1. Effects of Shading on Net Growth of H. hainanensis
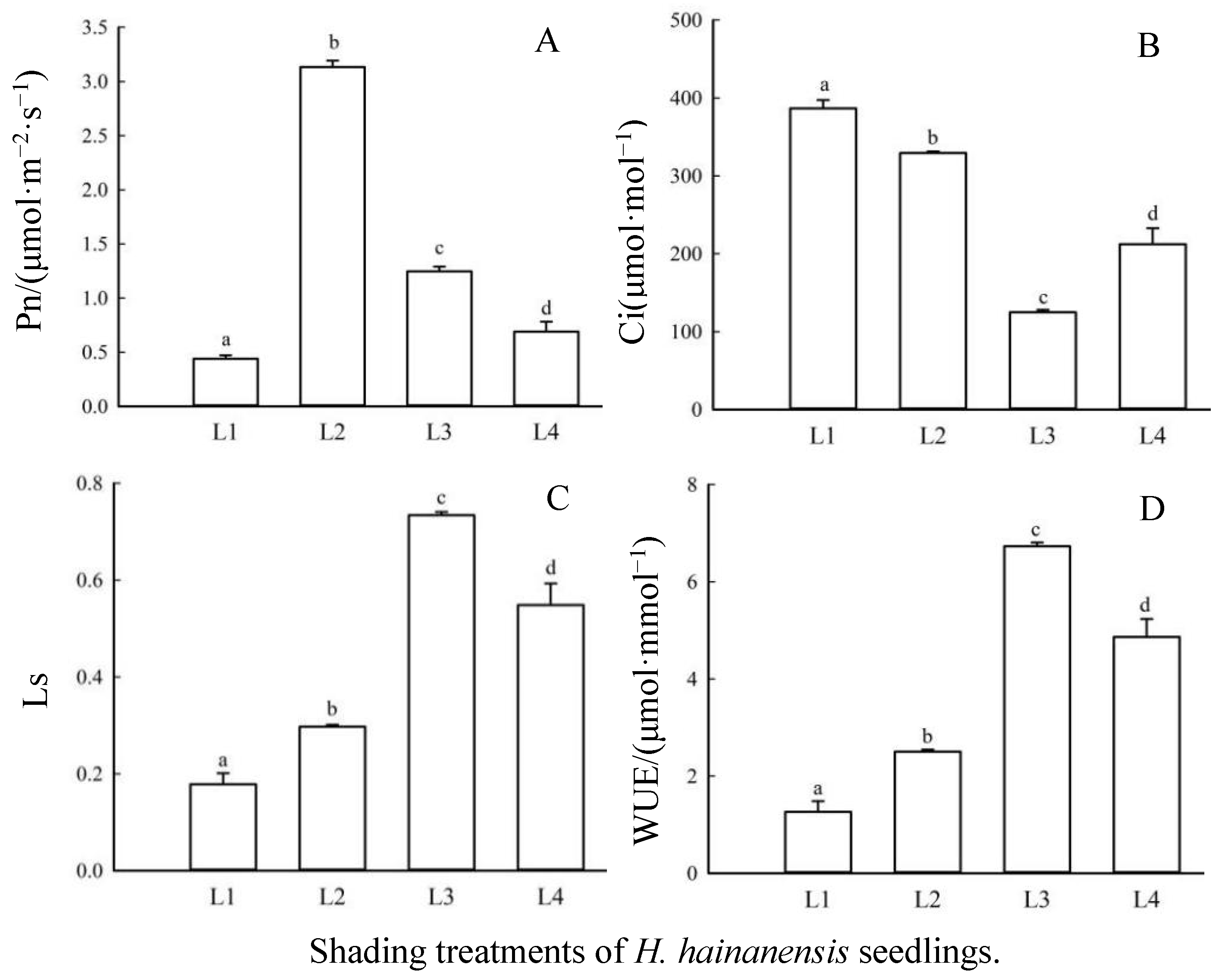
3.2. The Effects of Shading on Photosynthetic Gas Exchange Parameters in Leaves
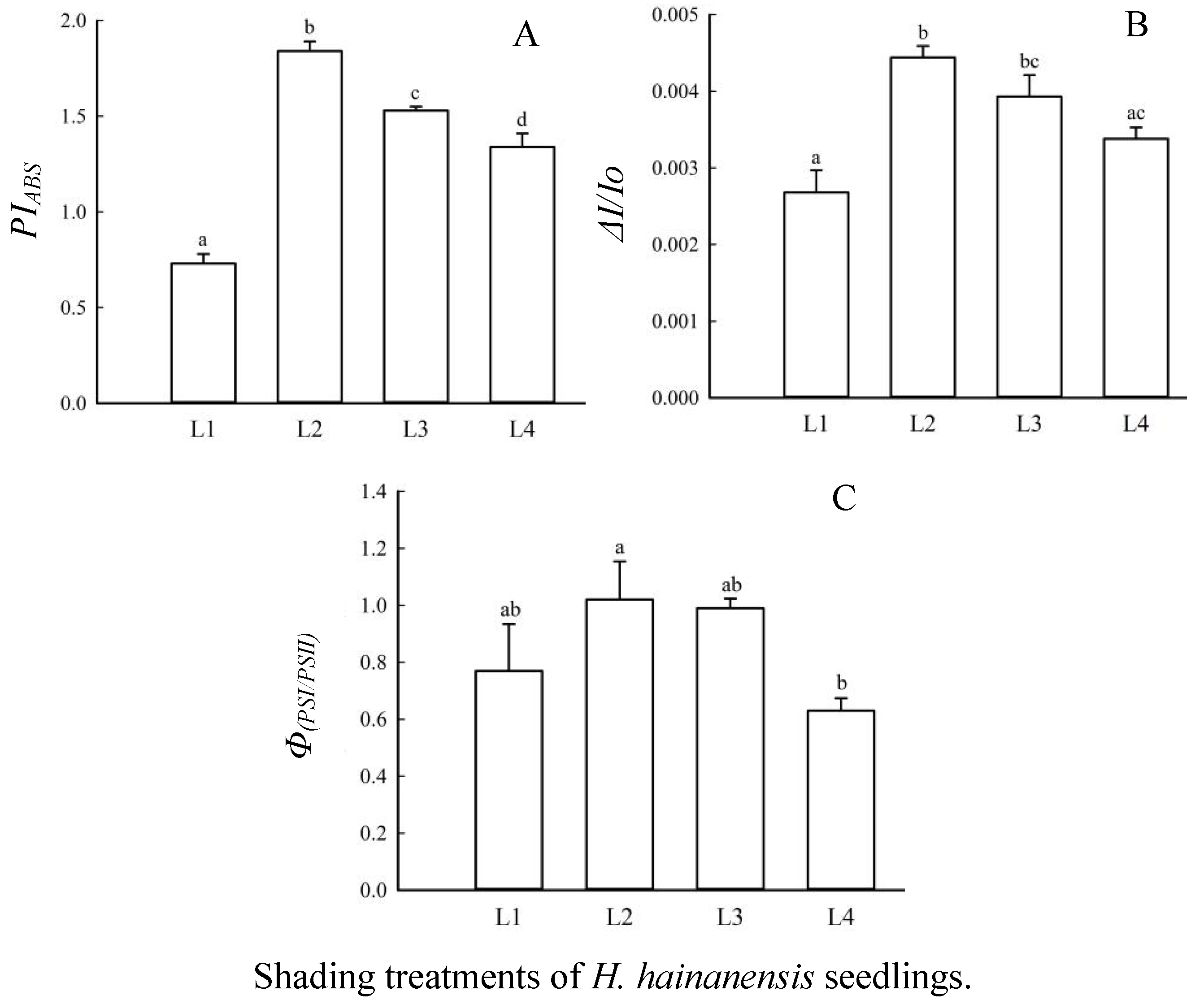
3.3. The Effects of Shading Treatments on Leaf Quantum Yield and Energy Distribution Ratio
3.4. The Effects of Shading on Leaf PSII Donor Side/Acceptor Side
3.5. Effects of Shading on the Overall Performance and Coordination of Leaf PSII/PSI
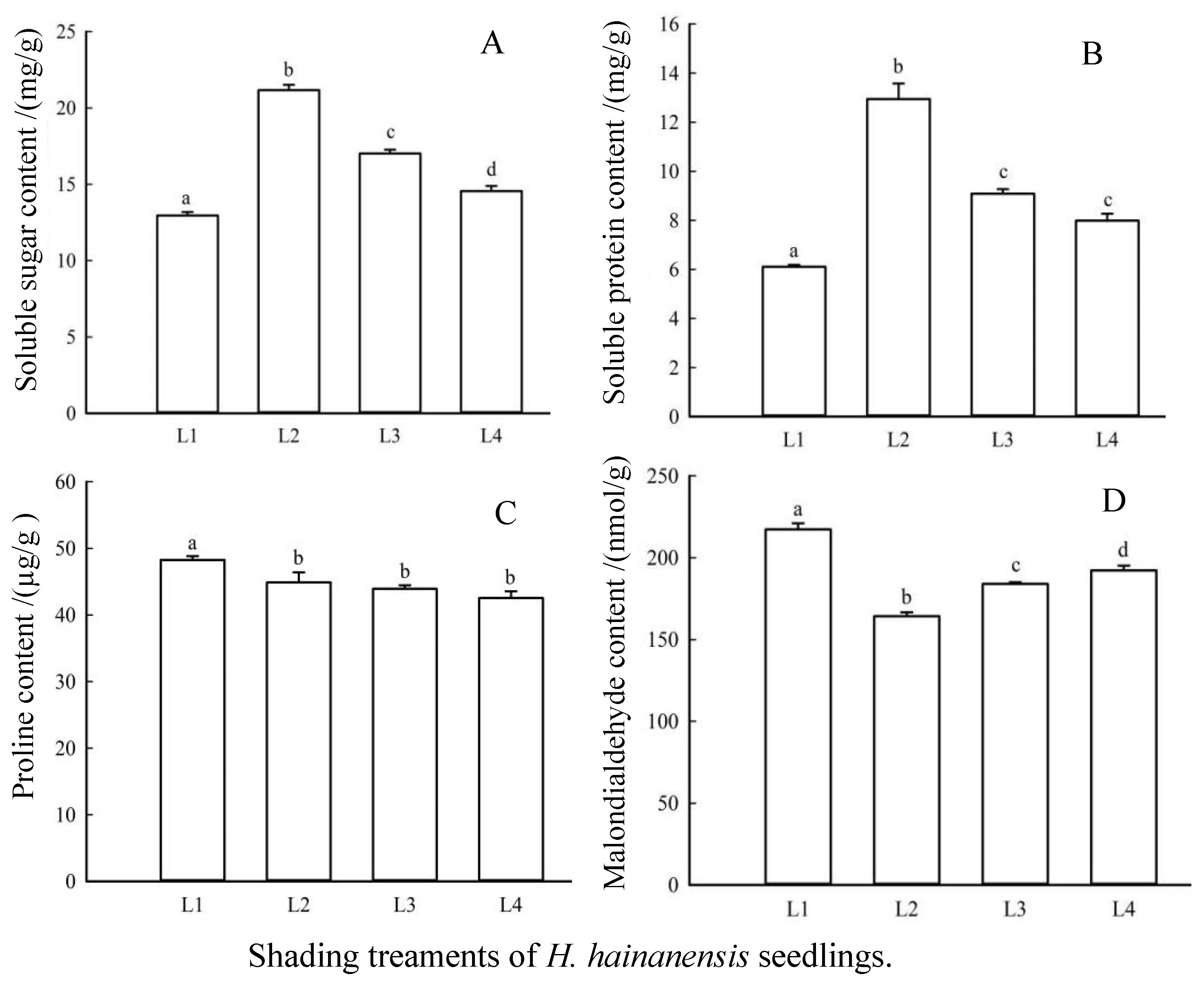
3.6. Effects of Shading on Osmotic Regulatory Substances and Membrane Lipid Peroxidation Products in Leaves of H. hainanensis Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sofo, A.; Dichio, B.; Montanaro, G.; Xiloyannis, C. Shade effect on photosynthesis and photoinhibition in olive during drought and rewatering. Agric. Water Manag. 2009, 96, 1201–1206. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Q.M.; Lu, H.F.; Liu, H.X.; Guo, Q.F.; Wang, J.; Jian, S.G.; Bao, H.O. Wild plant species with extremely small populations require conser-vation and reintroduction in China. Ambio 2012, 41, 913–917. [Google Scholar] [CrossRef]
- Li, L.J.; Gu, W.R.; Li, J.; Li, C.F.; Xie, T.L.; Qu, D.Y.; Meng, Y.; Li, C.F.; Wei, S. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol. Biochem. 2018, 129, 35–55. [Google Scholar] [CrossRef]
- Kim, J.H.; Glick, R.E.; Melis, A. Dynamics of photosystem stoichiometry adjustment by light quality in chloroplasts. Plant Physiol. 1993, 102, 181–190. [Google Scholar] [CrossRef]
- Vialet-Chabrand, S.; Matthews, J.S.; Simkin, A.J.; Raines, C.A.; Lawson, T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef]
- Barber, J.; Andersson, B. Too much of a good thing: Light can be bad for photosynthesis. Trends Biochem. Sci. 1992, 17, 61–66. [Google Scholar] [CrossRef]
- Niinemets, Ü. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef]
- Croce, R.; Amerongen, H. Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science 2020, 369, 2058. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wright, I.J.; Zhu, S.; Onoda, Y.; Liu, H.; Li, R.H.; Liu, X.R.; Hua, L.; Oyanoghafo, O.O.; Ye, Q. Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements. New Phytol. 2019, 223, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant re-sponses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Baltzer, J.L.; Thomas, S.C. Determinants of whole-plant light requirements in Bornean rain forest tree saplings. J. Ecol. 2007, 95, 1208–1221. [Google Scholar] [CrossRef]
- Portsmuth, A.; Niinemets, Ü. Structural and physiological plasticity in response to light and nutrients in five temperate decidu-ous woody species of contrasting shade tolerance. Funct. Ecol. 2007, 21, 61–77. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Xiang, W.H.; Jiang, Y.; He, Y.H.; Lin, J.Y. Floristic composition, structure and phytogeographic characteristics of Horsfieldia hainanensis community in Guangxi. J. Beijing For. Univ. 2016, 38, 74–82. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Liu, X.S.; Xiang, W.H.; Jiang, Y.; He, Y.H. Genetic diversity and structure analysis of the endangered plant species Horsfieldia hainanensis Merr. in China. Biotechnol. Biotechnol. Equip. 2018, 32, 95–101. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.P.; Cai, Y.T.; Yang, T.Y.; Yang, L.T.; Huang, Z.R.; Chen, L.S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol. 2018, 38, 1548–1565. [Google Scholar] [CrossRef]
- Guo, X.R.; Cao, K.F.; Xu, Z.F. Response of photosynthesis and antioxygenic enzymes in seedlings of three tropical forest tree species to different light environments. Chin. J. Appl. Ecol. 2004, 15, 377–381. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Xiao, Y.; Wang, Y.; Jiang, Y.; Jiang, Y. Soil seed burial and competition with surrounding plants determine the emer-gence and development of seedling of an endangered species Horsfieldia hainanensis Merr. in China. Sci. Rep. 2019, 9, 17970. [Google Scholar] [CrossRef]
- Liu, C.; Tian, T.; Li, S.; Wang, F.; Liang, Y. Growth response of Chinese woody plant seedlings to different light intensities. Acta Ecol. Sin. 2018, 38, 518–527. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimill-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescen cetransient. In Advances in Photosynthesis and Respiration; Papageorgiou, E., Govindjee, G.C., Eds.; KAP Press: Best, The Netherlands, 2004; pp. 1–42. [Google Scholar] [CrossRef]
- Li, P.M.; Gao, H.Y.; Strasser, R.J. Application of the fast chlorophyll fluorescence induction dynamics analysis in photosyn-thesis study. J. Plant Physiol. Mol. Biol. 2005, 31, 559–566. [Google Scholar] [CrossRef]
- Yang, J.J.; Lei, P.F.; Xiang, W.H.; Ouyang, S.; Hui, X.R. Growth Variations of Tree Saplings in Relation to Species Diversity and Functional Traits in a Tree Diversity Pot Experiment. Forests 2018, 9, 380. [Google Scholar] [CrossRef]
- Gonzalez, C.M.; Pignata, M.L. The Influence of Air Pollution on Soluble Proteins, Chlorophyll Degradation, MDA, Sulphur and Heavy Metals in A Transplanted Lichen. Chem. Ecol. 1994, 9, 105–113. [Google Scholar] [CrossRef]
- Monreal, J.A.; Jiménez, E.T.; Remesal, E.; Morillo-Velarde, R.; García-Maurino, S.; Echevarría, C. Proline content of sugar beet storage roots: Response to water deficit and nitrogen fertilization at field conditions. Environ. Exp. Bot. 2007, 60, 257–267. [Google Scholar] [CrossRef]
- Lassouane, N.; Aïd, F.; Lutts, S. Water stress impact on young seedling growth of Acacia arabica. Acta Physiol. Plant. 2013, 35, 2157–2169. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Toxic Effects, Oxidative Stress and Ultrastructural Changes in Moss Taxithelium Nepalense (Schwaegr.) Broth. Under Chromium and Lead Phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Haung, H.T.; Haung, J.J.; Cheng, J.; Cheng, Y.J.; Guan, D.S. Growth, physiological and biochemical response of Baccaurea ramiflora Lour. Seedlings to different shading environments. Chin. J. Ecol. 2020, 39, 1538–1547. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, P.; Yang, W.; Sui, X.; Li, X.; Li, W.; Zhang, R.; Gu, S.; Xu, N. Effects of flooding stress on the photosynthetic apparatus of leaves of two Physocarpus cultivars. J. For. Res. 2018, 29, 1049–1059. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.L.; Ye, Z.P.; Lu, Y.T.; Ma, X.F. Stomatal and non-stomatal limitations to photosynthesis in Sorghum bicolor at diferent temperatures. Plant Physiol. J. 2022, 58, 1245–1253. [Google Scholar] [CrossRef]
- Qin, P.; Zeng, S.H.; Liu, F.H.; Liu, X.L.; Zhou, W. Effect of different light intensity on photosynthesis of K326 vigorous florescence. J. Yunnan Univ. 2003, 1, 97–100. [Google Scholar]
- Zhang, J.; Liu, J.; Yang, C.; Du, S.; Yang, W. Photosynthetic performance of soybean plants to water deficit under high and low light intensity. S. Afr. J. Bot. 2016, 105, 279–287. [Google Scholar] [CrossRef]
- Miao, C.; Yang, S.; Xu, J.; Wang, H.; Zhang, Y.; Cui, J.; Zhang, H.; Jin, H.; Lu, P.; He, L.; et al. Effects of Light Intensity on Growth and Quality of Lettuce and Spinach Cultivars in a Plant Factory. Plants 2023, 12, 3337. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, Photosynthesis, and Nutrient Uptake at Different Light Intensities and Temperatures in Lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Zhang, R.; Kong, Z.; Chen, S.; Ran, Z.; Ye, M.; Xu, J.; Zhou, C.; Liao, K.; Cao, J.; Yan, X. The Comparative Study for Physiological and Biochemical Mechanisms of Thalassiosira Pseudonana and Chaetoceros Calcitrans in Response to Different Light Intensities. Algal Res. 2017, 27, 89–98. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.; Sukhova, E.; Gromova, E.; Mudrilov, M.; Zolin, Y.; Popova, A.; Nerush, V.; Pecherina, A.; Grishin, A.A.; Dorokhov, A.A. Effect of Duration of LED Lighting on Growth, Photosynthesis and Respiration in Lettuce. Plants 2023, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, R.L.; Knepper, C.; Cahn, M.D.; Mou, B. Screening of Lettuce Germplasm for Agronomic Traits under Low Water Conditions. HortScience 2016, 51, 669–679. [Google Scholar] [CrossRef]
- Cammarisano, L.; Körner, O. Response of Cyanic and Acyanic Lettuce Cultivars to an Increased Proportion of Blue Light. Biology 2022, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, M.; Gao, J.X.; Han, Y.W. Fast chlorophyll fluorescence kinetics and its application in plant physiology re-search. J. Biol. 2011, 28, 81–86. [Google Scholar] [CrossRef]
- Tikkanen, M.; Mekala, N.R.; Aro, E.M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta (BBA)–Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef]
- Cai, S.Q.; Xu, D.Q. Light intensity-dependent reversible down-regulation and irreversible damage of PSII in soybean leaves. Plant Sci. 2002, 163, 847–853. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, C.S.; Li, P.M.; Ma, W.F. Effects of drought on the photosynthetic apparatus in Malus hupehensis leaves explored by simultaneous measurement of prompt fluorescence, delayed fluorescence and modulated light reflection at 820 nm. Plant Physiol. J. 2013, 49, 551–560. [Google Scholar] [CrossRef]
- Lidon, F.J.C.; Reboredo, F.H.; Leitão, A.E.; Silva, M.M.A.; Duarte, M.P.; Ramalho, J.C. Impact of UV-B radiation on photosynthesis—An overview. Emir. J. Food Agric. 2012, 24, 546–556. [Google Scholar] [CrossRef]
- Jin, L.Q.; Che, X.K.; Zhang, Z.S.; Gao, Y.H. The relationship between the changes in Wk and different damage degree of PSII donor side and acceptor side under high temperature with high light in cucumber. Plant Physiol. J. 2015, 51, 969–976. [Google Scholar] [CrossRef]
- Chen, H.X.; Li, W.J.; An, S.Z.; Gao, H.Y. Characterization of PSII photochemistry and thermostability in salt-treated Rumex leaves. J. Plant Physiol. 2004, 161, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Strivastava, A.; Govindgee. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, Y.J.; Gao, H.Y.; Zhang, L.T.; Li, H.D.; Meng, Q.W. Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis sativus L.) leaves. Planta 2011, 234, 883–889. [Google Scholar] [CrossRef]
- Van Heerden, P.D.R.; Strasser, R.J.; Krüger, G.H.J. Reduction of dark chilling stress in N2-fixing soybean by nitrate as indi-cated by chlorophyll a fluorescence kinetics. Physiol. Plant. 2004, 121, 239–249. [Google Scholar] [CrossRef]
- Liu, M.S.; Huang, X.H.; Wang, R.J.; Xu, H.Y.; Zhu, F. Inhibition of photosynthesis in Melia azedarach and Ligustrum lucid-um induced by manganese toxicity using OJIP chlorophyll a fluorescence transient. Photosynthetica 2021, 59, 148–159. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820 nm reflection changes during drying and after rehydration of the resurrection plant haberlea rhodopensis. Biochim. Biophys. Acta (BBA)–Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Yan, K.; Zhao, S.J.; Xu, H.L.; Wu, C.W.; Chen, X.B. Effects of salt stress on photosynthetic characters in honeysuckle with different ploidies. Sci. Agric. Sin. 2015, 48, 3275–3286. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching: A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Li, Z.; Fan, F.; Liang, Y. Silicon ameliorates manganese toxicity by regulating both physiological processes and expression of genes associated with photosynthesis in rice (Oryza sativa L.). Plant Soil 2015, 397, 289–301. [Google Scholar] [CrossRef]
- Wan, P.; Xiong, X.Z.; Huang, X.H.; Wu, J.C.; Ou, Y.; Deng, X.M.; Liu, Y. Effects of pesticides stress on the chlorophyll fluo-rescence characteristics and growth of Vernicia fordII seedlings. Sci. Silvae Sin. 2016, 52, 22–29. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Li, J.; Guo, S.; Li, Y.; Jin, Z. Photosynthesis, Nitrogen Allocation, Non-Structural Carbohydrate Allocation, and C:N:P Stoichiometry of Ulmus elongata Seedlings Exposed to Different Light Intensities. Life 2022, 12, 1310. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.S.; Yun, F.; Shi, H.Z.; Wang, K.; Zhagn, C.H.; Song, J. Effects of Cooperation of Light and Nitrogen on the Content of Nitric Compound, Antioxidant System and Quality of Flue-cured Tobacco. Sci. Agric. Sin. 2010, 43, 3732–3741. [Google Scholar] [CrossRef]
- Luo, W.J. Effects of Light and Nitrogen Interaction on Growth and Physiology of Horsfieldia hainanensis Merr. Seedlings. Master’s Thesis, Guangxi University, Nanning, China, 2018. [Google Scholar] [CrossRef]
- Yuan, X.K.; Yang, Z.Q.; Li, Y.X.; Han, W. Effects of different levels of water stress on leaf photosynthetic characteristics and antioxidant enzyme activities of greenhouse tomato. Photosynthetica 2016, 54, 28–39. [Google Scholar] [CrossRef]
| Data extracted from recorded fluorescence transient OJIP | |
| Ft | Fluorescence at time t after onset of actinic illumination |
| F20μs | First reliable recording of FL fluorescence in 20 μs |
| F300μs | Fluorescence intensity at 300 μs |
| FJ = F2ms | Fluorescence intensity at the J-step (2 ms) of OJIP |
| FI = F30ms | Fluorescence intensity at the I-step (30 ms) of OJIP |
| FP | Maximal recorded FL fluorescence intensity, peak P of OJIP |
| tFM | Time to reach maximum value in milliseconds (ms) |
| Area | Total complementary area between FL fluorescence induction curve and F = FM |
| Fluorescence parameters derived from the extracted data | |
| FO ≌ F20μs | Minimal fluorescence when all RCs are on |
| FM | Maximum FL fluorescence when all RCs are on |
| FV = FM − FO | Maximum variable fluorescence |
| Wk = (Fk − FO)/(FJ − FO) | K-step relative variable fluorescence |
| VJ = (FJ − FO)/FM − FO) | J-step relative variable fluorescence |
| VI = (FI − FO)/(FM − FO) | I-step relative variable fluorescence |
| MO = 4(F300μs − FO)/(FM − FO) | Approximated initial slope (in ms−1) of the fluorescence transient normalized at the maximum variable fluorescence FV |
| SM = (Area)/(FM − FO) | Normalized area relative variable approximate initial slope of FL fluorescence at time t in ms−1 |
| Quantum yields and efficiency/probability | |
| φPo = TRO/ABS = [1 − (FO/FM)] | Maximum quantum yield of primary photochemistry at t = 0 |
| ΨO = ETO/TRO = (1 − VJ) | Probability of a captured excitation moving an electron into the electron transport chain outside of QA at t = 0 |
| φEo = ETO/ABS = [1 − (FO/FM)] · ΨO | Quantum yield of electron transport (at t = 0) |
| δ(Ro) = (1 − VI)/(1 − VJ) | Probability of an electron from the reducing-end electron acceptor at the QB to the reducing-end electron acceptor at the PS I acceptor (RE) |
| φDo = 1 − φPo = (FO/FM) | Quantum yield of energy dissipation (at t = 0) |
| Performance | |
| PIABS = (RC/ABS) · [φPo/(1 − φPo)] · [ΨO/(1 − ΨO)] | The performance index (potential) of PS II absorbing photon energy conservation to intersystem electron acceptor reduction |
| Calculated data for absorbance at 820 nm | |
| I0 = I0.7 | First reliable recorded fluorescence at 0.7 ms |
| Imin | Minimum fluorescence intensity |
| ΔI/I0 = (I0 − Imin)/I0 | 820 nm red light absorbance value |
| Data calculated from the recorded fluorescence transient OJIP and absorbance at 820 nm | |
| Φ(PSI/PSII) = (ΔI/Io)/ΨO | Coordination of photosystem II (PSII) and photosystem I (PSI) |
| Shading Treatment | φPo | Ψo | φEo | δ(Ro) | φDo |
|---|---|---|---|---|---|
| L1 | 0.71 ± 0.04 a | 0.37 ± 0.05 a | 0.27 ± 0.03 a | 0.07 ± 0.01 a | 0.29 ± 0.04 a |
| L2 | 0.81 ± 0.02 b | 0.45 ± 0.05 ab | 0.36 ± 0.03 b | 0.19 ± 0.02 b | 0.19 ± 0.02 b |
| L3 | 0.81 ± 0.01 b | 0.40 ± 0.02 a | 0.32 ± 0.01 ab | 0.19 ± 0.02 b | 0.19 ± 0.01 b |
| L4 | 0.75 ± 0.03 ab | 0.54 ± 0.03 b | 0.39 ± 0.02 b | 0.18 ± 0.01 b | 0.25 ± 0.03 ab |
| Shading Treatment | WK | VJ | Mo | SM |
|---|---|---|---|---|
| L1 | 0.53 ± 0.02 a | 0.63 ± 0.05 a | 1.08 ± 0.05 a | 5.23 ± 0.69 a |
| L2 | 0.43 ± 0.02 b | 0.55 ± 0.06 ab | 0.83 ± 0.06 bc | 11.92 ± 1.51 b |
| L3 | 0.39 ± 0.03 b | 0.60 ± 0.01 ab | 0.95 ± 0.02 ab | 11.01 ± 1.25 b |
| L4 | 0.45 ± 0.02 b | 0.49 ± 0.01 b | 0.76 ± 0.04 c | 12.8 ± 0.74 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Ma, J.; Huang, R.; Wang, Y.; Jiang, Y.; Ling, Y.; Yang, J.; Liang, H.; Liu, X.; Liao, N. The Effects of Shading on the Photosynthetic Performance of Endangered Plant Horsfieldia hainanensis Seedlings. Forests 2024, 15, 3. https://doi.org/10.3390/f15010003
Wang R, Ma J, Huang R, Wang Y, Jiang Y, Ling Y, Yang J, Liang H, Liu X, Liao N. The Effects of Shading on the Photosynthetic Performance of Endangered Plant Horsfieldia hainanensis Seedlings. Forests. 2024; 15(1):3. https://doi.org/10.3390/f15010003
Chicago/Turabian StyleWang, Renjie, Jinman Ma, Ronglin Huang, Yong Wang, Yi Jiang, Yaming Ling, Jisheng Yang, Huizi Liang, Xiongsheng Liu, and Nanyan Liao. 2024. "The Effects of Shading on the Photosynthetic Performance of Endangered Plant Horsfieldia hainanensis Seedlings" Forests 15, no. 1: 3. https://doi.org/10.3390/f15010003
APA StyleWang, R., Ma, J., Huang, R., Wang, Y., Jiang, Y., Ling, Y., Yang, J., Liang, H., Liu, X., & Liao, N. (2024). The Effects of Shading on the Photosynthetic Performance of Endangered Plant Horsfieldia hainanensis Seedlings. Forests, 15(1), 3. https://doi.org/10.3390/f15010003





