Tetramycin B3: An Effective and Biological Nematicide for Bursaphelenchus xylophilus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Nematodes
2.3. Nematicidal Activity Test
2.4. Effect of TB3 on Feeding and Population Growth Rate of B. xylophilus
2.5. Effect of TB3 on ROS Production and Lipid Accumulation in B. xylophilus
2.6. Effect of TB3 on POD, SOD, GST Activity, and CYP450 Content of B. xylophilus
2.7. Mechanism of Action of TB3 on B. xylophilus
2.8. Metabolomic Analysis of TB3 on B. xylophilus
2.9. Evaluation of the Prevention and Control Effect of TB3 on Pine Wilt Disease in Pots
2.9.1. Evaluation of the Safety of TB3 on Pine Trees
2.9.2. Assessment of the Preventive Effect of TB3 on Pine Wilt Disease
2.10. Statistical Analysis
3. Results
3.1. Nematicidal Activity
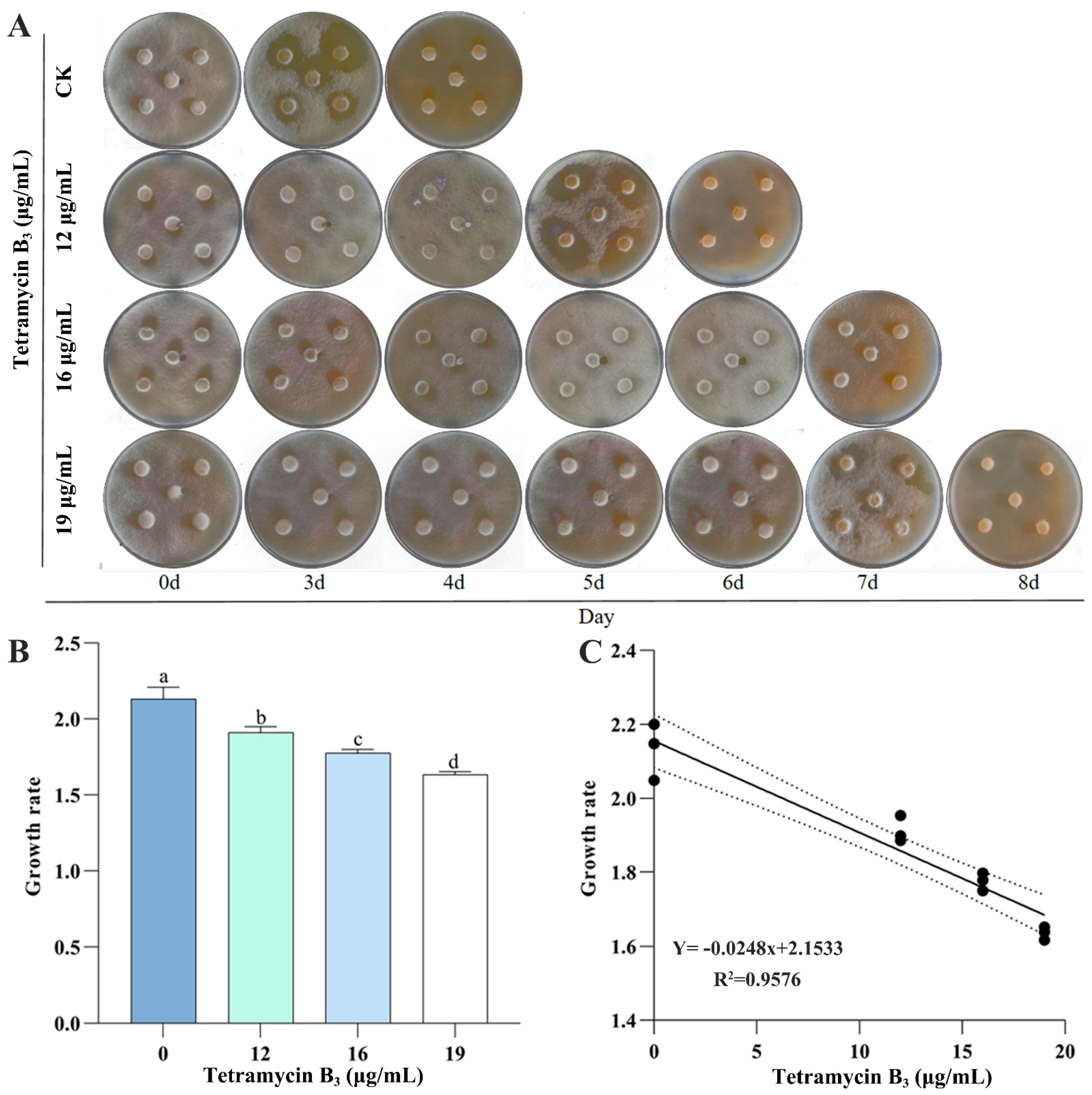
3.2. Feeding and Population Growth Rate of B. xylophilus
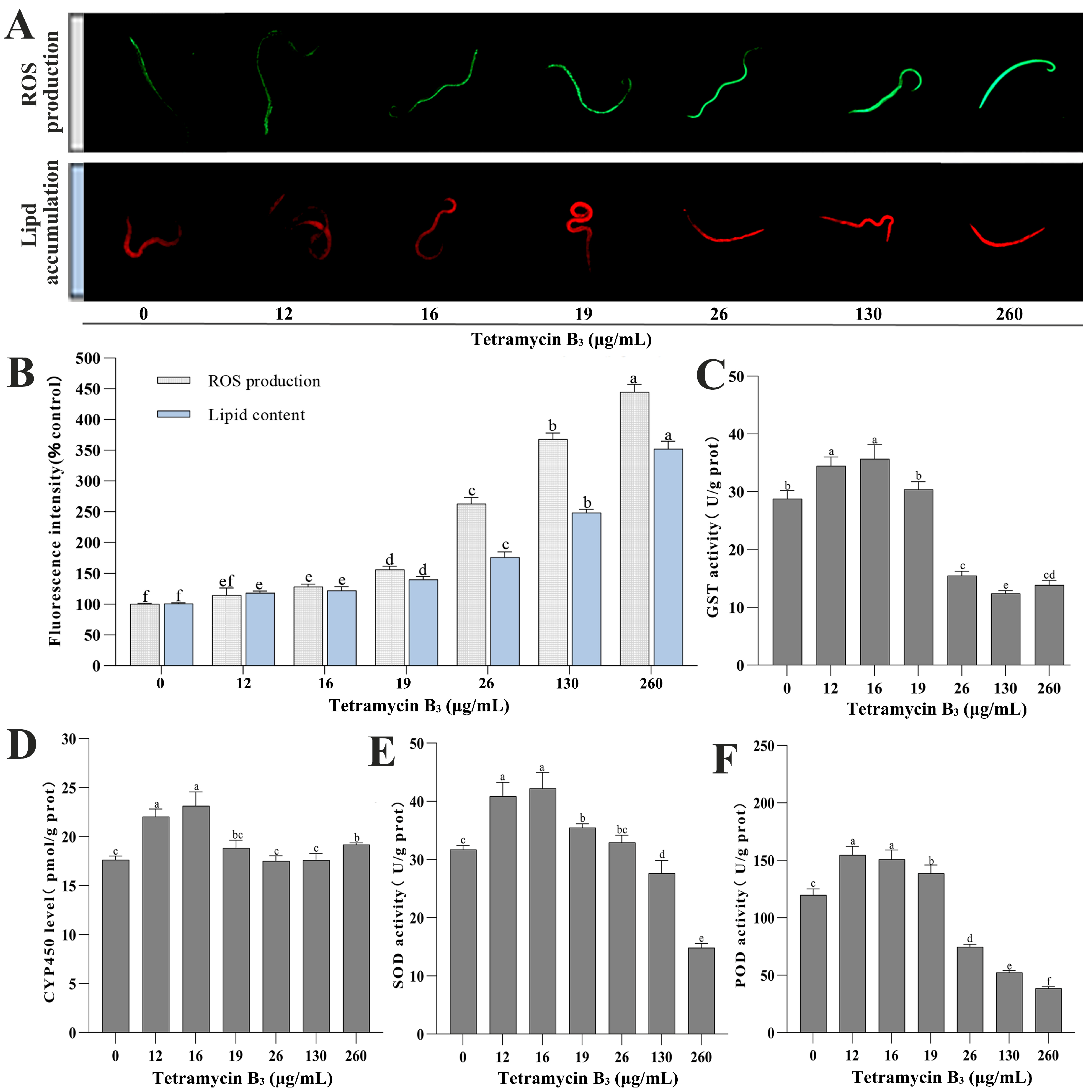
3.3. ROS Production and Lipid Accumulation in B. xylophilus
3.4. Effects of TB3 on POD, SOD, GST Activity, and CYP450 Content in B. xylophilus
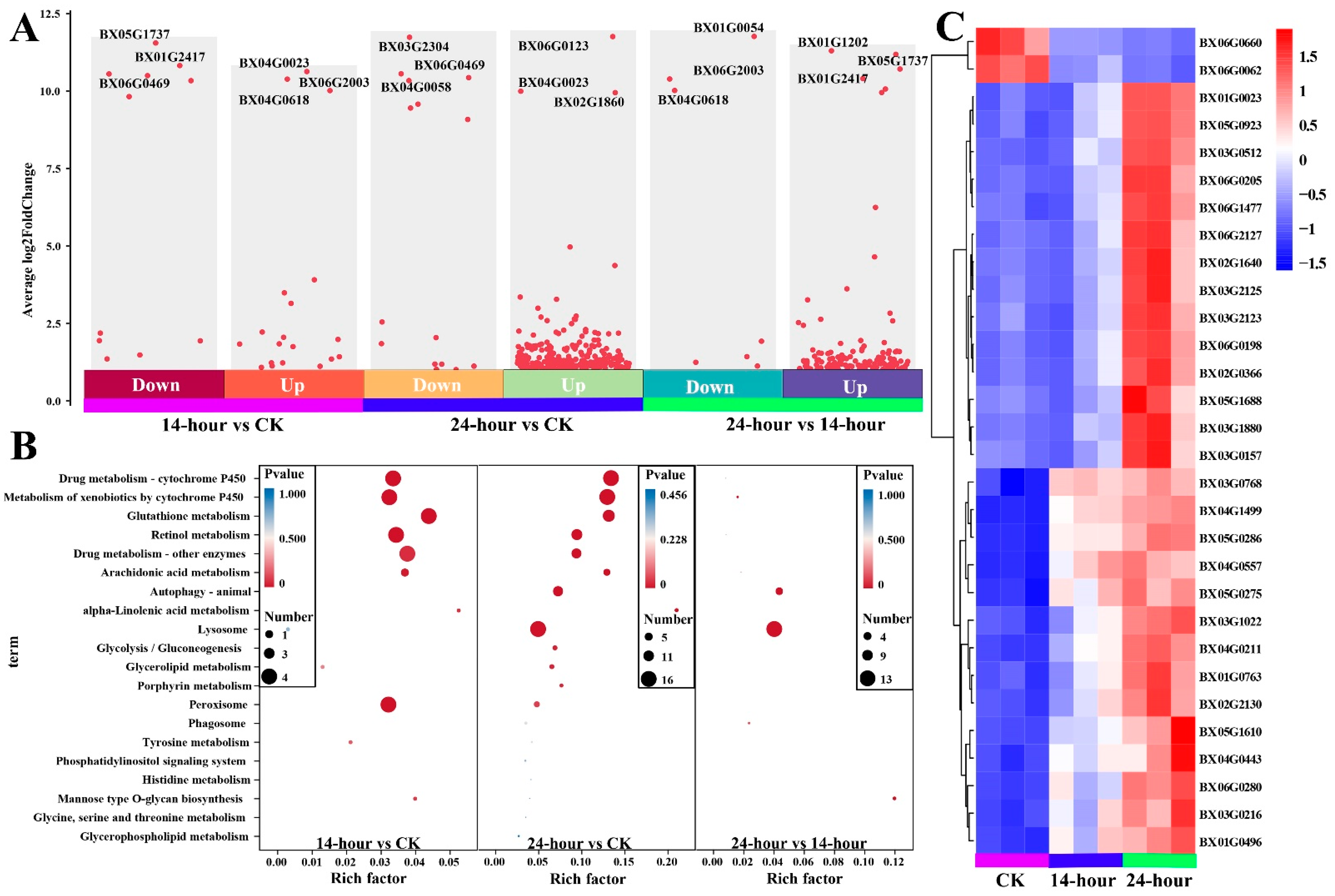
3.5. Effect of TB3 in B. xylophilus Based on Transcriptomics
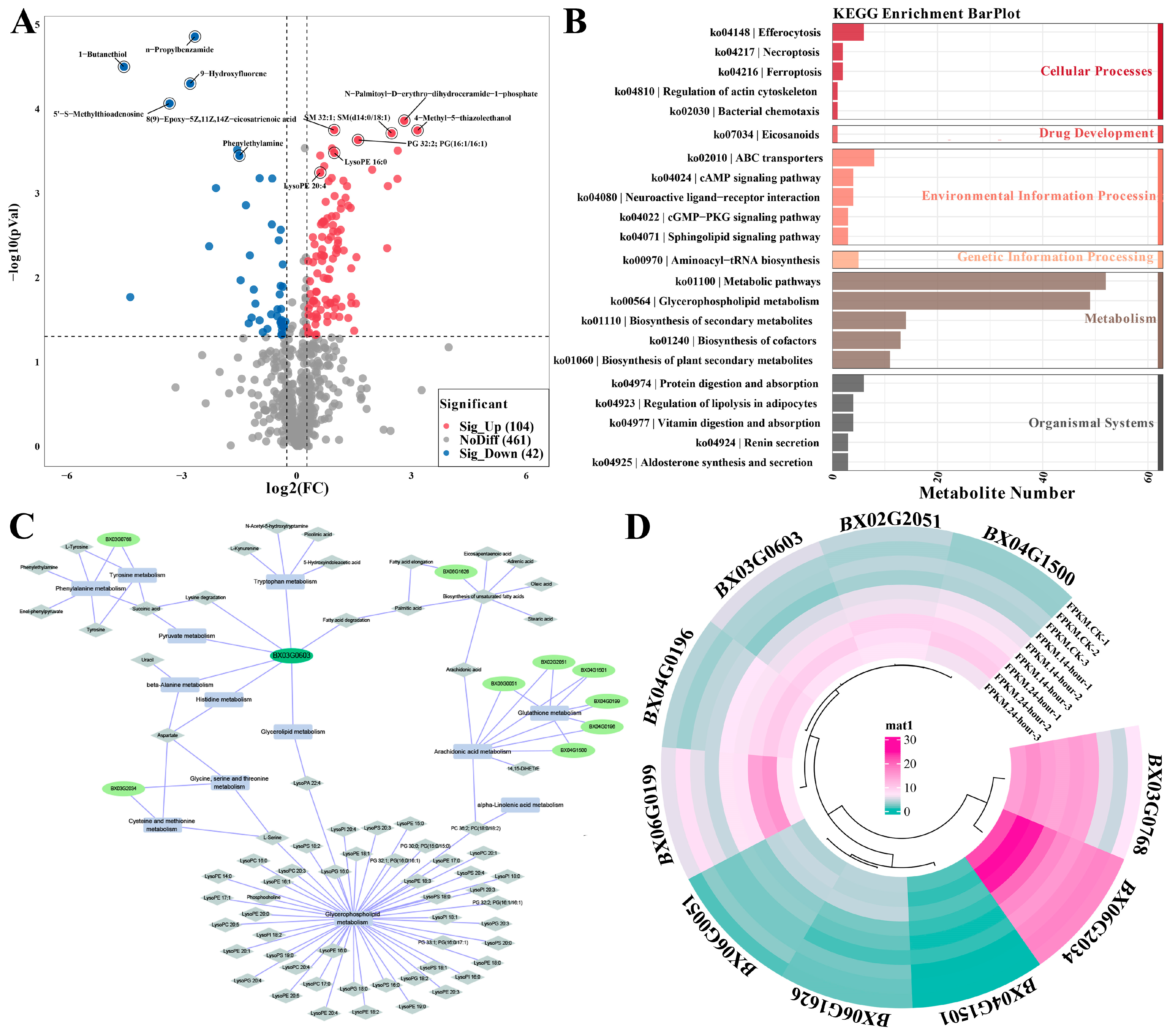
3.6. Effect of TB3 on the Expression of Metabolic Pathways in B. xylophilus Based on Metabolomics
3.7. Evaluation of the Prevention and Control Effect of TB3 on Pine Wilt Disease in Pot
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gaspar, D.; Trindade, C.; Usié, A.; Meireles, B.; Fortes, A.M.; Guimaraes, J.B.; Simoes, F.; Costa, R.L.; Ramos, A.M. Comparative transcriptomic response of two pinus species to infection with the pine wood nematode Bursaphelenchus xylophilus. Forests 2020, 11, 204. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef] [PubMed]
- Gruffudd, H.R.; Schröder, T.; Jenkins, T.A.R.; Evans, H.F. Modelling pine wilt disease (PWD) for current and future climate scenarios as part of a pest risk analysis for pine wood nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle in Germany. J. Plant Dis. Protect 2019, 126, 129–144. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, Q.Q.; Xie, N.; Yuan, G.Y.; Liao, M.Y.; Gui, Q.; Ding, G.J. Predicting the global potential distribution of Bursaphelenchus xylophilus using an ecological niche model: Expansion trend and the main driving factors. BMC. Ecol. Evol. 2024, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Jin, H.; Liu, Q.; Yan, Z.Q.; Ding, L.; Qin, B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest Manag. Sci. 2014, 70, 827–835. [Google Scholar] [CrossRef]
- Douda, O.; Stejskal, V.; Manasova, M.; Zouhar, M.; Hnatek, J. Inexpensive screening method to validate the efficacy of ethanedinitrile fumigant on the forest invasive nematode pest Bursaphelenchus xylophilus. Sustainability 2020, 12, 4765. [Google Scholar] [CrossRef]
- Wu, D.W.; Yu, L.F.; Yu, R.; Zhou, Q.; Li, J.X.; Zhang, X.D.; Ren, L.L.; Luo, Y.Q. Detection of the monitoring window for pine wilt disease using multi-temporal UAV-based multispectral imagery and machine learning algorithms. Remote Sens. 2023, 15, 444. [Google Scholar] [CrossRef]
- Iordache, M.D.; Mantas, V.; Baltazar, E.; Pauly, K.; Lewyckyj, N. A machine learning approach to detecting pine wilt disease using airborne spectral imagery. Remote Sens. 2020, 12, 2280. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, H.R.; Kim, D.S.; Kwon, J.H.; Huh, M.J.; Park, I.K. Emamectin benzoate 9.7% SL as a new formulation for a trunk-injections against pine wood nematode, Bursaphelenchus xylophilus. J. For. Res. 2020, 31, 1399–1403. [Google Scholar] [CrossRef]
- Lin, N.; Su, X.; Zhou, X.; Zhou, L.F.; Chen, A.L.; Hu, J.F.; Guo, K. Characteristics and conductivity of emamectin benzoate-inclusive nanocapsule in Pinus Massoniana Lamb. Forests 2024, 15, 444. [Google Scholar] [CrossRef]
- Lee, J.W.; Mwamula, A.O.; Choi, J.H.; Lee, H.W.; Kim, Y.S.; Kim, J.H.; Choi, Y.H.; Lee, D.W. Comparative bioactivity of emamectin benzoate formulations against the pine wood nematode, Bursaphelenchus xylophilus. Plant Pathol. J. 2023, 39, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ou, Y.Q.; Zhang, Q.; Gan, X.H. Discovery of 1,2,4-oxadiazole derivatives containing haloalkyl as potential acetylcholine receptor nematicides. Int. J. Mol. Sci. 2023, 24, 5773. [Google Scholar] [CrossRef] [PubMed]
- Espedal, P.G.; Glover, K.A.; Horsberg, T.E.; Nilsen, F. Emamectin benzoate resistance and fitness in laboratory reared salmon lice (Lepeophtheirus salmonis). Aquaculture 2013, 416, 111–118. [Google Scholar] [CrossRef]
- Ren, J.; Cui, Y.Q.; Zhang, F.; Cui, H.; Ni, X.P.; Chen, F.; Li, L.; Xia, H.Z. Enhancement of nystatin production by redirecting precursor fluxes after disruption of the tetramycin gene from Streptomyces ahygroscopicus. Microbiol. Res. 2014, 169, 602–608. [Google Scholar] [CrossRef]
- Song, Y.Y.; He, L.M.; Chen, L.L.; Ren, Y.P.; Lu, H.B.; Geng, S.; Mu, W.; Liu, F. Baseline sensitivity and control efficacy of antibiosis fungicide tetramycin against Botrytis cinerea. Eur. J. Plant Pathol. 2016, 146, 337–347. [Google Scholar] [CrossRef]
- Ma, D.C.; Zhu, J.M.; He, L.M.; Cui, K.D.; Mu, W.; Liu, F. Baseline sensitivity and control efficacy of tetramycin against Phytophthora capsici isolates in China. Plant Dis. 2018, 102, 863–868. [Google Scholar] [CrossRef]
- Gao, Y.Y.; He, L.F.; Li, X.X.; Lin, J.; Mu, W.; Liu, F. Toxicity and biochemical action of the antibiotic fungicide tetramycin on Colletotrichum scovillei. Pestic. Biochem. Phys. 2018, 147, 51–58. [Google Scholar] [CrossRef]
- Gao, J.T.; Lin, G.; Deng, X.M.; Zou, J.X.; Liu, Y.; Chen, X.J.; Liu, S.W. Development of tetramycin-loaded core-shell beads with hot-/wet-responsiverelease properties for control of bacterial wilt disease. Agronomy 2024, 14, 1199. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.T.; Wu, X.M.; Su, Y.; Long, Y.H. Co-Application of tetramycin and chitosan in controlling leaf spot disease of kiwifruit and enhancing its resistance, photosynthesis, quality and amino acids. Biomolecules 2022, 12, 500. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, S.; Cai, Q.F.; Song, H.Y.; Chen, J.X. Discovery and mechanism of a nematicide candidate (W3): A novel amide compound containing a cyclopropyl moiety. J. Agric. Food Chem. 2024, 72, 5585–5594. [Google Scholar] [CrossRef]
- Qu, M.; Xu, K.N.; Li, Y.H.; Wong, G.; Wang, D.Y. Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci. Total Environ. 2018, 643, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yang, J.; Li, H.; Guo, S.; Tariq, M.; Chen, H.B.; Wang, C.; Liu, Y.D. Chronic toxicity of hexabromocyclododecane (HBCD) induced by oxidative stress and cell apoptosis on nematode Caenorhabditis elegans. Chemosphere 2018, 208, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Yin, L.; Li, X.; Tang, M.; Zhang, T.; Wang, D.Y. Contributions of altered permeability of intestinal barrier and defecation behavior to toxicity formation from graphene oxide in nematode Caenorhabditis elegans. Nanoscale 2013, 5, 9934–9943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.P.; Wang, Y.; Liu, H.M.; Zhang, S.A.; Ji, X.X.; Qiao, K. Oxidative stress, intestinal damage, and cell apoptosis: Toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere 2022, 286, 131830. [Google Scholar] [CrossRef]
- Le Dang, Q.; Kim, W.K.; Cuong, M.N.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Park, M.S.; Lim, C.H.; Ngoc, H.L.; Kim, J.C. Nematicidal and antifungal activities of annonaceous acetogenins from Annona squamosa against various plant pathogens. J. Agric. Food Chem. 2011, 59, 11160–11167. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, K.T.; Park, Y.B.; Jin, G.H.; Ka, K.H.; Seo, S.T. Nematicidal effect of Sparassis latifolia-derived sparassol and disodium sparassol against Bursaphelenchus xylophilus. J. Asia-Pac. Entomol. 2016, 19, 81–84. [Google Scholar] [CrossRef]
- Cha, D.J.; Kim, J.; Kim, D.S. Nematicidal activities of three naphthoquinones against the pine wood nematode, Bursaphelenchus xylophilus. Molecules 2019, 24, 3634. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wu, X.Q.; Ye, J.R.; Li, C.Q.; Hu, L.J.; Rui, L.; Zhang, Y.; Shi, X.F.; Wang, L. Toxicity of an emamectin benzoate microemulsion against Bursaphelenchus xylophilus and its effect on the prevention of pine wilt disease. Forests 2023, 14, 1476. [Google Scholar] [CrossRef]
- Lee, J.W.; Mwamula, A.O.; Choi, J.H.; Lee, H.W.; Kim, Y.S.; Kim, J.H.; Lee, D.W. The Potency of Abamectin Formulations against the pine wood nematode, Bursaphelenchus xylophilus. Plant Pathol. J. 2023, 39, 290–302. [Google Scholar] [CrossRef]
- Liu, G.Y.; Lin, X.; Xu, S.Y.; Liu, G.; Liu, Z.Y.; Liu, F.; Mu, W. Efficacy of fluopyram as a candidate trunk-injection agent against Bursaphelenchus xylophilus. Eur. J. Plant Pathol. 2020, 157, 403–411. [Google Scholar] [CrossRef]
- Lou, I.C.; Zhao, Y.C.; Wu, Y.J.; Ricci, P.F. Systems cancer biology and the controlling mechanisms for the J-shaped cancer dose response: Towards relaxing the lnt hypothesis. Dose-Response 2013, 11, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.R.; Bhattacharyya, C.; Sarkar, A. Glutathione: Role in oxidative/nitrosative stress, antioxidant defense, and treatments. ChemistrySelect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Tang, H.Q.; Long, N.N.; Lin, L.; Liu, Y.; Li, J.L.; Sun, F.H.; Guo, L.J.; Zhang, F.; Dai, M. Effect of MRSA on CYP450: Dynamic changes of cytokines, oxidative stress, and drug-metabolizing enzymes in mice infected with MRSA. Infect. Drug Resist. 2018, 11, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Guo, K.; Chen, A.L.; Chen, S.N.; Lin, H.P.; Zhou, X. Transcriptomic profiling of effects of emamectin benzoate on the pine wood nematode Bursaphelenchus xylophilus. Pest Manag. Sci. 2020, 76, 747–757. [Google Scholar] [CrossRef]
- Li, X.M.; Wen, J.H.; Feng, Z.S.; Wu, Y.S.; Li, D.Y.; Liang, S.; Wu, D.; Wu, H.L.; Li, S.M.; Ye, Z.N.; et al. Effect of lacking ZKSCAN3 on autophagy, lysosomal biogenesis and senescence. Int. J. Mol. Sci. 2023, 24, 7786. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, C.; Wu, X.M.; Long, Y.H.; Su, Y. Chitosan augments tetramycin against soft rot in kiwifruit and enhances its improvement for kiwifruit growth, quality and aroma. Biomolecules 2021, 11, 1257. [Google Scholar] [CrossRef]


| Chemicals | Toxicity Regression Equation | Time | LC10 a | LC20 | LC30 | LC50 | LC90 | LC95 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| 1.5% Tetramycin | y = 1.3349 + 1.8616x | 24 h | 19.07 ± 0.1 Fa | 32.86 ± 1.1 Ea | 48.64 ± 3.2 Da | 93.05 ± 6.5 Ca | 169.37 ± 8.7 Ba | 197.84 ± 8.4 Aa | 0.9046 |
| y = 2.8635 + 1.6383x | 48 h | 3.32 ± 0.9 Ff | 6.17 ± 1.1 Ef | 9.63 ± 0.9 Df | 20.13 ± 0.4 Cf | 36.93 ± 3.2 Bf | 43.27 ± 4.2 Ae | 0.9920 | |
| y = 3.8689 + 1.0689x | 72 h | 0.72 ± 0.1 Fh | 1.86 ± 0.2 Eh | 3.69 ± 0.5 Di | 11.43 ± 1.9 Ci | 22.28 ± 1.6 Bh | 26.66 ± 0.24 Ag | 0.9507 | |
| Tetramycin B3 (TB3) | y = −1.4170 + 4.4524x | 24 h | 12.82 ± 2.8 Fc | 16.44 ± 2.1 Ed | 19.68 ± 1.1 Dd | 26.49 ± 1.2 Cd | 44.71 ± 6.4 Bd | 50.46 ± 8.3 Ad | 0.9837 |
| y = 2.9165 + 1.5221x | 48 h | 3.36 ± 0.4 Fe | 6.54 ± 1.1 Ee | 10.57 ± 2.1 De | 23.37 ± 0.4 Ce | 31.77 ± 0.8 Bg | 36.78 ± 0.5 Af | 0.9894 | |
| y = 3.8871 + 1.0053x | 72 h | 0.67 ± 0.2 Fi | 1.86 ± 0.4 Eh | 3.84 ± 0.5 Dh | 12.79 ± 0.1 Ch | 20.94 ± 0.9 Bi | 24.99 ± 1.1 Ag | 0.9989 | |
| 2% Emamectin benzoate | y = 0.6187 + 2.4559x | 24 h | 18.28 ± 2.5 Fb | 27.62 ± 2.6 Eb | 37.81 ± 2.4 Db | 60.81 ± 0.8 Cb | 117.96 ± 6.1 Bb | 138.15 ± 8.6 Ab | 0.9590 |
| y = −0.1082 + 3.4853x | 48 h | 12.53 ± 2.1 Fd | 16.75 ± 1.9 Ec | 20.66 ± 1.6 Dc | 29.21 ± 0.5 Cc | 56.11 ± 3.4 Bc | 64.98 ± 4.7 Ac | 0.9837 | |
| y = 2.9558 + 1.6770x | 72 h | 2.84 ± 0.1 Fg | 5.21 ± 0.3 Eg | 8.05 ± 0.4 Dg | 16.55 ± 0.6 Cg | 40.79 ± 0.1 Be | 48.57 ± 0.6 Ad | 0.9830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Li, W.; Ju, K.; Xiong, X.; Li, J.; Yu, C.; Tian, Y.; Liu, H. Tetramycin B3: An Effective and Biological Nematicide for Bursaphelenchus xylophilus. Forests 2024, 15, 1699. https://doi.org/10.3390/f15101699
Sun S, Li W, Ju K, Xiong X, Li J, Yu C, Tian Y, Liu H. Tetramycin B3: An Effective and Biological Nematicide for Bursaphelenchus xylophilus. Forests. 2024; 15(10):1699. https://doi.org/10.3390/f15101699
Chicago/Turabian StyleSun, Shuaibin, Wenchao Li, Kunyang Ju, Xiong Xiong, Jie Li, Chengming Yu, Yehan Tian, and Huixiang Liu. 2024. "Tetramycin B3: An Effective and Biological Nematicide for Bursaphelenchus xylophilus" Forests 15, no. 10: 1699. https://doi.org/10.3390/f15101699





