Visible Light-Induced Photocatalytic Degradation of Methylene Blue Using Copper-Doped Carbon Dots One-Step Derived from CCA-Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Copper-Doped CDs
2.3. Degradation of Methylene Blue
2.4. Characterizations of CDs and MB Degradation Products
3. Results and Discussion
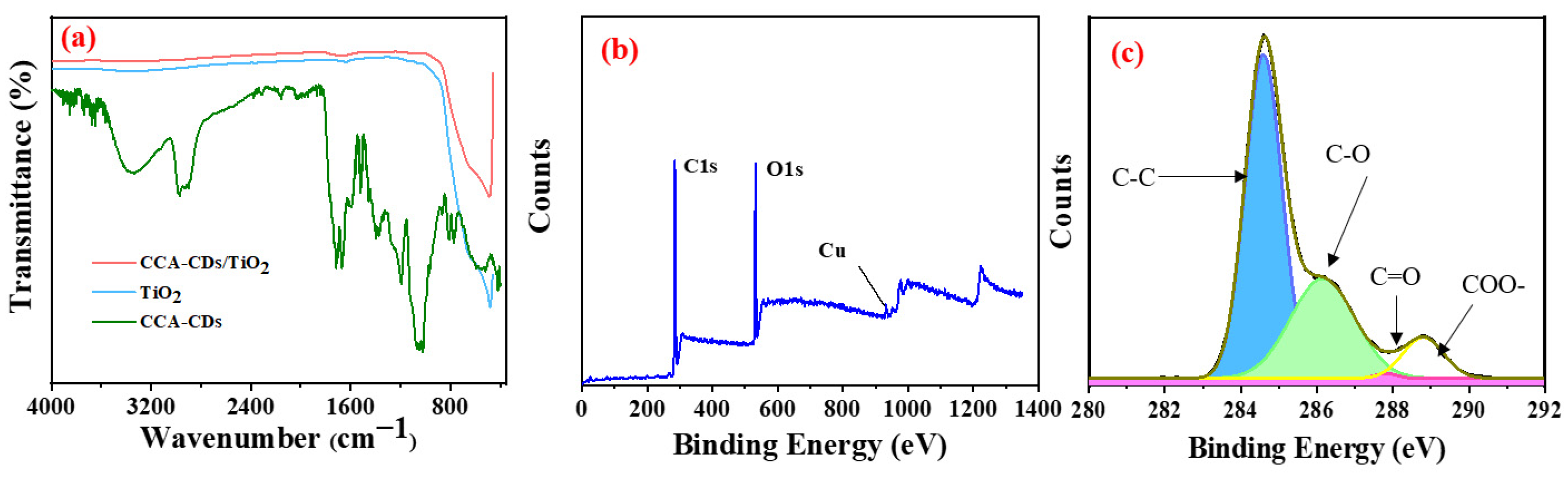
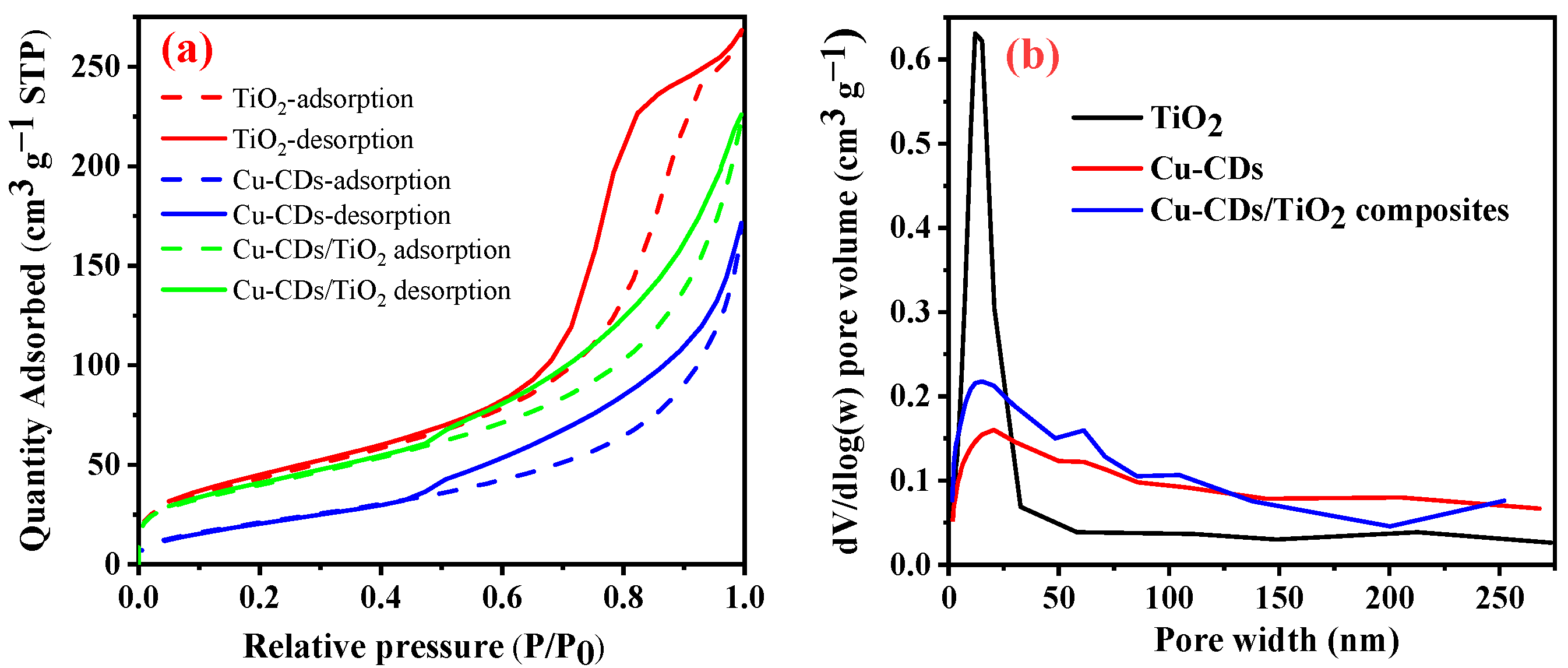
3.1. Characteristics of the Synthesized Samples
3.2. Photocatalysis Degradation of MB
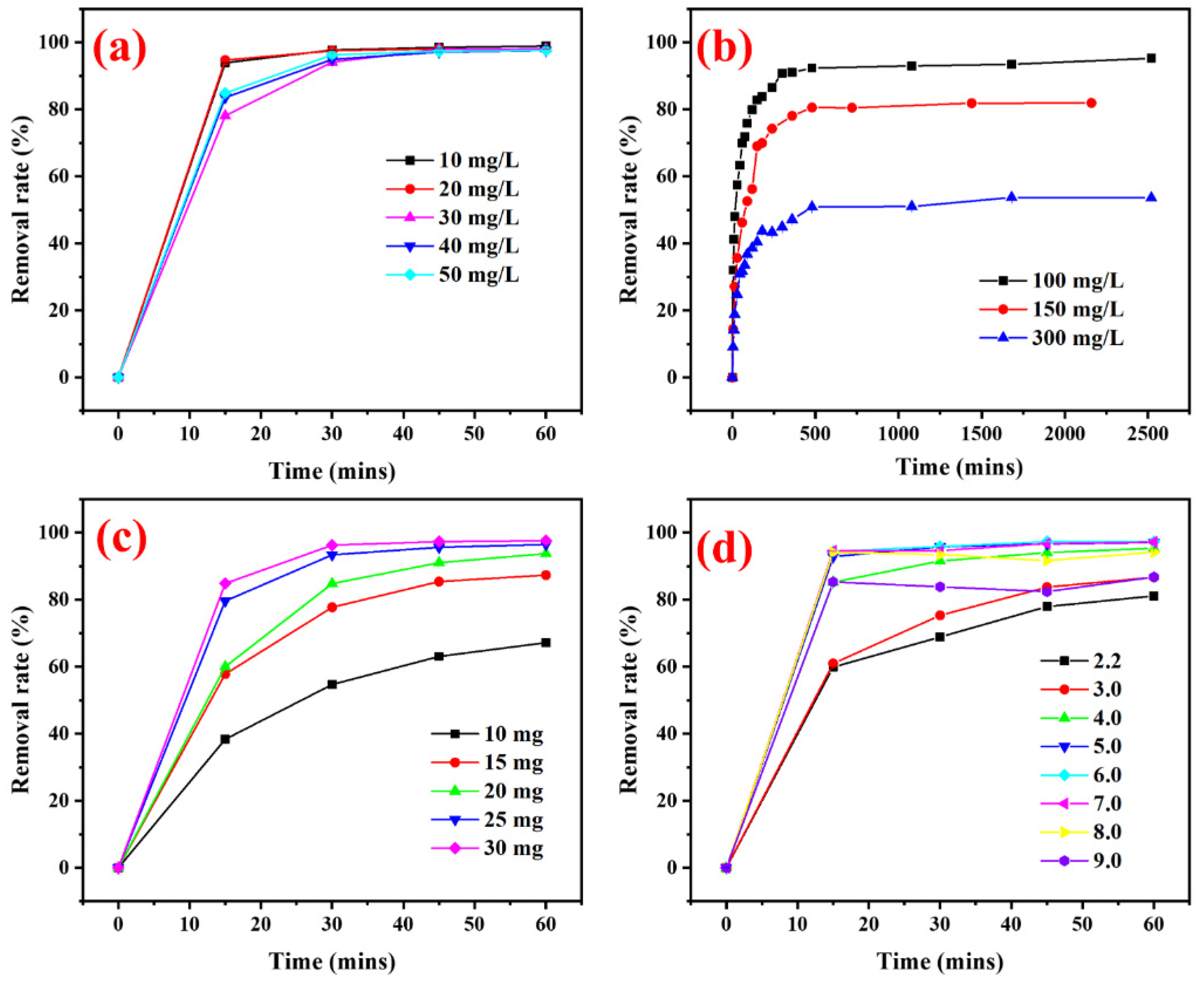
3.2.1. Influence of Photocatalyst Types
3.2.2. Influence of MB Concentrations, CCA-CDs Contents, and pH
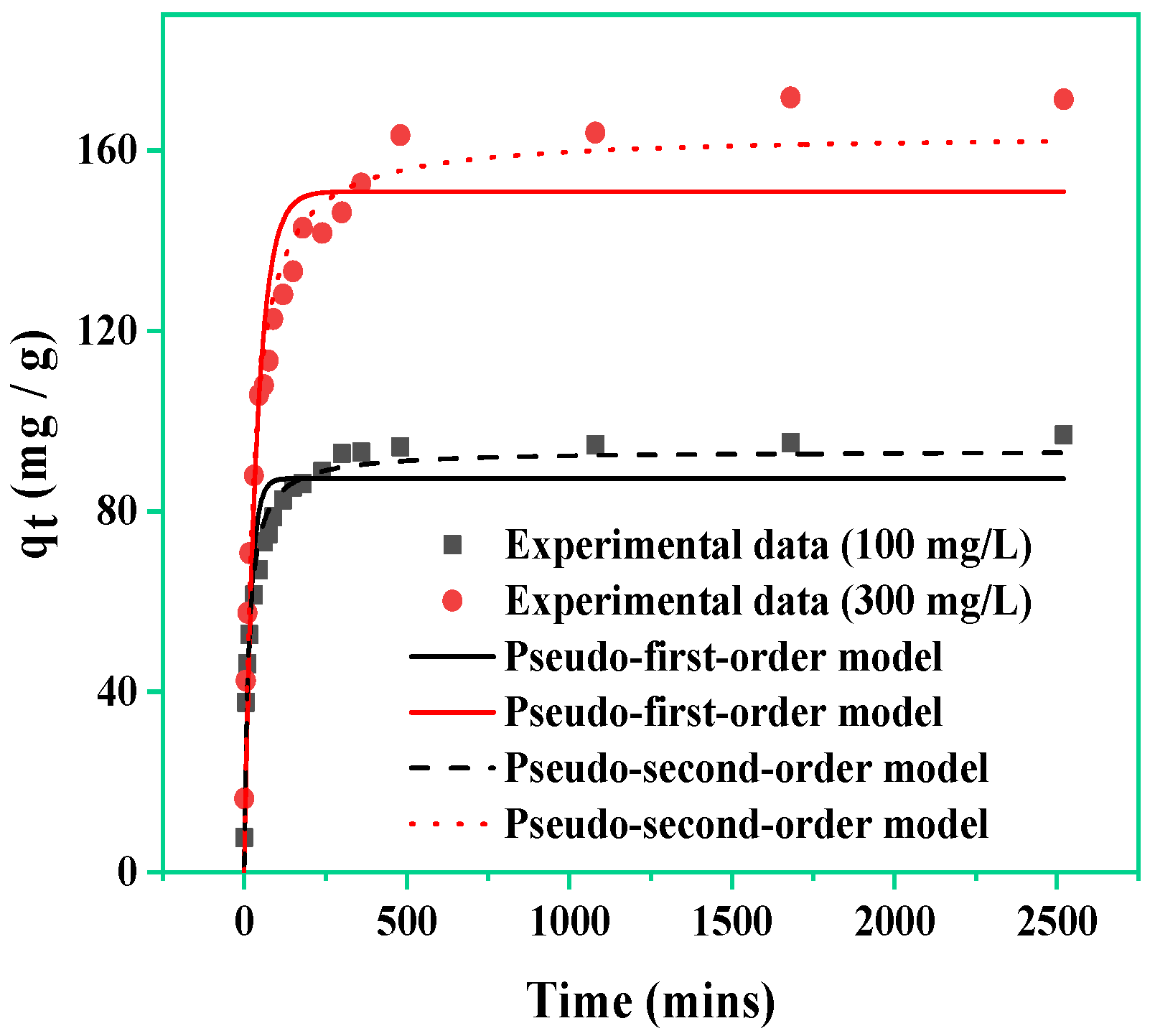
3.3. Kinetic Studies
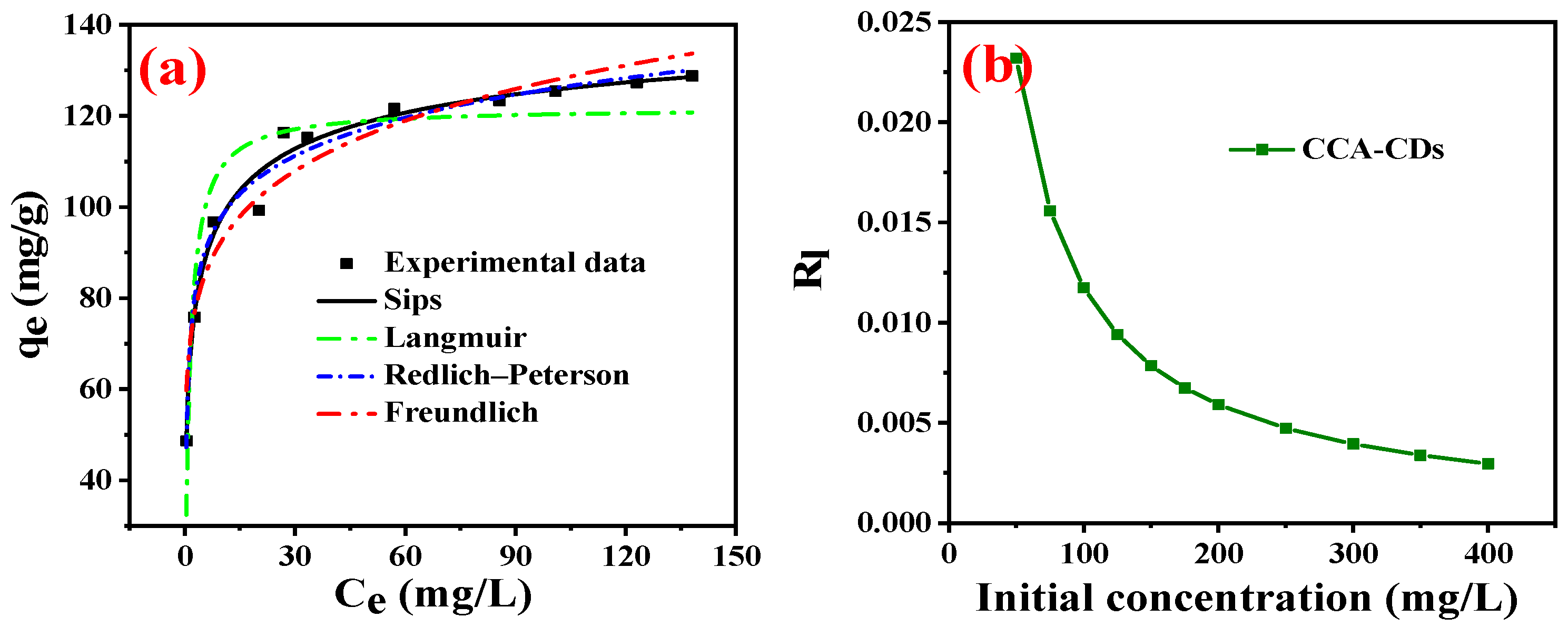
3.4. Isotherm Studies
3.5. Mechanism of MB Dye Photodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiam, S.-L.; Pung, S.-Y.; Yeoh, F.-Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, H.; Emran, M.Y.; El-Gammal, M.A. Biodegradation of azo dye pollutants using microorganisms. In Handbook of Biodegradable Materials; Springer: Cham, Switzerland, 2023; pp. 781–809. [Google Scholar]
- Zhang, X.; Li, Y.; Li, M.; Zheng, H.; Du, Q.; Li, H.; Wang, Y.; Wang, D.; Wang, C.; Sui, K.; et al. Removal of methylene blue from aqueous solution using high performance calcium alginate/activated carbon membrane. Int. J. Cloth. Sci. Technol. 2020, 32, 307–321. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute toxicity of textile dye Methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Mills, A.; Grosshans, P.; Hazafy, D. A novel reversible relative-humidity indicator ink based on methylene blue and urea. Analyst 2010, 135, 33–35. [Google Scholar] [CrossRef]
- Nyankson, E.; Kumar, R.V. Removal of water-soluble dyes and pharmaceutical wastes by combining the photocatalytic properties of Ag3PO4 with the adsorption properties of halloysite nanotubes. Mater. Today Adv. 2019, 4, 100025. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Jothi, V.K.; Ganesan, K.; Natarajan, A.; Rajaram, A. Green Synthesis of Self-Passivated Fluorescent Carbon Dots Derived from Rice Bran for Degradation of Methylene Blue and Fluorescent Ink Applications. J. Fluoresc. 2021, 31, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Wozniak, A.; Pietrzak, R. Adsorption of organic and inorganic pollutants on activated bio-carbons prepared by chemical activation of residues of supercritical extraction of raw plants. Chem. Eng. J. 2020, 393, 124785. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, N.; Jiang, Y.; Han, J.; Feng, G.; Shi, Z.; He, C. Rapid photodegradation of methylene blue by laser-induced plasma. RSC Adv. 2022, 12, 21056–21065. [Google Scholar] [CrossRef]
- Juang, R.S.; Ju, Y.C.; Liao, C.S.; Lin, K.S.; Lu, H.C.; Wang, S.F.; Sun, A.C. Synthesis of Carbon Dots on Fe3O4 Nanoparticles as Recyclable Visible-Light Photocatalysts. IEEE Trans. Magn. 2017, 53, 5200404. [Google Scholar] [CrossRef]
- Singh, P.; Mohan, B.; Madaan, V.; Ranga, R.; Kumari, P.; Kumar, S.; Bhankar, V.; Kumar, P.; Kumar, K. Nanomaterials photocatalytic activities for waste water treatment: A review. Environ. Sci. Pollut. Res. 2022, 29, 69294–69326. [Google Scholar] [CrossRef] [PubMed]
- Akbar, K.; Moretti, E.; Vomiero, A. Carbon dots for photocatalytic degradation of aqueous pollutants: Recent advancements. Adv. Opt. Mater. 2021, 9, 2100532. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Hebbar, A.; Selvaraj, R.; Vinayagam, R.; Varadavenkatesan, T.; Kumar, P.S.; Duc, P.A.; Rangasamy, G. A critical review on the environmental applications of carbon dots. Chemosphere 2023, 313, 137308. [Google Scholar] [CrossRef]
- Song, Z.; Shang, Y.; Lou, Q.; Zhu, J.; Hu, J.; Xu, W.; Li, C.; Chen, X.; Liu, K.; Shan, C.X. A molecular engineering strategy for achieving blue phosphorescent carbon dots with outstanding efficiency above 50%. Adv. Mater. 2023, 35, 2207970. [Google Scholar] [CrossRef]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-based carbon dots: Current development and future perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Singh, P.; Kumar, S.; Kumar, P.; Bhankar, V.; Kumar, K. Plant-mediated synthesis of nanoparticles and their applications: A review. Mater. Res. Bull. 2023, 163, 112233. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Pang, T.; Teng, Q.; Li, C.; Jiang, N.; Zheng, S.; Zhang, R.; Zheng, Y.; Chen, D. Ultra-Narrow-Bandwidth Deep-Red Electroluminescence Based on Green Plant-Derived Carbon Dots. Adv. Mater. 2023, 35, 2302275. [Google Scholar] [CrossRef]
- Kaur, I.; Batra, V.; Bogireddy, N.K.R.; Landa, S.D.T.; Agarwal, V. Detection of organic pollutants, food additives and antibiotics using sustainable carbon dots. Food Chem. 2023, 406, 135029. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, M.; Wang, Y.; Li, C.; Zhang, X.; Weng, S.; Li, Y. Carbon dots with selective fluorescence response to hydroxyl radical for sensitive detection of bleomycin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 306, 123582. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, Y.; Zhang, C.; Zhang, J.; Liu, S.; Zhang, K.; Lan, M. Recent advances of solvent-engineered carbon dots: A review. Carbon 2023, 204, 76–93. [Google Scholar] [CrossRef]
- Abbasi, A.; Abushad, M.; Khan, A.; Bhat, Z.U.H.; Hanif, S.; Shakir, M. Bare undoped nontoxic carbon dots as a visible light photocatalyst for the degradation of methylene blue and congo red. Carbon Trends 2023, 10, 100238. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Kim, Y.; Park, M.J.; Hong, B.H.; Jang, D.-J. Graphene quantum dots-decorated ZnS nanobelts with highly efficient photocatalytic performances. RSC Adv. 2016, 6, 24115–24120. [Google Scholar] [CrossRef]
- Bokare, A.; Chinnusamy, S.; Erogbogbo, F. TiO2-graphene quantum dots nanocomposites for photocatalysis in energy and biomedical applications. Catalysts 2021, 11, 319. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, T.; Chen, G.; Alborzi, E.; Yong, X.; Wang, Y. N, P co-doped carbon quantum dots bridge g-C3N4 and SnO2: Accelerating charge transport in S-scheme heterojunction for enhanced photocatalytic hydrogen production. J. Alloys Compd. 2024, 971, 172667. [Google Scholar] [CrossRef]
- Kaur, I.; Batra, V.; Bogireddy, N.K.; Baveja, J.; Kumar, Y.; Agarwal, V. Chemical-and green-precursor-derived carbon dots for photocatalytic degradation of dyes. Iscience 2024, 27, 108920. [Google Scholar] [CrossRef]
- Bhati, A.; Anand, S.R.; Gunture; Garg, A.K.; Khare, P.; Sonkar, S.K. Sunlight-induced photocatalytic degradation of pollutant dye by highly fluorescent red-emitting Mg-N-embedded carbon dots. ACS Sustain. Chem. Eng. 2018, 6, 9246–9256. [Google Scholar] [CrossRef]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Gallareta-Olivares, G.; Rivas-Sanchez, A.; Cruz-Cruz, A.; Hussain, S.M.; González-González, R.B.; Cárdenas-Alcaide, M.F.; Iqbal, H.M.; Parra-Saldívar, R. Metal-doped carbon dots as robust nanomaterials for the monitoring and degradation of water pollutants. Chemosphere 2023, 312, 137190. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Koubaa, A.; Tao, Y.; Magdouli, S.; Li, P.; Bouafif, H.; Zhang, J. Copper-Doped Carbon Nanodots with Superior Photocatalysis, Directly Obtained from Chromium-Copper-Arsenic-Treated Wood Waste. Polymers 2023, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Xin, X.; Zhang, Y.; Meng, L.Y. Facile synthesis of nitrogen-doped carbon dots (N-CDs) and N-CDs/NiO composite as an efficient electrocatalyst for oxygen evolution reaction. Carbon Lett. 2021, 31, 695–706. [Google Scholar] [CrossRef]
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu2+. Appl. Surf. Sci. 2019, 476, 468–480. [Google Scholar] [CrossRef]
- Zhuang, L.; Song, X.J.; Xu, Y.H. Study on the Infrared Spectral Characteristic of Tetracentron sinense Wood. Hubei Agric. Sci. 2017, 7, 1334–1339. [Google Scholar]
- Arul, V.; Sethuraman, M.G. Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Opt. Mater. 2018, 78, 181–190. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, D.; Gao, Y.; Tong, B.; Li, Y.; Zhu, Y. Non-thermal effect of microwave on the chemical structure and luminescence properties of biomass-derived carbon dots via hydrothermal method. Appl. Surf. Sci. 2021, 552, 149503. [Google Scholar] [CrossRef]
- Ramezani, Z.; Qorbanpour, M.; Rahbar, N. Green synthesis of carbon quantum dots using quince fruit (Cydonia oblonga) powder as carbon precursor: Application in cell imaging and As3+ determination. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 58–66. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Boyd, I.W.; O’Sullivan, B.; Hurley, P.; Kelly, P.; Senateur, J.-P. Nanocrystalline TiO2 films studied by optical, XRD and FTIR spectroscopy. J. Non-Cryst. Solids 2002, 303, 134–138. [Google Scholar] [CrossRef]
- Abd El Khalk, A.A.; Betiha, M.A.; Mansour, A.S.; Abd El Wahed, M.G.; Al-Sabagh, A.M. High degradation of methylene blue using a new nanocomposite based on zeolitic imidazolate framework-8. ACS Omega 2021, 6, 26210–26220. [Google Scholar] [CrossRef]
- Zulfajri, M.; Dayalan, S.; Li, W.-Y.; Chang, C.-J.; Chang, Y.-P.; Huang, G.G. Nitrogen-doped carbon dots from Averrhoa carambola fruit extract as a fluorescent probe for methyl orange. Sensors 2019, 19, 5008. [Google Scholar] [CrossRef]
- Van Luam, P.; Kim, D.G.; Ko, S.O. Mechanisms of Methylene Blue Degradation by Nano-Sized β-MnO2 Particles. KSCE J. Civ. Eng. 2020, 24, 1385–1394. [Google Scholar]
- Ning, R.; Ge, C.; Liu, Q.; Tian, J.; Asiri, A.M.; Alamry, K.A.; Li, C.M.; Sun, X. Hierarchically porous N-doped carbon nanoflakes: Large-scale facile synthesis and application as an oxygen reduction reaction electrocatalyst with high activity. Carbon 2014, 78, 60–69. [Google Scholar] [CrossRef]
- Jagiello, J.; Chojnacka, A.; Pourhosseini, S.E.M.; Wang, Z.; Beguin, F. A dual shape pore model to analyze the gas adsorption data of hierarchical micro-mesoporous carbons. Carbon 2021, 178, 113–124. [Google Scholar] [CrossRef]
- Umrao, S.; Sharma, P.; Bansal, A.; Sinha, R.; Singh, R.K.; Srivastava, A. Multi-layered graphene quantum dots derived photodegradation mechanism of methylene blue. RSC Adv. 2015, 5, 51790–51798. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Lee, J.-W.; Shim, W.-G.; Jang, H.D.; Lee, S.-I.; Yoo, S.-J. Adsorption and photocatalysis of nanocrystalline TiO2 particles prepared by sol–gel method for methylene blue degradation. Adv. Powder Technol. 2012, 23, 414–418. [Google Scholar] [CrossRef]
- Guo, H.; Ke, Y.; Wang, D.; Lin, K.; Shen, R.; Chen, J.; Weng, W. Efficient adsorption and photocatalytic degradation of Congo red onto hydrothermally synthesized NiS nanoparticles. J. Nanopart. Res. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- An, T.; An, J.; Yang, H.; Li, G.; Feng, H.; Nie, X. Photocatalytic degradation kinetics and mechanism of antivirus drug-lamivudine in TiO2 dispersion. J. Hazard. Mater. 2011, 197, 229–236. [Google Scholar] [CrossRef]
- Liu, L.; Fan, S.; Li, Y. Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism. Int. J. Environ. Res. Public Health 2018, 15, 1321. [Google Scholar] [CrossRef]
- Bechambi, O.; Jlaiel, L.; Najjar, W.; Sayadi, S. Photocatalytic degradation of bisphenol A in the presence of Ce–ZnO: Evolution of kinetics, toxicity and photodegradation mechanism. Mater. Chem. Phys. 2016, 173, 95–105. [Google Scholar] [CrossRef]
- Zhu, M.; Agustiono, K.T.; Song, F.; Tong, O.; Dzarfan, O.M.H.; Mashallah, R.; Saeed, S. Applicability of BaTiO3/graphene oxide (GO) composite for enhanced photodegradation of methylene blue (MB) in synthetic wastewater under UV–vis irradiation. Environ. Pollut. 2019, 255, 113182. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Khraisheh, M.A.; Ahmad, M.N.; Allen, S. Adsorption behaviour of methylene blue onto Jordanian diatomite: A kinetic study. J. Hazard. Mater. 2009, 165, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Shankang, T.; Qijin, G.; Gang, L.; Xiao, W.; Renwu, Y. Investigation into salt effect and kinetics of photocatalytic degradation of reactive dye K-GL. Chem. Ind. Eng. Prog. 2021, 33, 1908. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef]
- Anwar, D.I.; Mulyadi, D. Synthesis of Fe-TiO2 Composite as a Photocatalyst for Degradation of Methylene Blue. Procedia Chem. 2015, 17, 49–54. [Google Scholar] [CrossRef]
- Devadi, M.A.H.; Krishna, M.; Murthy, H.N.; Sathyanarayana, B. Statistical optimization for photocatalytic degradation of methylene blue by Ag-TiO2 nanoparticles. Procedia Mater. Sci. 2014, 5, 612–621. [Google Scholar] [CrossRef]





| Samples | SBET (m2/g) | Sext (m2/g) | Vmi (cm3/g) | Vme (cm3/g) | VT (cm3/g) |
|---|---|---|---|---|---|
| TiO2 | 158.25 | 108.24 | 0.011 | 0.395 | 0.406 |
| CCA-CDs | 80.50 | 55.38 | 0.016 | 0.227 | 0.243 |
| CCA-CDs/TiO2 | 144.63 | 98.56 | 0.007 | 0.326 | 0.333 |
| Models | Parameters | Samples | |
|---|---|---|---|
| 100.000 mg/L | 300.000 mg/L | ||
| qe,exp (mg/g) | 95.280 | 171.660 | |
| Pseudo-first-order | qe,cal (mg/g) | 87.290 | 150.850 |
| k1 (min−1) | 0.055 | 0.026 | |
| R2 | 0.870 | 0.883 | |
| Pseudo-second-order | qe,cal (mg/g) | 93.430 | 163.620 |
| k2 (g/(mg·min)) | 0.087 × 10−2 | 0.024 × 10−2 | |
| R2 | 0.963 | 0.964 | |
| Models | Parameters | CCA-CDs |
|---|---|---|
| Langmuir | qmax (mg/g) | 121.81 |
| KL (L/mg) | 0.82 | |
| R2 | 0.877 | |
| Freundlich | Kf (mg/g) | 67.05 |
| 1/n | 0.14 | |
| R2 | 0.940 | |
| Redlich–Peterson | Kr (L/g) | 290.52 |
| ar (L/mg) | 3.58 | |
| β | 0.91 | |
| R2 | 0.980 | |
| Sips | qs (mg/g) | 151.07 |
| Ks (L/g) | 0.68 | |
| ns | 0.43 | |
| R2 | 0.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, D.; Zhang, J.; Magdouli, S.; Tao, Y.; Li, P.; Bouafif, H.; Koubaa, A. Visible Light-Induced Photocatalytic Degradation of Methylene Blue Using Copper-Doped Carbon Dots One-Step Derived from CCA-Wood. Forests 2024, 15, 680. https://doi.org/10.3390/f15040680
Xing D, Zhang J, Magdouli S, Tao Y, Li P, Bouafif H, Koubaa A. Visible Light-Induced Photocatalytic Degradation of Methylene Blue Using Copper-Doped Carbon Dots One-Step Derived from CCA-Wood. Forests. 2024; 15(4):680. https://doi.org/10.3390/f15040680
Chicago/Turabian StyleXing, Dan, Jingfa Zhang, Sara Magdouli, Yubo Tao, Peng Li, Hassine Bouafif, and Ahmed Koubaa. 2024. "Visible Light-Induced Photocatalytic Degradation of Methylene Blue Using Copper-Doped Carbon Dots One-Step Derived from CCA-Wood" Forests 15, no. 4: 680. https://doi.org/10.3390/f15040680
APA StyleXing, D., Zhang, J., Magdouli, S., Tao, Y., Li, P., Bouafif, H., & Koubaa, A. (2024). Visible Light-Induced Photocatalytic Degradation of Methylene Blue Using Copper-Doped Carbon Dots One-Step Derived from CCA-Wood. Forests, 15(4), 680. https://doi.org/10.3390/f15040680









