The Potential Threats of Spodoptera frugiperda on Six Economic Tree Species in the Tropical Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Feeding Patterns and Developmental Characteristics of Spodoptera frugiperda
2.3. Determination of Enzyme Activity
2.4. Feeding Preferences
2.5. Data Analysis
3. Results
3.1. Variability in Spodoptera frugiperda Survival among Various Plant Species
3.2. Leaves Consumption of Spodoptera frugiperda
3.3. Performance of Spodoptera frugiperda on Different Diets
3.4. Enzyme Activity
3.5. Feeding Preference Results of Spodoptera frugiperda
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, A.N. A Review of the Biology of the Fall Armyworm. Fla. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Shi, W.P. How greedy are the fall armyworm? J. Plant Prot. 2020, 47, 687–691. [Google Scholar] [CrossRef]
- Kenis, M. Prospects for Classical Biological Control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Invaded Areas Using Parasitoids from the Americas. J. Econ. Entomol. 2023, 116, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Gadratagi, B.-G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable Management of Invasive Fall Armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall Armyworm: Impacts and Implications for Africa. Outlook Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the Global Extent of Invasion of the Cereal Pest Spodoptera frugiperda, the Fall Armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef]
- Ganiger, P.C.; Yeshwanth, H.M.; Muralimohan, K.; Vinay, N.; Kumar, A.R.; Chandrashekara, K.J. Occurrence of the New Invasive Pest, Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), in the Maize Fields of Karnataka, India. Curr. Sci. 2018, 115, 621–623. [Google Scholar] [CrossRef]
- Tang, J.; Lu, B.; Lu, H.; Ji, X.; Yang, P.; Su, H.; Cai, B. Investigation and Preliminary Study of Biological Characteristic of Parasitic Wasps of Spodoptera frugiperda in Hainan. Chin. J. Trop. Crop. 2020, 41, 1189–1195. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.Y.; Wu, Q.L.; Zhao, S.Y.; Li, H. Characteristics of Spodoptera frugiperda infestation in winter and spring and its trend in the second half of the year in China. China Plant Prot. 2019, 39, 36–38+49. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.; Jiang, Y.Y.; Liu, J.; Wu, K.M.; Xiao, Y.T. Molecular characterization analysis of fall armyworm populations in China. Plant Prot. 2019, 45, 20–27. [Google Scholar] [CrossRef]
- Tay, W.T.; Rane, R.V.; James, W.; Gordon, K.H.J.; Downes, S.; Kim, J.; Kuniata, L.; Walsh, T.K. Resistance Bioassays and Allele Characterization Inform Analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) Introduction Pathways in Asia and Australia. J. Econ. Entomol. 2022, 115, 1790–1805. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Meagher, R.L.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, Evolution, and Management Options of an Invasive Species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Liu, J.; Xie, M.C.; Li, Y.H.; Yang, J.J.; Zhang, M.L.; Qiu, K. Observation on law of diffusion damage of Spodoptera frugiperda in China in 2019. Plant Prot. 2019, 45, 10–19. [Google Scholar] [CrossRef]
- Zacarias, D.A. Global Bioclimatic Suitability for the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), and Potential Co-Occurrence with Major Host Crops under Climate Change Scenarios. Clim. Chang. 2020, 161, 555–566. [Google Scholar] [CrossRef]
- Fiteni, E.; Durand, K.; Gimenez, S.; Meagher, R.L.; Legeai, F.; Kergoat, G.J.; Nègre, N.; d’Alençon, E.; Nam, K. Host-Plant Adaptation as a Driver of Incipient Speciation in the Fall Armyworm (Spodoptera frugiperda). BMC Ecol. Evol. 2022, 22, 133. [Google Scholar] [CrossRef]
- Gouin, A.; Bretaudeau, A.; Nam, K.; Gimenez, S.; Aury, J.-M.; Duvic, B.; Hilliou, F.; Durand, N.; Montagné, N.; Darboux, I.; et al. Two Genomes of Highly Polyphagous Lepidopteran Pests (Spodoptera frugiperda, Noctuidae) with Different Host-Plant Ranges. Sci. Rep. 2017, 7, 11816. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hu, C.; Jia, H.; Wu, Q.; Shen, X.; Zhao, S.; Jiang, Y.; Wu, K. Case Study on the First Immigration of Fall Armyworm, Spodoptera Frugiperda Invading into China. J. Integr. Agric. 2021, 20, 664–672. [Google Scholar] [CrossRef]
- Wu, K.M. Management strategies of fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 1–5. [Google Scholar] [CrossRef]
- Fen, R.S. Characteristics of the Occurrence and Control Measures of Spodoptera frugiperda. Seed Sci. 2023, 41, 116–118. [Google Scholar] [CrossRef]
- Casmuz, A.; Juárez, M.L.; Socías, M.G.; Murúa, M.G.; Prieto, S.; Medina, S.; Willink, E. Revisión de los hospederos del gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Arg. 2010, 69, 3–4. Available online: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0373-56802010000200007&lng=es&tlng=es (accessed on 26 December 2023).
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Sun, R.; Gao, L.; Mi, Z.; Zheng, Y.; Li, D. CnMADS1, a MADS Transcription Factor, Positively Modulates Cell Proliferation and Lipid Metabolism in the Endosperm of Coconut (Cocos nucifera L.). Planta 2020, 252, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Planting the Six Trees and Finding the Power Points. Hainan Daily. 2022, p. A05. Available online: http://news.hndaily.cn/html/2022-04/27/content_58468_14759402.htm (accessed on 9 September 2023).
- Wu, C.B.; Ren, C.C.; Zhu, M.J.; Du, R.K.; Yan, C.Z.; Han, S.; Rui, K.; Lu, C.J. Efficacy of a combination of carbofuran and azoxystrobin against red-veined spike borer of betel nut. China Plant Prot. 2022, 42, 83–85. [Google Scholar] [CrossRef]
- Meng, X.L.; Song, W.W.; Tang, Q.H.; Niu, X.Q.; Li, C.X.; Zhong, B.Z.; Lv, C.J.; Huang, S.C.; Qin, W.Q. Advances in Main Diseases and Insect Pests of Areca Palm. Chin. J. Trop. Crop. 2021, 42, 3055–3065. [Google Scholar] [CrossRef]
- Tang, Q.B.; Wang, C.Z. Leaf disctest used in caterpillar feeding preference study. Chin. Bull. Entomol. 2007, 44, 912–915. [Google Scholar] [CrossRef]
- Yong, Z.; Hao-Ru, T.; Ya, L. Variation in Antioxidant Enzyme Activities of Two Strawberry Cultivars with Short-Term Low Temperature Stress. World J. Agric. Sci. 2008, 4, 458–462. Available online: https://www.idosi.org/wjas/wjas4(4)/8.pdf (accessed on 27 December 2023).
- Loh, K.P.; Qi, J.; Tan, B.K.H.; Liu, X.H.; Wei, B.G.; Zhu, Y.Z. Leonurine Protects Middle Cerebral Artery Occluded Rats Through Antioxidant Effect and Regulation of Mitochondrial Function. Stroke 2010, 41, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Xiao, Y.; Cao, L.-L.; Yan, X.; Li, C.; Shi, H.-Y.; Wang, J.-W.; Ye, Y.-H. Cerebroside C Increases Tolerance to Chilling Injury and Alters Lipid Composition in Wheat Roots. PLoS ONE 2013, 8, e73380. [Google Scholar] [CrossRef]
- Su, X.N.; Li, C.Y.; Xu, Y.J.; Huang, S.H.; Liu, W.L.; Liao, Z.X.; Zhang, Y.P. Feeding preference and adaptability of fall armyworm Spodoptera frugiperda on five species of host plants and six weeds. J. Environ. Entomol. 2022, 44, 263–272. [Google Scholar] [CrossRef]
- Chen, Y.; Romeis, J.; Meissle, M. Performance of Daphnia Magna on Flour, Leaves, and Pollen from Different Maize Lines: Implications for Risk Assessment of Genetically Engineered Crops. Ecotoxicol. Environ. Saf. 2021, 212, 111967. [Google Scholar] [CrossRef] [PubMed]
- McKnight, P.E.; Najab, J. Mann-Whitney U Test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; p. 1. ISBN 978-0-470-47921-6. [Google Scholar]
- CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora. Available online: https://www.cites.org/eng/disc/what.php (accessed on 27 December 2023).
- Song, J.; Zhang, L.N.; Zhang, Z.H.; Zhou, Z.Z.; Liang, J.F.; Lu, J.K. High-throughput Sequencing Analysis of Fungal Diversity in Agar-wood Wound Locations. Chin. J. Trop. Crop. 2020, 42, 3358–3368. [Google Scholar] [CrossRef]
- Zhou, Y.K.; Qiao, H.L.; Zhan, Q.Q.; Zhao, X.S.; Lu, L.L.; Chen, J. Occurrence and Control of the Disease and Pests Damage on Aquilaria Siensis in Hainan. Mod. Chin. Med. 2017, 19, 1102–1105. [Google Scholar] [CrossRef]
- Silva, D.M.D.; Bueno, A.D.F.; Andrade, K.; Stecca, C.D.S.; Neves, P.M.O.J.; Oliveira, M.C.N.D. Biology and Nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) Fed on Different Food Sources. Sci. Agric. 2017, 74, 18–31. [Google Scholar] [CrossRef]
- Carrasco, D.; Larsson, M.C.; Anderson, P. Insect Host Plant Selection in Complex Environments. Curr. Opin. Insect Sci. 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Hou, M.L.; Wen, J.H.; Li, J.W. Effects of plant volatiles on herbivorous insects. Plant Prot. 2007, 33, 7–11. [Google Scholar] [CrossRef]
- Moreau, J.; Benrey, B.; Thiéry, D. Grape Variety Affects Larval Performance and Also Female Reproductive Performance of the European Grapevine Moth Lobesia Botrana (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2006, 96, 205–212. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Zhu, H.; Romeis, J.; Li, Y.; Peng, Y.; Chen, X. Effects of Straw Leachates from Cry1C-Expressing Transgenic Rice on the Development and Reproduction of Daphnia Magna. Ecotoxicol. Environ. Saf. 2018, 165, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vajpayee, P.; Ali, M.B.; Tripathi, R.D.; Singh, N.; Rai, U.N.; Singh, S.N. Biochemical Responses of Cassia Siamea Lamk. Grown on Coal Combustion Residue (Fly-Ash). Bull. Environ. Contam. Toxicol. 2002, 68, 675–683. [Google Scholar] [CrossRef]
- Marciniak, B.; Grabowicz, W.; Ferenc, T. Evaluation of Micronuclei in Mice Bone Marrow and Antioxidant Systems in Erythrocytes Exposed to A-Amanitin. Toxicon 2013, 63, 147–153. [Google Scholar] [CrossRef]
- Vuleta, A. Adaptive Flexibility of Enzymatic Antioxidants SOD, APX and CAT to High Light Stress: The Clonal Perennial Monocot Iris Pumila as a Study Case. Plant Physiol. Biochem. 2016, 100, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, M.M.S.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of pH on Antioxidants Production by Spirulina (Arthrospira) Platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Alyokhin, A.; Benkovskaya, G.; Udalov, M. Chapter 4—Colorado Potato Beetle. In Insect Pests of Potato, 2nd ed.; Alyokhin, A., Rondon, S.I., Gao, Y., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 29–43. ISBN 978-0-12-821237-0. [Google Scholar] [CrossRef]
- Jernelöv, A. The Colorado (Potato) Beetle. In The Long-Term Fate of Invasive Species: Aliens Forever or Integrated Immigrants with Time? Jernelöv, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 105–116. ISBN 978-3-319-55396-2. [Google Scholar] [CrossRef]
- Chen, Z.M. Chemical Ecology of Tea Tree Pests; Shanghai Scientific & Technical Publishers: Shanghai, China, 2013. [Google Scholar]
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.A.G.; Guo, Y. Mirid Bug Outbreaks in Multiple Crops Correlated with Wide-Scale Adoption of Bt Cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, W.; Tao, X.; Wang, D.; Lu, L.; He, Y. Using Two-Sex Life Table Traits to Assess the Fruit Preference and Fitness of Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2018, 111, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, W.; Saeed, S.; Saeed, Q.; Naqqash, M.N.; Sial, M.U.; Aine, Q.U.; Yanyuan, L.; Rui, Z.; He, Y.; Lu, L. Effects of Three Different Cultivars of Cruciferous Plants on the Age-Stage, Two-Sex Life Table Traits of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Entomol. Res. 2019, 49, 151–157. [Google Scholar] [CrossRef]
- Naqqash, M.; Saeed, S.; Jaleel, W.; Zaka, M.; Saeed, Q. Effect of Host Plants on Life History Traits of Dysdercus Koenigii (Hemiptera: Pyrrhocoridae). J. Biodivers. Environ. Sci. 2014, 4, 187–194. [Google Scholar]
- Rwomushana, I. Spodoptera Frugiperda (Fall Armyworm). CABI Compend. 2019, 29810. [Google Scholar] [CrossRef]
- Heppner, J.B. Lepidoptera of Florida, Part 1: Introduction and Catalog. Arthropods Fla. Neighboring Land Areas 2007, 17, 1–670. [Google Scholar]
- Silva, A.G.A.; Goncalves, C.R.; Galvão, D.M.; Gonçalves, A.J.L.; Gomes, J.; Silva, M.N.; Simoni, L.; Silva, R.; Galvao, D.; Gonçalves, M. Quarto Catálogo Dos Insetos Que Vivem Nas Plantas Do Brasil. Seus Parasitos e Predadores: Parte 2, Tomo 1o, Insetos, Hospedeiros e Inimigos Naturais—ScienceOpen; Ministério da Agricultura: Rio de Janeiro, Brazil, 1968. [Google Scholar]
- Wang, Z.; Cao, H. The Complete Mitochondrial Genome Sequence of Aquilaria Sinensis. Mitochondrial DNA Part B Resour. 2021, 6, 381–383. [Google Scholar] [CrossRef]
- Zheng, M. Identification of Insecticidal Activity of Areca Nut Alkaloids Components and Their Formulation Study. Master’s Thesis, Yangzhou University, Yangzhou, China, 2021. [Google Scholar]
- Cui, C.; Yang, Y.; Zhao, T.; Zou, K.; Peng, C.; Cai, H.; Wan, X.; Hou, R. Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia oleifera. Molecules 2019, 24, 4518. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huang, B.Q. Determination of the synergistic effect of tea saponins on several pesticides. J. South China Agric. Univ. 1999, 20, 32–35. [Google Scholar] [CrossRef]
- Hao, W.N.; Zeng, Y.; Hu, M.Y.; Li, F.; Ji, D.Q. Progress of tea saponins in pesticide applications. Pesticides 2010, 49, 90–93+96. [Google Scholar] [CrossRef]
- Kong, D.L. A Study on the Resistance of Five Species of Dalbergia to Plecoptera oculata Moore. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2022. [Google Scholar]
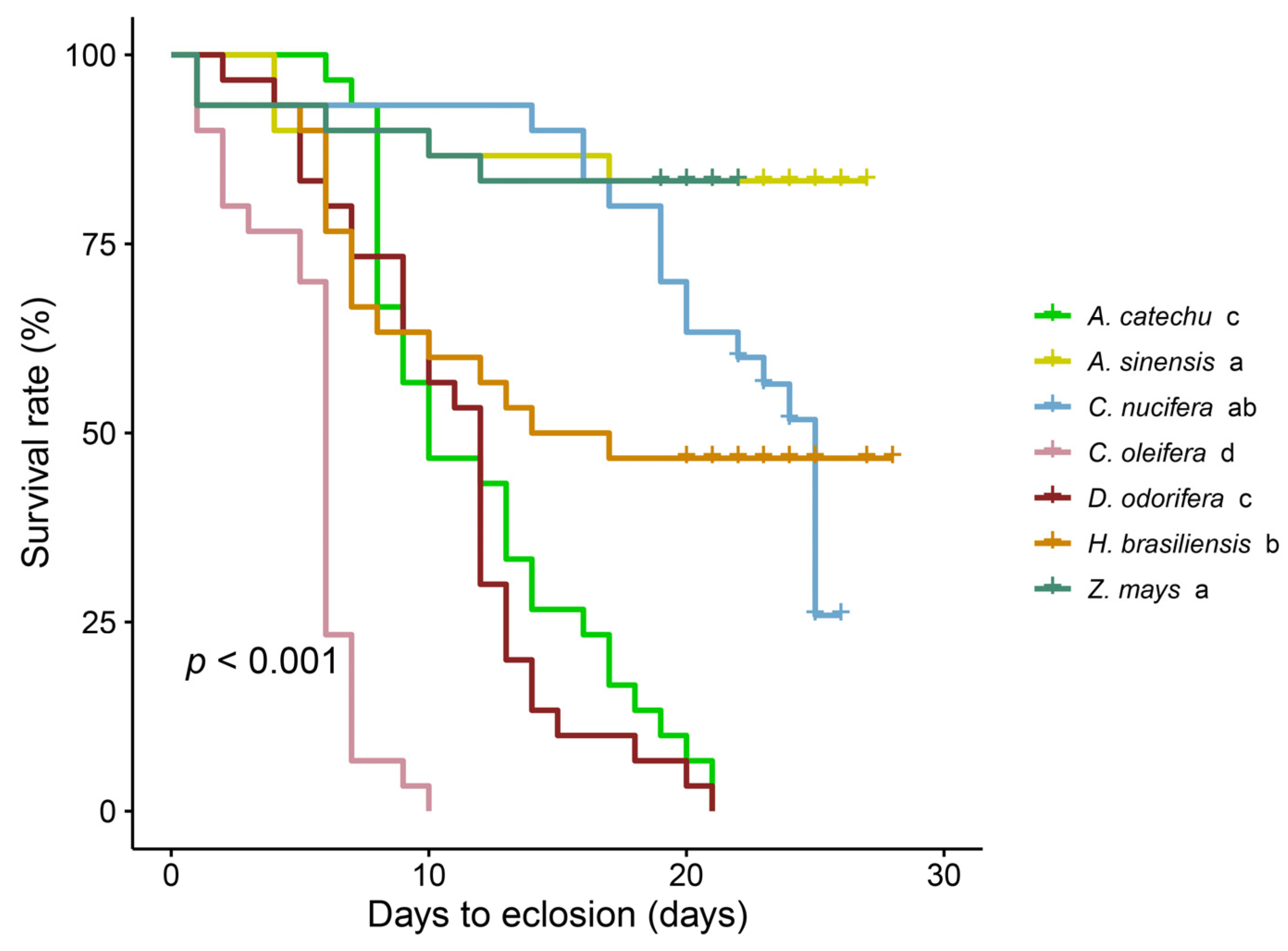
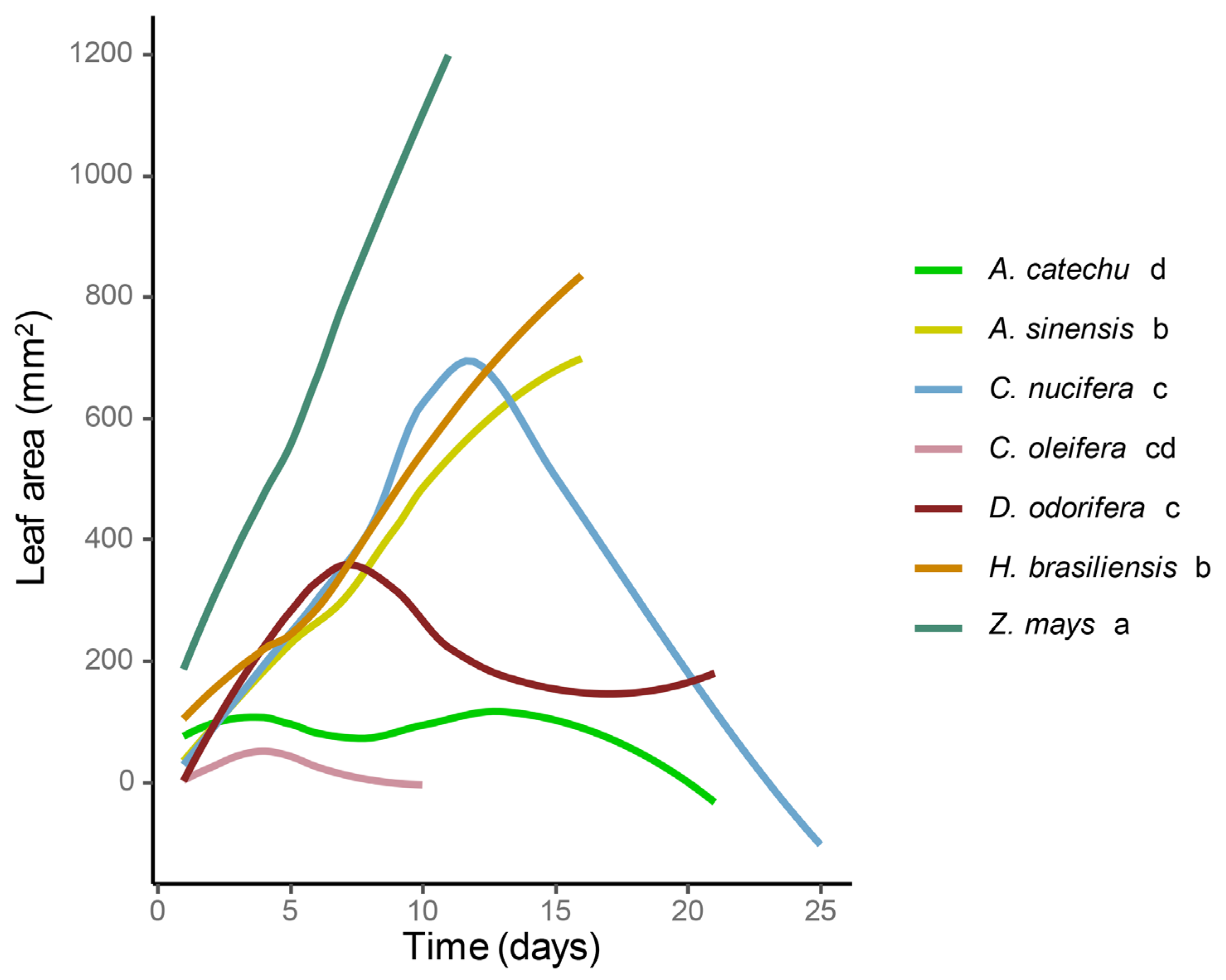
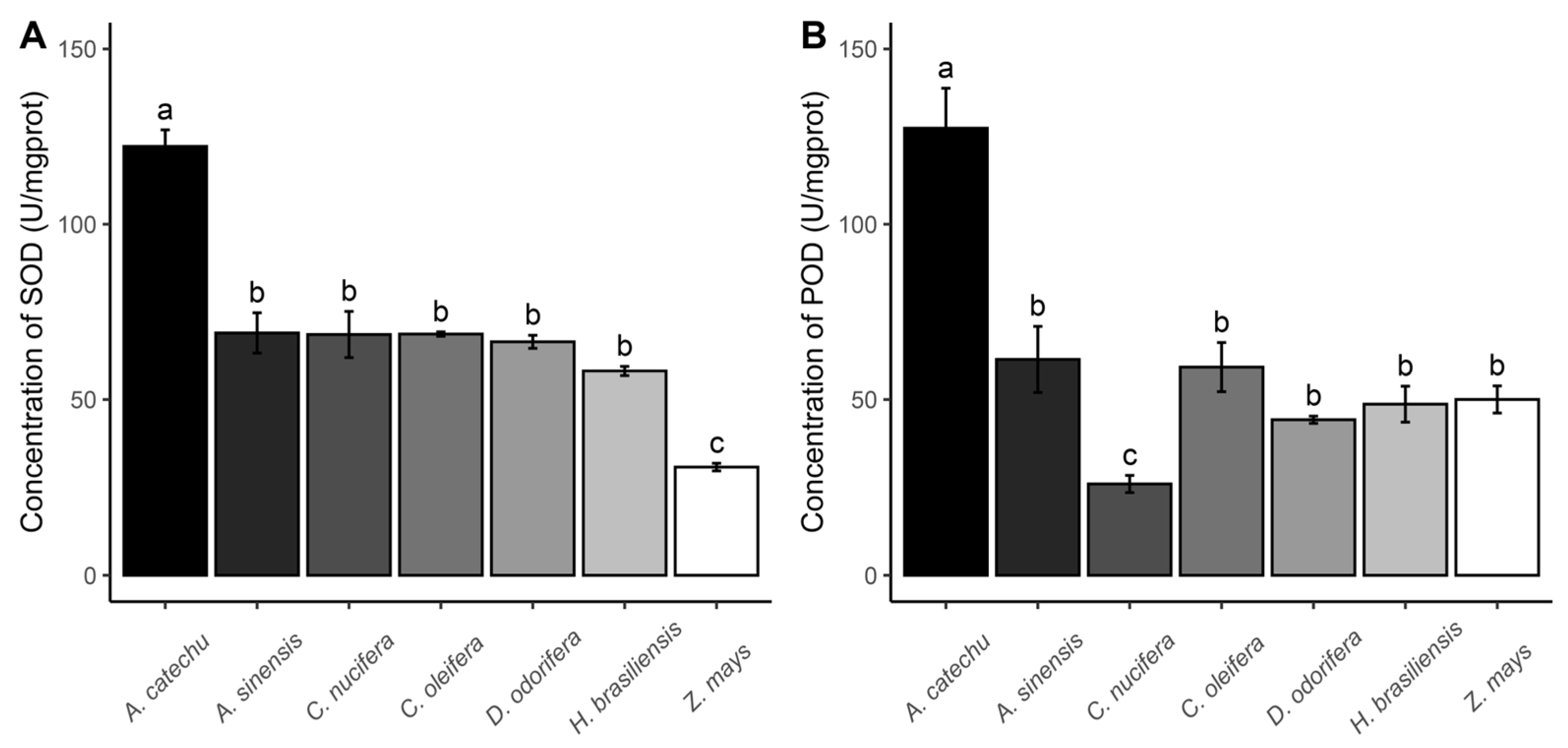
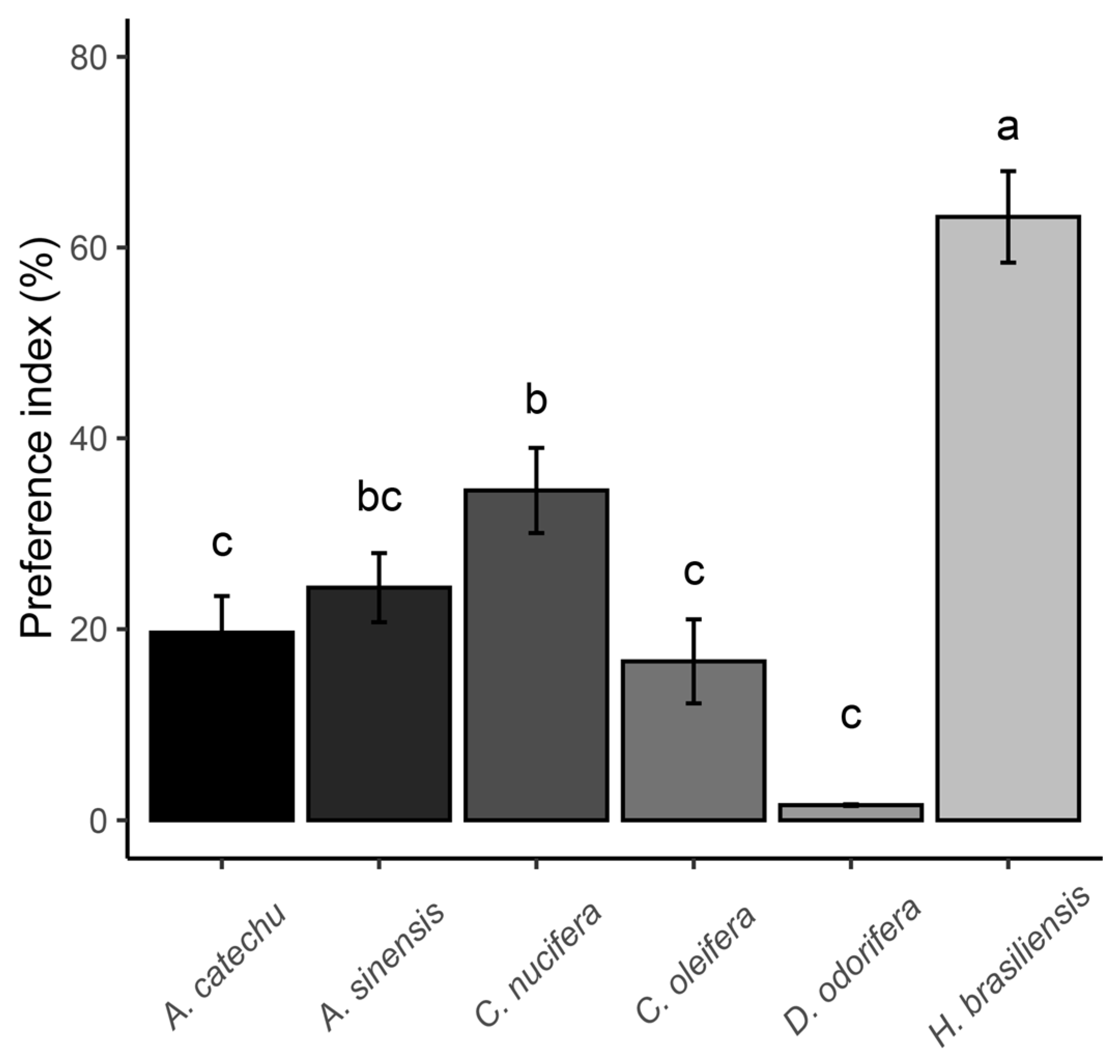
| Parameter | Z. mays | H. brasiliensis | A. sinensis | C. nucifera | D. odorifera | A. catechu | C. oleifer |
|---|---|---|---|---|---|---|---|
| Pupation rate (%) a | 90.00 (30) a | 50.00 (30) b | 86.67 (30) a | 76.67 (30) ab | 0 (30) c | 3.33 (30) c | 0 (30) c |
| Eclosion rate (%) a | 83.33 (30) a | 46.67 (30) b | 83.33 (30) a | 46.67 (30) b | 0 (30) c | 0 (30) c | 0 (30) c |
| Larval development time (d) b | 10.18 ± 0.21 (27) a | 13.27 ± 0.57 (15) b | 14.36 ± 0.32 (26) bc | 16.13 ± 0.55 (23) c | - | 14 (1) | - |
| Pupal development time (d) b | 10.04 ± 0.12 (25) a | 10.79 ± 0.21 (14) b | 10.72 ± 0.13 (25) b | 9.00 ± 0.15 (14) c | - | - | - |
| Pupal length (mm) c | 15.34 ± 0.20 (25) a | 16.11 ± 0.38 (14) a | 15.81 ± 0.23 (25) a | 15.38 ± 0.27 (14) a | - | 12.00 (1) | - |
| Female pupal fresh weight (mg) c | 120.72 ± 2.84 (11) a | 150.00 ± 6.06 (6) b | 130.50 ± 5.08 (6) ab | 111.67 ± 4.00 (12) a | - | - | - |
| Male pupal fresh weight (mg) c | 131.00 ± 3.18 (14) a | 133.00 ± 7.94 (8) a | 143.47 ± 3.00 (19) a | 127.00 ± 3.00 (2) a | - | - | - |
| Pupal mean fresh weight (mg) | 126.48 ± 3.17 (25) a | 140.29 ± 5.56 (14) b | 140.36 ± 3.70 (25) b | 113.86 ± 0.3.73 (14) a | |||
| Females rate (%) a | 44.00 (25) a | 42.86 (14) a | 24.00 (25) a | 85.71 (14) a | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Chen, Y.; Kong, X.; Jia, R.; Jiang, X.; Guo, J.; Guo, Y.; Yang, Y. The Potential Threats of Spodoptera frugiperda on Six Economic Tree Species in the Tropical Region. Forests 2024, 15, 701. https://doi.org/10.3390/f15040701
Xue J, Chen Y, Kong X, Jia R, Jiang X, Guo J, Guo Y, Yang Y. The Potential Threats of Spodoptera frugiperda on Six Economic Tree Species in the Tropical Region. Forests. 2024; 15(4):701. https://doi.org/10.3390/f15040701
Chicago/Turabian StyleXue, Jiabao, Yi Chen, Xiangyi Kong, Ruizong Jia, Xiaoqi Jiang, Jingyuan Guo, Yunling Guo, and Yan Yang. 2024. "The Potential Threats of Spodoptera frugiperda on Six Economic Tree Species in the Tropical Region" Forests 15, no. 4: 701. https://doi.org/10.3390/f15040701
APA StyleXue, J., Chen, Y., Kong, X., Jia, R., Jiang, X., Guo, J., Guo, Y., & Yang, Y. (2024). The Potential Threats of Spodoptera frugiperda on Six Economic Tree Species in the Tropical Region. Forests, 15(4), 701. https://doi.org/10.3390/f15040701





