Variability of Water Use Efficiency of Gmelina arborea Plantations in the Tropical Dry Forest of Colombia
Abstract
:1. Introduction
2. Materials and Methods
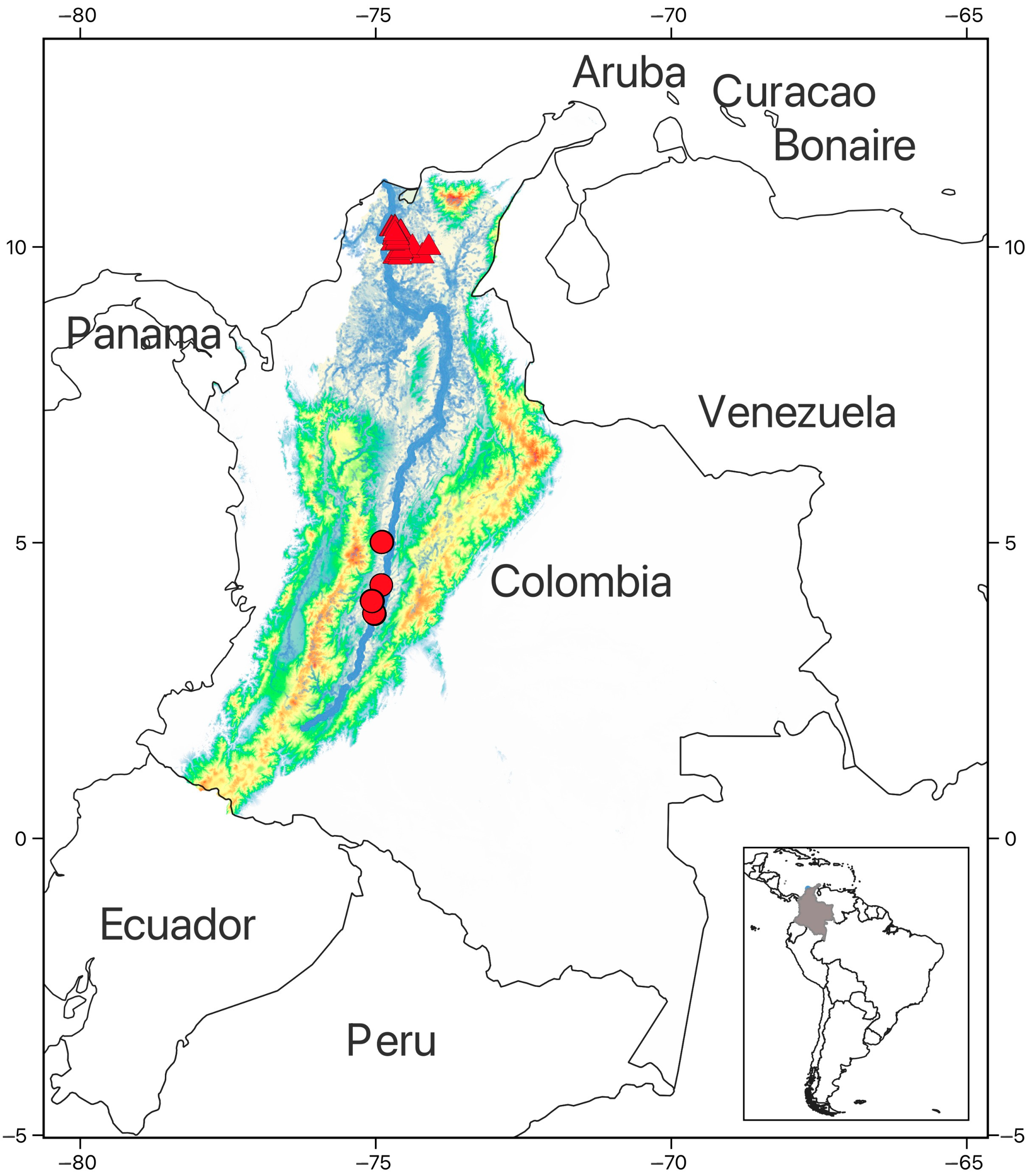
2.1. Study Area
2.2. Permanent Sampling Plots
2.3. Stand Variables
2.4. Site Variables
2.4.1. Climatic Variables
2.4.2. Soil Variables
2.5. Water Use Efficiency
2.6. Data Analysis
3. Results
3.1. Regional Variations in Stand and Site Variables
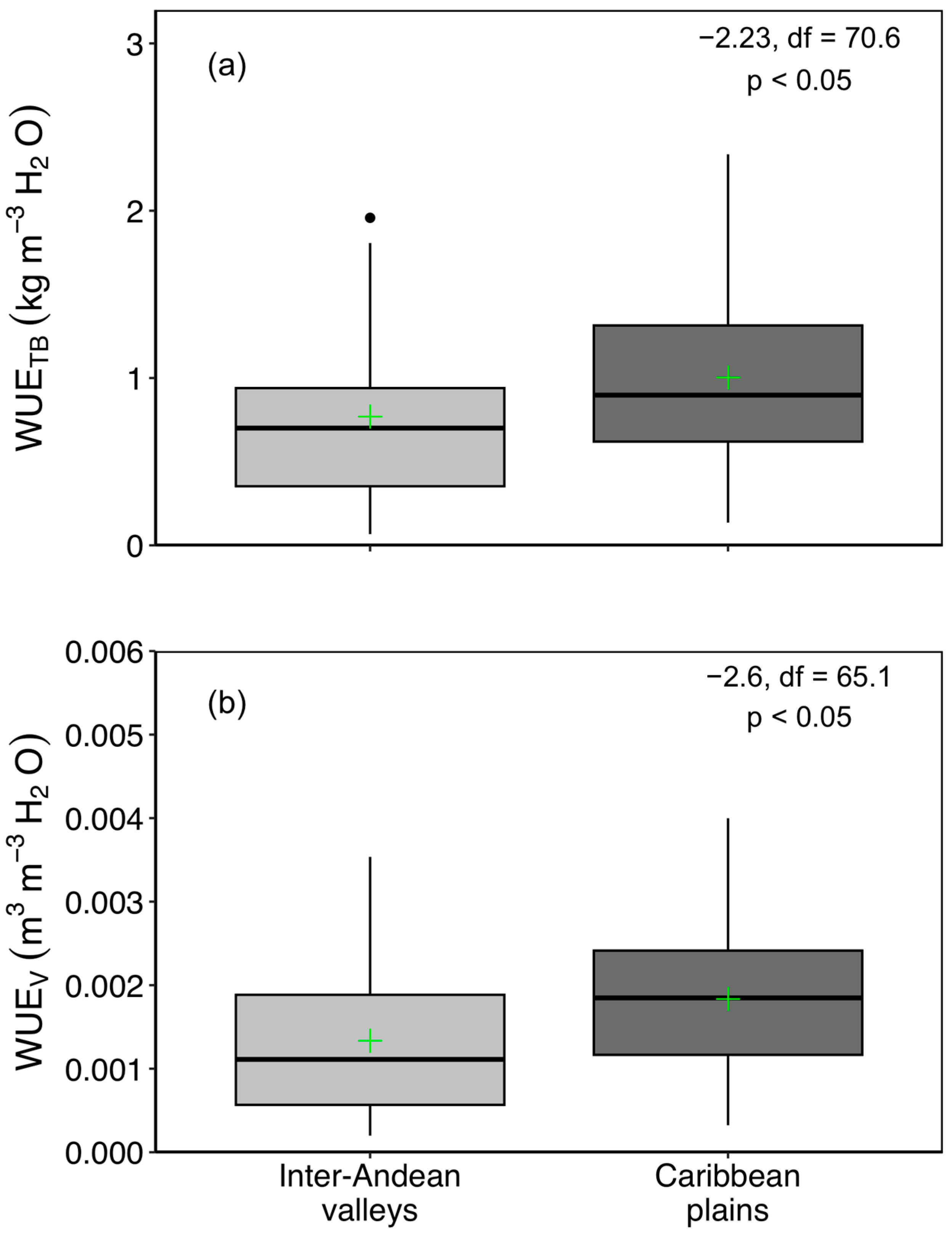
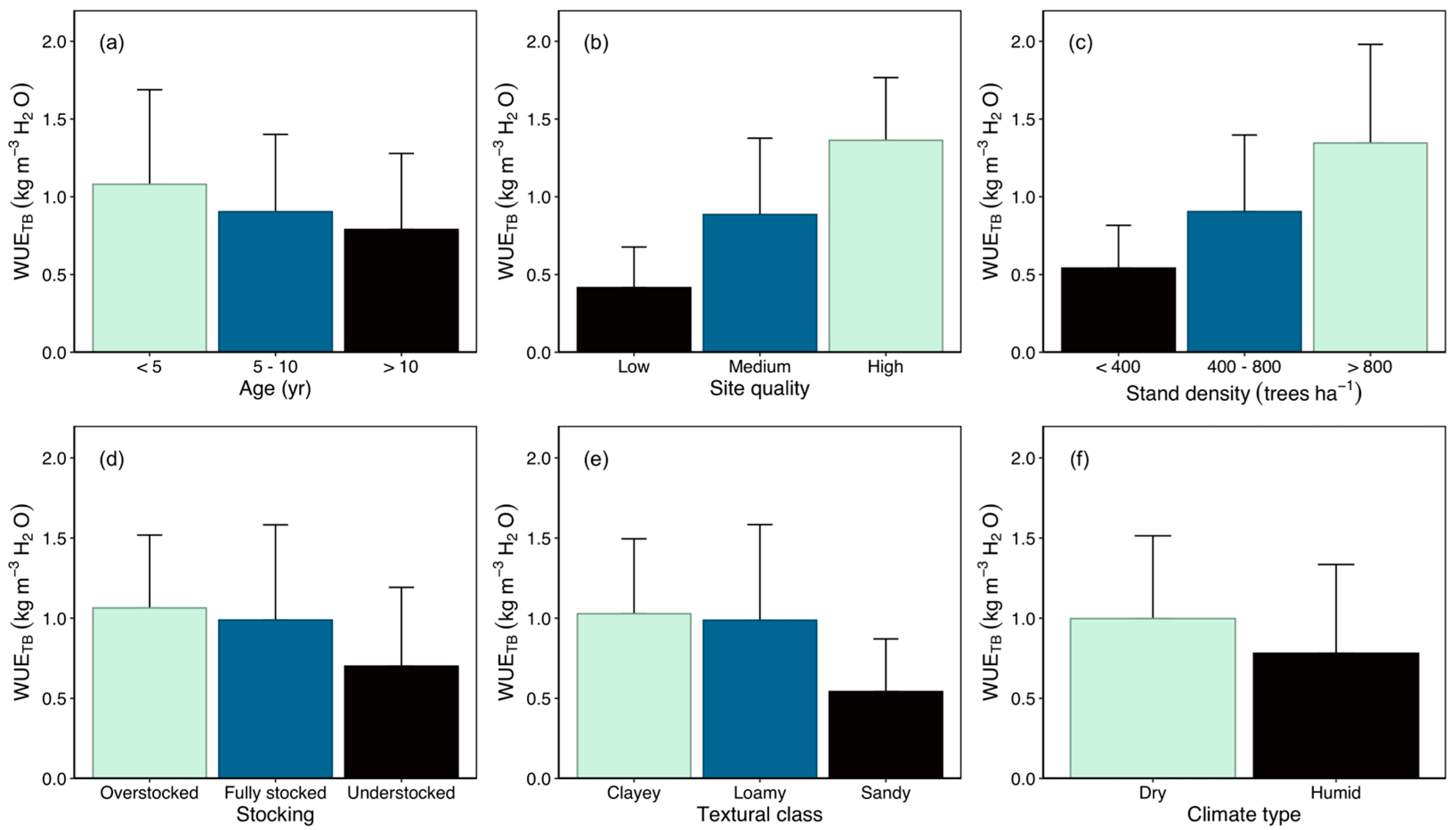
3.2. WUE Variation
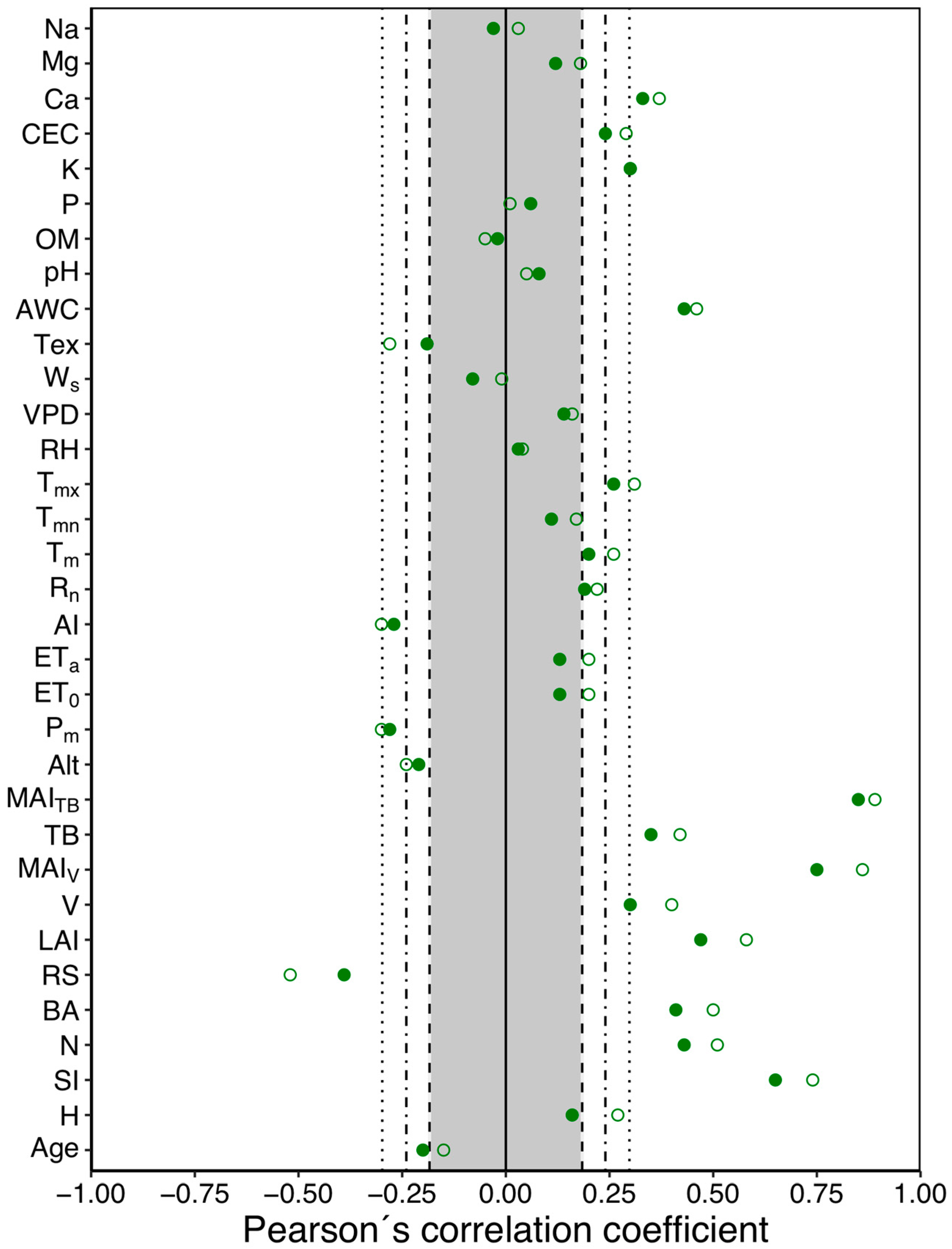
3.3. Relationship between WUE and Stand and Site Variables
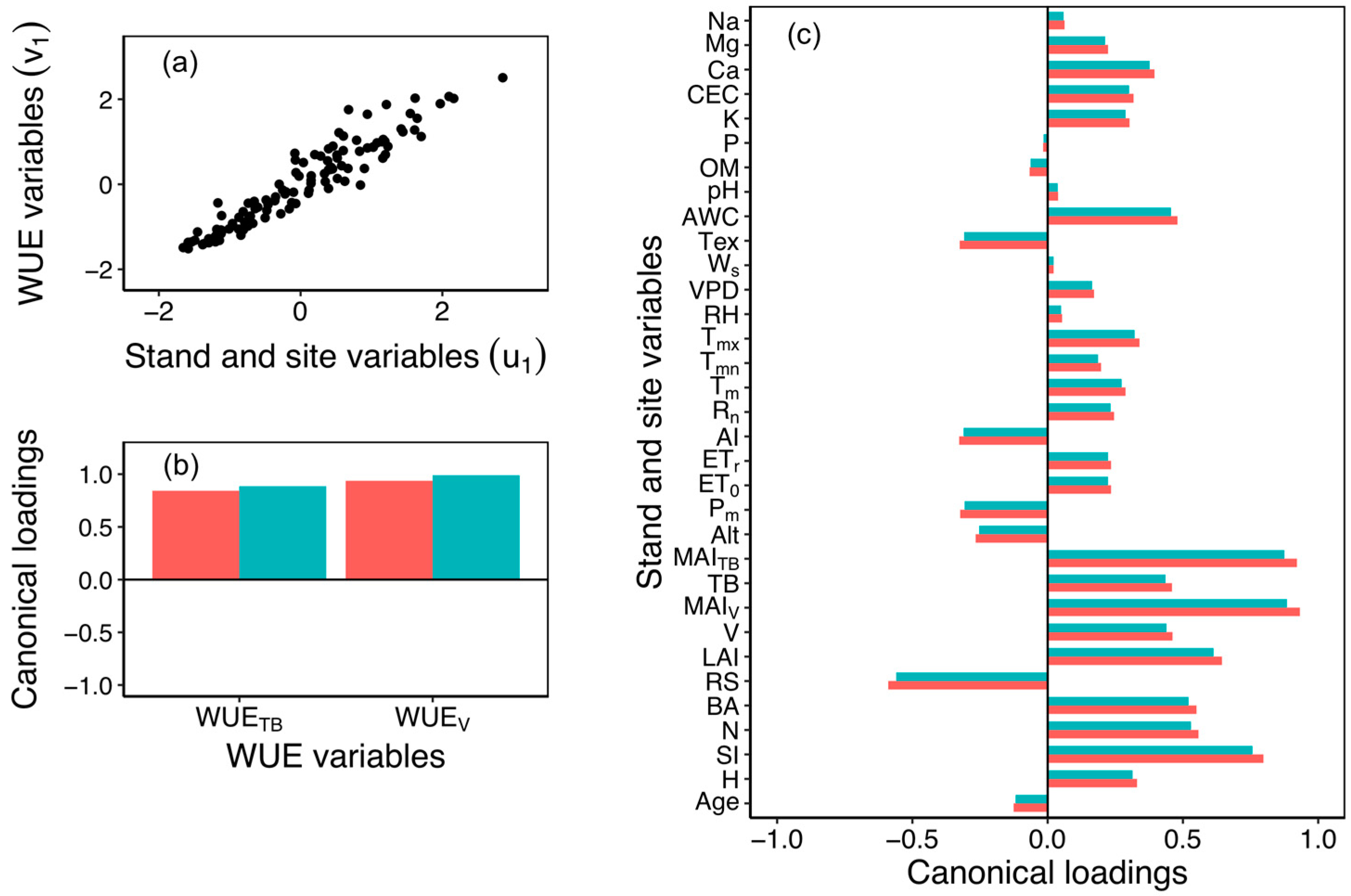
3.4. Canonical Correlations
3.5. Main Driver of WUE
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Loustau, D.; Bosc, A.; Colin, A.; Ogee, J.; Davi, H.; Francois, C.; Dufrene, E.; Deque, M.; Cloppet, E.; Arrouays, D.; et al. Modeling Climate Change Effects on the Potential Production of French Plains Forests at the Sub-Regional Level. Tree Physiol. 2005, 25, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report; Pachauri, R.K., Mayer, L.A., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2015; ISBN 978-92-9169-143-2. [Google Scholar]
- Kwon, H.; Law, B.E.; Thomas, C.K.; Johnson, B.G. The Influence of Hydrological Variability on Inherent Water Use Efficiency in Forests of Contrasting Composition, Age, and Precipitation Regimes in the Pacific Northwest. Agric. For. Meteorol. 2018, 249, 488–500. [Google Scholar] [CrossRef]
- Bhattacharya, A. Changing Climate and Resource Use Efficiency in Plants; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-816209-5. [Google Scholar]
- Asensio, V.; Domec, J.-C.; Nouvellon, Y.; Laclau, J.-P.; Bouillet, J.-P.; Jordan-Meille, L.; Lavres, J.; Rojas, J.D.; Guillemot, J.; Abreu-Junior, C.H. Potassium Fertilization Increases Hydraulic Redistribution and Water Use Efficiency for Stemwood Production in Eucalyptus grandis Plantations. Environ. Exp. Bot. 2020, 176, 104085. [Google Scholar] [CrossRef]
- Plomion, C.; Bartholomé, J.; Bouffier, L.; Brendel, O.; Cochard, H.; De Miguel, M.; Delzon, S.; Gion, J.-M.; Gonzalez-Martinez, S.C.; Guehl, J.-M.; et al. Understanding the Genetic Bases of Adaptation to Soil Water Deficit in Trees through the Examination of Water Use Efficiency and Cavitation Resistance: Maritime Pine as a Case Study. J. Plant Hydraul. 2016, 3, e008. [Google Scholar] [CrossRef]
- Hakamada, R.E.; Hubbard, R.M.; Moreira, G.G.; Stape, J.L.; Campoe, O.; Ferraz, S.F.D.B. Influence of Stand Density on Growth and Water Use Efficiency in Eucalyptus Clones. For. Ecol. Manag. 2020, 466, 118125. [Google Scholar] [CrossRef]
- Binkley, D.; Stape, J.L.; Ryan, M.G. Thinking about Efficiency of Resource Use in Forests. For. Ecol. Manag. 2004, 193, 5–16. [Google Scholar] [CrossRef]
- Waghorn, M.J.; Whitehead, D.; Watt, M.S.; Mason, E.G.; Harrington, J.J. Growth, Biomass, Leaf Area and Water-Use Efficiency of Juvenile Pinus radiata in Response to Water Deficits. N. Z. J. For. Sci. 2015, 45, 3. [Google Scholar] [CrossRef]
- Berner, L.T.; Law, B.E.; Hudiburg, T.W. Water Availability Limits Tree Productivity, Carbon Stocks, and Carbon Residence Time in Mature Forests across the Western US. Biogeosciences 2017, 14, 365–378. [Google Scholar] [CrossRef]
- Pereira, J.S.; Chaves, M.; Caldeira, M.; Correia, A.V. Water Availability and Productivity. In Plant Growth and Climate Change; Morison, J.I.L., Morecroft, M.D., Eds.; Wiley: Hoboken, NJ, USA, 2006; pp. 118–145. ISBN 978-1-4051-3192-6. [Google Scholar]
- Guedes, B.S.; Olsson, B.A.; Sitoe, A.A.; Egnell, G. Net Primary Production in Plantations of Pinus taeda and Eucalyptus cloeziana Compared with a Mountain Miombo Woodland in Mozambique. Glob. Ecol. Conserv. 2018, 15, e00414. [Google Scholar] [CrossRef]
- Sohel, S.I. Systematic Review and Meta-Analysis Reveals Functional Traits and Climate Are Good Predictors of Tropical Tree Water Use. Trees For. People 2022, 8, 100226. [Google Scholar] [CrossRef]
- Li, X.; Farooqi, T.J.A.; Jiang, C.; Liu, S.; Sun, O.J. Spatiotemporal Variations in Productivity and Water Use Efficiency across a Temperate Forest Landscape of Northeast China. For. Ecosyst. 2019, 6, 22. [Google Scholar] [CrossRef]
- Wullschleger, S.; Meinzer, F.; Vertessy, R. A Review of Whole-Plant Water Use Studies in Tree. Tree Physiol. 1998, 18, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H.; Flexas, J.; Ribas-Carbó, M.; Gulías, J. Measuring Water Use Efficiency in Grapevines. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 123–134. ISBN 978-90-481-9282-3. [Google Scholar]
- Fernandes, T.J.G.; Del Campo, A.D.; Herrera, R.; Molina, A.J. Simultaneous Assessment, through Sap Flow and Stable Isotopes, of Water Use Efficiency (WUE) in Thinned Pines Shows Improvement in Growth, Tree-Climate Sensitivity and WUE, but Not in WUEi. For. Ecol. Manag. 2016, 361, 298–308. [Google Scholar] [CrossRef]
- Medrano Gil, H.; Bota Salort, J.; Cifre Llompart, J.; Flexas Sans, J.; Ribas Carbó, M.; Gulías León, J. Eficiencia En El Uso Del Agua Por Las Plantas. Investig. Geogr. 2007, 43, 63. [Google Scholar] [CrossRef]
- Delrot, S.; Medrano, H.; Or, E.; Bavaresco, L.; Grando, S. Methodologies and Results in Grapevine Research; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-9282-3. [Google Scholar]
- Hasanuzzaman, M. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Springer: Singapore, 2020; ISBN 9789811521553. [Google Scholar]
- Farquhar, G.; Richards, R. Isotopic Composition of Plant Carbon Correlates with Water-Use Efficiency of Wheat Genotypes. Funct. Plant Biol. 1984, 11, 539. [Google Scholar] [CrossRef]
- Bouvet, J.-M.; Makouanzi Ekomono, C.G.; Brendel, O.; Laclau, J.-P.; Bouillet, J.-P.; Epron, D. Selecting for Water Use Efficiency, Wood Chemical Traits and Biomass with Genomic Selection in a Eucalyptus Breeding Program. For. Ecol. Manag. 2020, 465, 118092. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G.; Do Nascimento Gomes, A. Water Use, Water Limitation, and Water Use Efficiency in a Eucalyptus Plantation. Bosque Valdivia 2004, 25, 35–41. [Google Scholar] [CrossRef]
- Otto, M.S.G.; Hubbard, R.M.; Binkley, D.; Stape, J.L. Dominant Clonal Eucalyptus grandis × urophylla Trees Use Water More Efficiently. For. Ecol. Manag. 2014, 328, 117–121. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Delgado-Rojas, J.S.; Christina, M.; Nouvellon, Y.; Bouillet, J.-P.; Piccolo, M.D.C.; Moreira, M.Z.; Gonçalves, J.L.D.M.; Roupsard, O.; Laclau, J.-P. Potassium Fertilization Increases Water-Use Efficiency for Stem Biomass Production without Affecting Intrinsic Water-Use Efficiency in Eucalyptus grandis Plantations. For. Ecol. Manag. 2016, 364, 77–89. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Xu, H.; Creed, I.F.; Blanco, J.A.; Wei, X.; Sun, G.; Asbjornsen, H.; Bishop, K. Forest Water-Use Efficiency: Effects of Climate Change and Management on the Coupling of Carbon and Water Processes. For. Ecol. Manag. 2023, 534, 120853. [Google Scholar] [CrossRef]
- Chen, S.; Wei, W.; Tong, B.; Chen, L. Effects of Soil Moisture and Vapor Pressure Deficit on Canopy Transpiration for Two Coniferous Forests in the Loess Plateau of China. Agric. For. Meteorol. 2023, 339, 109581. [Google Scholar] [CrossRef]
- Rojas Rodríguez, F.; Dagoberto, A.; Aguilar, R.; Roque, R.; Meza, A.; Olman, M.; Gamboa, M.; Arguedas, M. Manual Para Productores de Melina Gmelina arborea en Costa Rica; FONAFIFO: Cartago, Costa Rica, 2004. [Google Scholar]
- Ministerio de Agricultura y Desarrollo Rural (MADR). Boletín Estadístico Forestal Marzo 2023; MADR: Bogotá, Colombia, 2023. [Google Scholar]
- Guzman, R.Z. Site Productivity Evaluation of Some Yemane (Gmelina arborea Roxb.) Plantations in the Philippines, Ph.D. Thesis, University of the Philippines, Los Baños, Laguna, 1988.
- Barrios Trilleras, A.; López Aguirre, A.M.; Báez Aparicio, C.A. Modelamiento de La Productividad de Gmelina arborea Roxb. Con Base En Variables Biofísicas y de Rodal. Colomb. For. 2021, 24, 71–87. [Google Scholar] [CrossRef]
- Melo-Cruz, O.; Martínez-Bustamante, E.; Correa-Londoño, G. Indicadores Morfométricos Foliares Para La Detección Temprana Del Estrés Hídrico de Gmelina arborea Roxb. En Sistemas Agroforestales, Santafé de Antioquia. Colomb. For. 2012, 15, 215. [Google Scholar] [CrossRef]
- Rojas, A.; Moreno, L.; Melgarejo, L.M.; RodrÃguez, M.M.A. Physiological Response of Gmelina (Gmelina arborea Roxb.) to Hydric Conditions of the Colombian Caribbean. Agron.-Colomb. 2012, 30, 52–58. [Google Scholar]
- Arguedas, M.; Rodriguez-Solis, M.; Moya, R.; Berrocal, A. Gmelina arborea “Death Disease” in Fast-Growth Plantations: Effects of Soil and Climatic Conditions on Severity and Incidence and Its Implications for Wood Quality. For. Syst. 2018, 27, e003. [Google Scholar] [CrossRef]
- Coy-Rodríguez, V.A.; Vargas-Osorio, L.J.; Varón-López, M.; López-Cardona, N. Identificación de Hongos y Bacterias Asociados a Fustes de Melina (Gmelina arborea Roxb.) En El Departamento Del Tolima, Colombia. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2018, 42, 343. [Google Scholar] [CrossRef]
- Fernandez, A.; Fernandez, F.; Imitola, S. Diseño de Un Sistema de Riego Para la Plantación de Gmelina arborea de Aw Faber Castell & TH Reforestación SAS. Bachelor’s Thesis, Universidad del Norte, Barranquilla, Colombia, 2018. [Google Scholar]
- Rodriguez, J.; Urueña, E.; Borralho, N. Mejoramiento de Semillas Para Incrementar Las Plantaciones de Teca (Tectona grandis L.f.) y Melina (Gmelina arborea Roxb.) En El Departamento de Tolima; Corporación Nacional de Investigación y Fomento Forestal (CONIF): Bogotá, Colombia, 2011. [Google Scholar]
- Niño Lopez, G.S.; Ramos Molina, P.A.; Barrios Trilleras, A.; López Aguirre, A.M. Modelos Compatibles de Ahusamiento-Volumen Para Árboles de Gmelina arborea Roxb. En El Alto Magdalena, Colombia. Colomb. For. 2018, 21, 174–187. [Google Scholar] [CrossRef]
- Collazos, J.C.; Barrios Trilleras, A.; Diaz Sierra, D.R. Modelos de Altura Dominante Para Gmelina arborea Roxb. En Colombia. Colomb. For. 2024, 27, e20569. [Google Scholar] [CrossRef]
- Obando, D. Interceptación de La Radiación, Acumulación y Distribución de Biomasa y Contenido de Carbono En Gmelina arborea Roxb. y Pochota Quinata (Jacq) Dugand. Bachelor’s Thesis, Universidad del Tolima, Ibagué, Colombia, 2004. [Google Scholar]
- Rodriguez, N. Guía Para La Cuantificación de La Biomasa y El Carbono Forestal, Generación de Modelos y Uso de Herramientas Para Su Estimación; Corporación Autonóma Regional de Cundinamarca—CAR: Bogotá, Colombia, 2013. [Google Scholar]
- López-Aguirre, A.M.; Barrios-Trilleras, A.; Melo-Cruz, O.A. Balancing Wood Production and Water Use Efficiency in the Selection of Open-Pollinated Families of Gmelina arborea. New For. 2024, 55, 961–978. [Google Scholar] [CrossRef]
- Arias, D.; Calvo-Alvarado, J.; Dohrenbusch, A. Calibration of LAI-2000 to Estimate Leaf Area Index (LAI) and Assessment of Its Relationship with Stand Productivity in Six Native and Introduced Tree Species in Costa Rica. For. Ecol. Manag. 2007, 247, 185–193. [Google Scholar] [CrossRef]
- Barrios, A.; Trincado, G.; Garreaud, R. Alternative Approaches for Estimating Missing Climate Data: Application to Monthly Precipitation Records in South-Central Chile. For. Ecosyst. 2018, 5, 28. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Si, J.H.; Feng, Q.; Yu, T.F.; Li, P.D. Comparative Study of Daytime and Nighttime Sap Flow of Populus euphratica. Plant Growth Regul. 2017, 82, 353–362. [Google Scholar] [CrossRef]
- Allen, R.G. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2000; ISBN 978-92-5-104219-9. [Google Scholar]
- La Manna, L.; Tarabini, M.; Gomez, F.; Noli, P.A.; Vogel, B.; Buduba, C.G. Estimación de La Capacidad de Retención de Agua de Suelos Volcánicos En Función de Variables de Fácil Determinación a Campo. Cienc. Suelo 2018, 36, 23–29. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC.: Boston, MA, USA, 2020. [Google Scholar]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Análise Multivariada de Dados; Artmed: Porto Alegre, Brazil, 2021; ISBN 978-85-363-0482-3. [Google Scholar]
- Badii, M.H.; Castillo, J. Análisis de Correlación Canónica (ACC) e Investigación Científica. Rev. Innovaciones Neg. 2017, 4, 405–422. [Google Scholar] [CrossRef]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis, 6th ed.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2007; ISBN 978-0-13-187715-3. [Google Scholar]
- Everitt, B.S. An R and S-PLUS® Companion to Multivariate Analysis; Springer Texts in Statistics; Springer: London, UK, 2005; ISBN 978-1-85233-882-4. [Google Scholar]
- Maier, C.A.; Albaugh, T.J.; Cook, R.I.; Hall, K.; McInnis, D.; Johnsen, K.H.; Johnson, J.; Rubilar, R.A.; Vose, J.M. Comparative Water Use in Short-Rotation Eucalyptus benthamii and Pinus taeda Trees in the Southern United States. For. Ecol. Manag. 2017, 397, 126–138. [Google Scholar] [CrossRef]
- Albaugh, J.M.; Dye, P.J.; King, J.S. Eucalyptus and Water Use in South Africa. Int. J. For. Res. 2013, 2013, 852540. [Google Scholar] [CrossRef]
- Skubel, R.; Arain, M.A.; Peichl, M.; Brodeur, J.J.; Khomik, M.; Thorne, R.; Trant, J.; Kula, M. Age Effects on the Water-use Efficiency and Water-use Dynamics of Temperate Pine Plantation Forests. Hydrol. Process. 2015, 29, 4100–4113. [Google Scholar] [CrossRef]
- Gyenge, J.; Fernández, M.E.; Sarasola, M.; Schlichter, T. Testing a Hypothesis of the Relationship between Productivity and Water Use Efficiency in Patagonian Forests with Native and Exotic Species. For. Ecol. Manag. 2008, 255, 3281–3287. [Google Scholar] [CrossRef]
- Melo-Cruz, O.; Guzman Betancourt, A.; Rojas Urquiza, A. Ecología y Funcionalidad de La Gmelina arborea Roxb.: Aplicada a La Silvicultura En Áreas de Bosque Seco Tropical; Sello Editorial Universidad del Tolima: Ibagué, Colombia, 2019; ISBN 978-958-55-6939-3. [Google Scholar]
- Fardusi, M.J.; Ferrio, J.P.; Comas, C.; Voltas, J.; Resco De Dios, V.; Serrano, L. Intra-Specific Association between Carbon Isotope Composition and Productivity in Woody Plants: A Meta-Analysis. Plant Sci. 2016, 251, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Geng, X.; Yin, G.; Hänninen, H.; Hao, F.; Zhang, X.; Fu, Y.H. Legacy Effect of Spring Phenology on Vegetation Growth in Temperate China. Agric. For. Meteorol. 2020, 281, 107845. [Google Scholar] [CrossRef]
- Zhang, Q.; Ficklin, D.L.; Manzoni, S.; Wang, L.; Way, D.; Phillips, R.P.; Novick, K.A. Response of Ecosystem Intrinsic Water Use Efficiency and Gross Primary Productivity to Rising Vapor Pressure Deficit. Environ. Res. Lett. 2019, 14, 074023. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Poree, F.; Schaeufele, R.; Helmke, H.; Frackenpohl, J.; Lehr, S.; Von Koskull-Döring, P.; Christmann, A.; Schnyder, H.; et al. Abscisic Acid Receptors and Coreceptors Modulate Plant Water Use Efficiency and Water Productivity. Plant Physiol. 2019, 180, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xu, T.; Liu, L.; Zhou, S.; Zhao, J.; Liu, S.; Xu, Z.; Mao, K.; He, X.; Zhu, Z.; et al. Modeling Transpiration with Sun-Induced Chlorophyll Fluorescence Observations via Carbon-Water Coupling Methods. Remote Sens. 2021, 13, 804. [Google Scholar] [CrossRef]
- Hakamada, R.; Prados-Coronado, J.; Lages, C.; Vrechi, A.; Zuñiga-Grajeda, V.; Villota-Gonzalez, F.H.; Sulbaran-Rangel, B. Initial Growth of Gmelina arborea and Efficacy of RGB Image to Capture Canopy Area in a Large Range of Stockings. Remote Sens. 2023, 15, 4751. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D. Insights from Full-Rotation Nelder Spacing Trials with Eucalyptus in São Paulo, Brazil. South. For. J. For. Sci. 2010, 72, 91–98. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Warren, C.R.; Baker, T.G. Effect of Thinning, Pruning and Nitrogen Fertiliser Application on Transpiration, Photosynthesis and Water-Use Efficiency in a Young Eucalyptus nitens Plantation. For. Ecol. Manag. 2012, 266, 286–300. [Google Scholar] [CrossRef]
- Yu, G.; Song, X.; Wang, Q.; Liu, Y.; Guan, D.; Yan, J.; Sun, X.; Zhang, L.; Wen, X. Water-use Efficiency of Forest Ecosystems in Eastern China and Its Relations to Climatic Variables. New Phytol. 2008, 177, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ji, X.; Ye, L.; Jiang, J. Inherent Water-Use Efficiency of Different Forest Ecosystems and Its Relations to Climatic Variables. Forests 2022, 13, 775. [Google Scholar] [CrossRef]
- Henri, C.J. Soil-Site Productivity of Gmelina arborea, Eucalyptus urophylla and Eucalyptus grandis Forest Plantations in Western Venezuela. For. Ecol. Manag. 2001, 144, 255–264. [Google Scholar] [CrossRef]
- Binkley, D.; Fisher, R.F. Ecology and Management of Forest Soils, 1st ed.; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-45565-3. [Google Scholar]
- White, P.J. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhou, Y.-B.; Li, H.; Zhang, S.-Z.; Fang, Y.; Zhang, Y.-J.; Zou, X. Linking Tree Water Use Efficiency with Calcium and Precipitation. Tree Physiol. 2022, 42, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Roshetko, J.M. Agroforestry Species and Technologies: A Compilation of the Highlights and Factsheets Published by NFTA and FACT Net 1985–1999; Winrock: Morrilton, AR, USA, 2001; ISBN 978-1-57360-032-3. [Google Scholar]
- Xue, B.-L.; Guo, Q.; Otto, A.; Xiao, J.; Tao, S.; Li, L. Global Patterns, Trends, and Drivers of Water Use Efficiency from 2000 to 2013. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Barrios Trilleras, A.; López Aguirre, A.M.; Nieto, V. Experiencias y Avances en el Manejo Silvícola de Plantaciones Forestales Comerciales; Corporación Nacional de Investigación y Fomento Forestal—CONIF: Bogotá, Colombia, 2011; ISBN 978-958-99597-6. [Google Scholar]
- Calvo-Alvarado, J.; Dagoberto, A.; Jimenez Rodriguez, C.; Montero, J. Efecto de Cinco Sustratos En El Contenido Foliar de Nutrientes y Crecimiento Inicial de Tres Especies Forestales Empleadas En Mesoamérica. Rev. For. Mesoam. Kurú 2008, 5, 1–15. [Google Scholar]
- Barrios, A.; López, A.M.; Nieto, V.; Burgos, N.; Yaya, M.; González, I. Efecto Del Control de Malezas y Fertilización Sobre El Crecimiento Inicial de Gmelina arborea Roxb. En El Departamento Del Tolima, Colombia. Colomb. For. 2011, 14, 31. [Google Scholar] [CrossRef]
- Urueña Lozano, H. Assessing Different Stocking Regimes in a Plantation of Gmelina arborea in Northern Colombia. New For. 2004, 28, 287–291. [Google Scholar] [CrossRef]


| Stand Variable | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Age (yr) | 7.2 | 3.5 | 2.2 | 15.5 |
| Dominant height (H, m) | 17.1 | 5.8 | 6.3 | 27.9 |
| Site index (SI, m) | 21.9 | 3.6 | 13.1 | 29.0 |
| Density (N, trees ha−1) | 660.0 | 171.4 | 225.0 | 1020.0 |
| Basal area (BA, m2 ha−1) | 16.7 | 7.8 | 2.7 | 34.2 |
| Relative spacing (RS) | 0.3 | 0.1 | 0.1 | 0.6 |
| Leaf area index (LAI, m2 m−2) | 6.5 | 2.7 | 0.7 | 11.8 |
| Volume (V, m3 ha−1) | 114.3 | 76.6 | 6.1 | 290.8 |
| MAI in volume (MAIV, m3 ha−1 yr−1) | 15.6 | 7.6 | 1.6 | 30.8 |
| Total biomass (TB, Mg ha−1) | 71.2 | 39.9 | 11.1 | 171.4 |
| MAI in TB (MAITB, Mg ha−1 yr−1) | 10.4 | 4.4 | 2.8 | 21.8 |
| Variable | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Annual precipitation (Pm, mm yr−1) | 1566.8 | 435.7 | 854.0 | 3218.1 |
| Reference evapotranspiration (ET0, mm yr−1) | 1508.0 | 134.1 | 1292.0 | 2599.4 |
| Actual evapotranspiration (ETa, mm yr−1) | 1313.5 | 116.8 | 1125.3 | 2264.1 |
| Aridity index (AI, mm mm−1) | 1.06 | 0.35 | 0.44 | 2.28 |
| Net solar radiation (Rn, MJ m−2 yr−1) | 3759.9 | 232.1 | 3229.4 | 4217.3 |
| Mean air temperature (Tm, °C) | 28.2 | 0.6 | 26.7 | 29.7 |
| Minimum air temperature (Tmn, °C) | 22.7 | 0.6 | 21.3 | 24.1 |
| Maximum air temperature (Tmx, °C) | 33.7 | 0.9 | 31.5 | 35.6 |
| Relative humidity (RH, %) | 76.9 | 3.0 | 65.8 | 83.6 |
| Vapor pressure deficit (VPD, kPa) | 1.15 | 0.14 | 0.87 | 1.56 |
| Wind speed (Ws, m s−1) | 1.89 | 0.64 | 1.19 | 8.87 |
| Variables | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Soil texture (Tex) | 6.0 | 4.1 | 1.0 | 11.0 |
| Available water content (AWC, cm3 cm−3) | 0.12 | 0.02 | 0.07 | 0.16 |
| Soil pH | 5.93 | 0.93 | 4.12 | 7.60 |
| Organic matter (OM, %) | 2.25 | 1.43 | 0.02 | 7.21 |
| Phosphorus (P, ppm) | 23.0 | 19.4 | 1.7 | 86.5 |
| Potassium (K, meq 100 g−1) | 0.20 | 0.17 | 0.03 | 0.70 |
| Cation exchange capacity (CEC, meq 100 g−1) | 23.4 | 15.1 | 4.6 | 60.0 |
| Calcium (Ca, meq 100 g−1) | 14.3 | 9.7 | 1.0 | 38.2 |
| Magnesium (Mg, meq 100 g−1) | 8.1 | 8.0 | 0.2 | 34.4 |
| Sodium (Na, meq 100 g−1) | 0.40 | 0.50 | 0.05 | 2.67 |
| Canonical Variable Pairs | Canonical Correlation (R) | Canonical R2 | Mult. F | df1 | df2 | p-Value |
|---|---|---|---|---|---|---|
| (u1, v1) | 0.949 | 0.901 | 11.862 | 66 | 158 | <0.001 |
| (u2, v2) | 0.845 | 0.714 | 6.253 | 32 | 80 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Aguirre, A.M.; Barrios Trilleras, A. Variability of Water Use Efficiency of Gmelina arborea Plantations in the Tropical Dry Forest of Colombia. Forests 2024, 15, 1192. https://doi.org/10.3390/f15071192
López Aguirre AM, Barrios Trilleras A. Variability of Water Use Efficiency of Gmelina arborea Plantations in the Tropical Dry Forest of Colombia. Forests. 2024; 15(7):1192. https://doi.org/10.3390/f15071192
Chicago/Turabian StyleLópez Aguirre, Ana Milena, and Alonso Barrios Trilleras. 2024. "Variability of Water Use Efficiency of Gmelina arborea Plantations in the Tropical Dry Forest of Colombia" Forests 15, no. 7: 1192. https://doi.org/10.3390/f15071192





