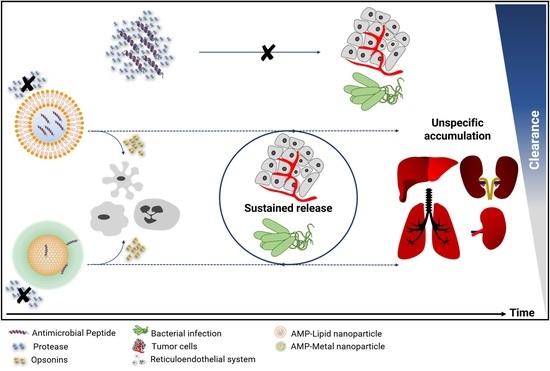
Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery
Abstract
1. Introduction
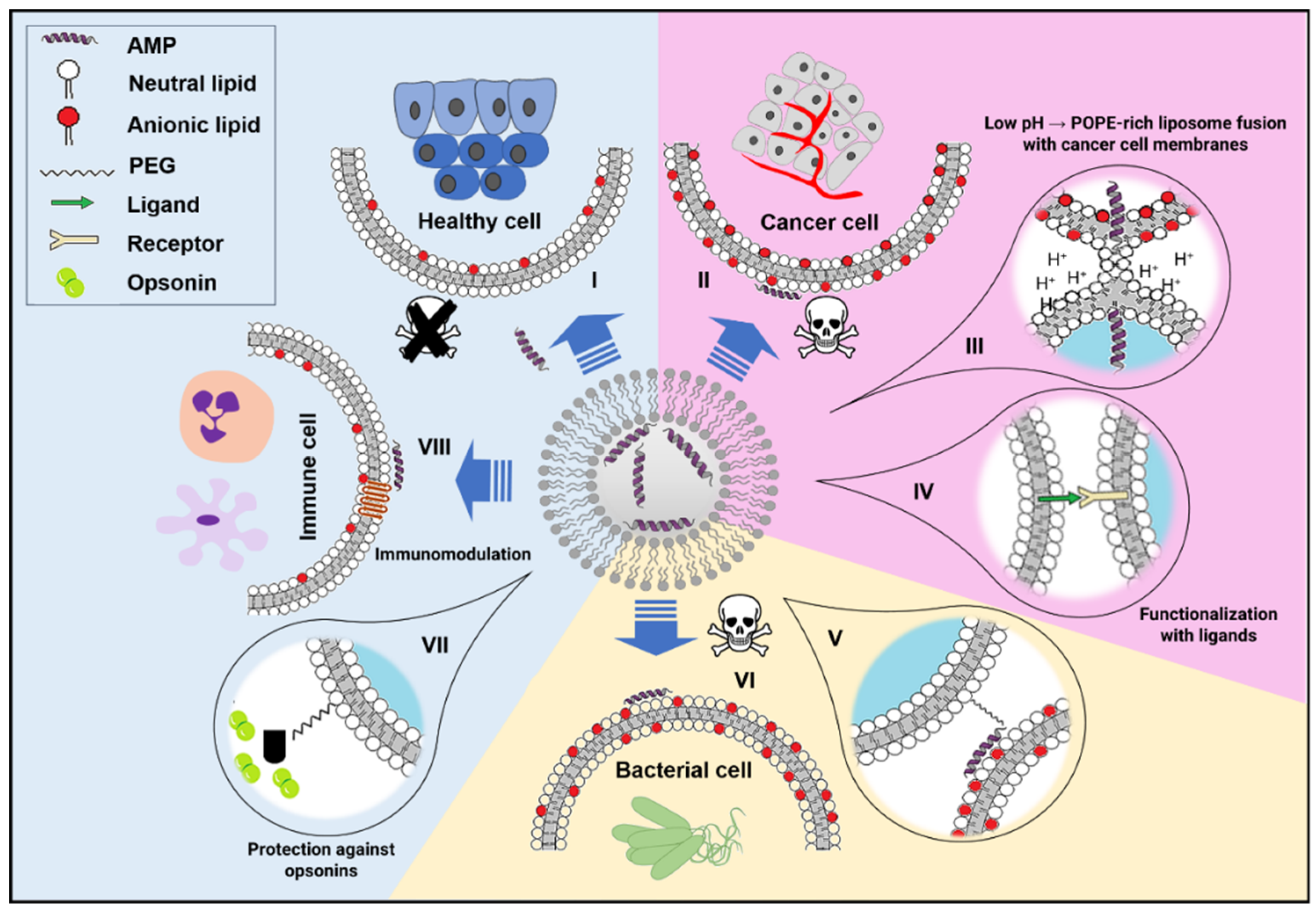
2. Lipid-Based Nanoparticles
2.1. Liposomes
2.1.1. Liposomal Antimicrobial Peptide (AMP) Formulations against Bacteria Infections
2.1.2. Anticancer Liposomal AMPs
2.2. Lyotrophic Liquid Crystals
2.3. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
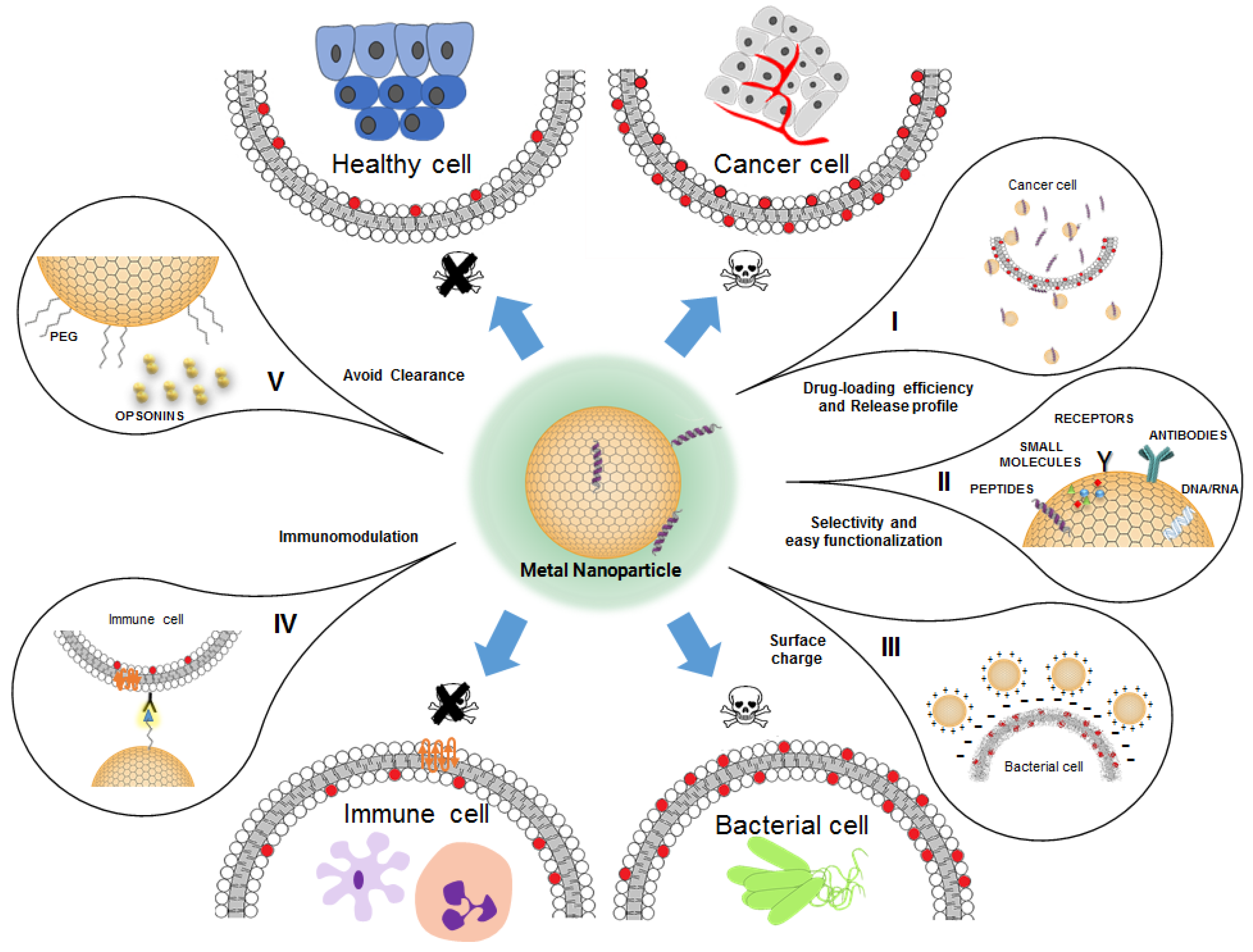
3. Metal Nanoparticles
3.1. Antimicrobial Peptide (AMP)-Conjugated Metal Nanoparticles against Bacteria Infections
3.2. Anticancer Antimicrobial Peptide (AMP)-Conjugated Metal Nanoparticles
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ag | Silver |
| AMP | Antimicrobial peptide |
| AMR | Antimicrobial resistance |
| ATP | Adenosine triphosphate |
| Au | Gold |
| AY1 | Andersonin-Y1 |
| Chol | Cholesterol |
| CL | Cardiolipin |
| Clav A | Clavanin A |
| CS | Chitosan |
| Cys | Cysteine |
| DC | Dendritic cell |
| DDS | Drug delivery system |
| DLS | Dynamic light scattering |
| DNA | Deoxyribonucleic acid |
| DPPC | Dipalmitoylphosphatidylcholine |
| Epi | Epirubicin |
| EPR | Enhanced permeability and retention |
| FDA | USA Food and Drug Administration |
| GDNF | Glial-derived growth neurotrophic factor |
| GMO | Glycerol monooleate |
| HDP | Host defense peptide |
| IL | Interleukin |
| LC | Liquid crystal |
| LCNP | Liquid crystal nanoparticle |
| LNP | Lipid nanoparticle |
| LPS | Lipopolysaccharide |
| MIC | Minimum inhibitory concentration |
| mPEG | Methylpolyethylene glycol |
| MNPs | Metal-based nanoparticle |
| MRSA | Multi-resistant Staphylococcus aureus |
| NLC | Nanostructured lipid carrier |
| NP | Nanoparticle |
| OA1 | Odorranain-A-OA1 |
| OA | Oleic acid |
| PB | Polymyxin B |
| PC | Phosphatidylcholine |
| PDT | Photodynamic therapy |
| PE | Phosphatidylethanolamine |
| PEG | Polyethylene glycol |
| PG | Phosphatidylglycerol |
| POPC | 1-palmitoyl-2-oleoylphosphatidylcholine |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| ROS | Reactive oxygen specie |
| SLN | Solid lipid nanoparticle |
| TGEV | Transmissible gastroenteritis virus |
| TGF-β | Transforming growth factor β |
| TNF-α | Tumor necrosis factor α |
| UHT | Ultra-high temperature processed |
Appendix A
| AMPs | NPs | Shape | Diameter (nm) | Applications | Reference |
|---|---|---|---|---|---|
| Polymyxin B Gramicidin S | AgNPs | - | 25 | Synergic activity against E. coli, Acinetobacter calcoaceticus, Enterobacter helveticus, Aeromonas bestiarum, Proteus myxofaciens, Pseudomonas fluorescens, Bacillus subtilis, Kocuria rhizophila and Micrococcus luteus Synergic activity against E. helveticus, P. myxofaciens and P. fluorescens | [180] |
| Polymyxin B | AuNPs | Spherical | 2.7 ± 0.7 | Maintains the same antimicrobial activity as the free form of polymyxin B against E. coli and methicillin-resistant S. aureus (MRSA) | [208] |
| Nisin | AuNPs | Spherical | 12.0 ± 2.0 | M. luteus | [209] |
| NK-2 LLKKK-18 | AgNPs-Alstonia macrophylla biomass AgNPs-Trichoderma sp. Biomass | Spherical | 50 and 100 | M. smegmatis M. smegmatis and M. marinum | [182] |
| Bacitracin A and polymyxin E | AgNPs | Spherical | 3.1 | E. coli, B. amyloliquefaciens, P. aeruginosa and S. aureus Promotes healing of infected wounds | [181] |
| G3R6TAT | AgNPs-citrate AgNPs-SDS and Au@Ag-BSA | Triangular Spherical | 30–70 30 | B. subtilis, E. coli and C. albicans | [165] |
| LL-37 CYS-modified (LL37-SH) | AgNPs | Spherical | 5.3 ± 1.8 | No anti-proliferative effect on primary skin cells; promotes wound healing, preventing potential infection by E. coli, Staphylococcus epidermidis, S. aureus, and free living and biofilm forms of P. aeruginosa | [210] |
| Nisin | AgNPs incorporated in poly(d,l-lactide) (PDLLA) and poly(ethylene oxide) (PEO) nanofibers | - | 21.81 ± 5.5 | P. aeruginosa, Klebsiella pneumoniae, E. coli, S. typhimurium and S. aureus | [211] |
| RPT-0001 | AgNPs | Spherical | 20–30 | Against food-borne bacterial pathogens: L. monocytogenes, Cronobacter sakazakii, S. enterica subsp. enterica and E. coli | [212] |
| Indolicidin | COOH-functionalized AuNPs | Spherical | 3 | Immuno suppressive action by downregulation of IFNβ expression and increase of IL-10 in RAW264.7 murine macrophage cells and THP-1 human monocyte cell lines | [213] |
| OA1 | AgNPs-citrate | Spherical | 10 | E. coli | [183] |
| PEP (a peptide sequence from lactoferrin) | AuNPs-polyethylenimine (PEI) | Spherical | - | Carrier for in vivo gene delivery vector in MSCs cells. Antibacterial activity againstS. Aureus, both in vitro and in vivo. | [214] |
| Nisin | AgNPs | Spherical | 10.1 ± 1.7 | B. subtilis, E. coli, Proteus vulgaris and S. aureus | [215] |
| LL37-SH | AgNPs with type I collagen as capping agent | Spherical | 4 | Sprayed formulation against free living and biofilm forms of P. aeruginosa | [216] |
| Hexahistidine-tagged A3-APO (A3-APOHis) | AuNPs-DNA aptamer | Spherical | 15 | Deliver of AMPs to Salmonella enterica and Typhimurium-infected HeLa cells | [217] |
| α-lipoic acid-peptide (LA-WKRAKLAK) | CTABI-capped AuNPs | Spherical Rod | 28.1 and 49.7 20 and 40 | Resistant cancer cells MCF-7 and metastatic T47D breast cancer cell line | [218] |
| Cecropin-mellitin | AuNP-coated SPIONsII | Quasi-spherical | 12 ± 2 (gold layer: 3) | E. coli and S. aureus | [219] |
| Cecropin-melittin (CM) CM-SH(cysteine at C-terminus) | AuNPs | - | 14 | S. aureus, E. coli, P. aeruginosa and K. pneumoniae | [190] |
| Cecropin-melittin | AuNPs-cysteamine AuNPs-PEG-NH2 | spherical | 20 | Coating based on CM peptide on AuNPs immobilized glass surfaces against S. aureus and E. coli | [220] |
| Cecropin-melitti CM-SH (cysteine at C-terminus) | AuNPs | - | - | Adsorption process of CM peptides onto a gold surface based on all-atom molecular dynamics simulations | [221] |
| CYRGRKKRRQRRR containing domain of trans-activator of transcription (TAT) (ANSIII-TAT) | AuNPs | Spherical | 3.8 ± 0.7 and 22.1 ± 3.6 | Cancer cells HepG2, MCF-7 and resistant cancer cell line MCF-7/ADR | [222] |
| Esculentin-1a(1–21)NH2 | AuNPs@PEGIV | Spherical | 14 | Free living and biofilm forms of P. aeruginosa | [191] |
| Clavanin A | AuNPs-Cys | Spherical | 10 | Sensitive biosensor for Gram-negative bacteria detection: S. aureus, E. faecalis, P. aeruginosa, S.Typhimurium and E. coli (higher levels of response were observed for the last two) | [192] |
| Ubiquicidin 29–41 | AgNPs | Spherical | 12.3 ± 3.9 | E. coli and P. aeruginosa | [223] |
| l-Arg-l-Arg-OMe l-His-l-Arg-OMe l-His-l-His-OMe | AgNPs AuNPs | Spherical | 12 ± 2 14 ± 2 | AgNPs have additive effect and enhance the antimicrobial activity of the peptides, whereas AuNPs reduce their activity against E. coli, S. aureus and S. Typhimurium | [142] |
| LL37 | AuNPs | Spherical | 15–25 | Enhances the migratory properties of keratinocytes in vitro and has higher wound healing activity in vivo (skin wound healing) | [224] |
| Polymyxin B | AgNPs | Spherical | 2 | Inhibited the growth of polymyxin B-resistant P. aeruginosa isolates from patients with acute exacerbations of cystic fibrosis | [225] |
| x-PGLa x-MSI103 x-MAP x-BP100 x-TP10 | AuNPs | Spherical | 5–7 | The peptides change to α-helical conformation onto the NPs surface in the presence of model membranes and maintain the same antimicrobial activity as in the free form against E. coli, B. subtilis, S. aureus and M. luteus | [226] |
| Lycosin-I | AuNPs | Spherical Rods | 60.88 ± 0.48 65.80 ± 3.18 | Efficient selectivity and cellular internalization for cancer cells in vitro, and efficient accumulation in tumors in vivo Can translocate specifically into cancer cells and kill by photothermal effect under near infrared (NIR; 808 nm) irradiation in vitro and in vivo | [227] |
| HPA3PHis | AuNPs-DNA aptamer | Spherical | 15 | Vibrio vulnificus | [189] |
| VG16KRKP | AuNPs | Spherical | 20 | Potent in vitro and in vivo anti-Salmonella typhi activity. The conjugate can penetrate into host epithelial and macrophage cells, and lysis the internalized pathogen. | [228] |
| LL37 Cys-modified (LL37-SH) | AuNPs | - | - | Computational study on the interaction of the AMP with a AuNP, showing that the cysteine may have an effect on the formation of the conjugate | [229] |
| Human β-defensin 3 (hBD3) | AuNPs | Spherical | 45 | Promotes the osteogenic differentiation of human periodontal ligament cells | [230] |
| Nisin | AgNP (green synthesis) | Spherical | 233 | Induce inflammatory response via increasing IL-12 without changes on the production of TNF-α by macrophage cells | [231] |
| Indolicidin | AuNPs | Spherical | 5 | Biofilm formation of C. albicans and Candida tropicalis multi-resistant clinical isolates | [194] |
| LL37 | AuNPs with poly(ethylene imine) as capping agent | Spherical | 7 | Bactericidal effect in vitro with MRSA from human isolates from ulcers in diabetic patients and in vivo with diabetic wound healing models. Combined with pro-angiogenic (VEGF) plasmids, the conjugate prevented MRSA infection in wound sites. | [232] |
| 1018-derivative peptide (1018K6) | AuNPs | Spherical | 8 ± 2 | Bacterial killing ability against L. monocytogenes (food-isolated) and Salmonella typhi | [233] |
| Andersonin-Y1 (AY1) CAY1 (cysteine at C-terminus) AY1C (cysteine at N-terminus) | AgNPs | Spherical | 10 | Better MICV with cysteine tagged nanoconjugates against E. coli and multidrug resistant strains of P. aeruginosa, Salmonella typhi and K. pneumoniae | [184] |
| Daptomycin | AuNPs | Spherical | 6 | Causes bacterial genomic DNA fragmentation in MRSA | [234] |
| Motif (Pep-H) of human neutrophil peptide-1 | AuNPs | Spherical | 20 | Antimicrobial activity against intracellular M. tuberculosis in infected monocyte-derived macrophages | [235] |
References
- Barriere, S.L. Clinical, economic and societal impact of antibiotic resistance. Expert Opin. Pharmacother. 2015, 16, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Available online: http://amr-review.org/sites/default/files/160525_Final paper_with cover.pdf (accessed on 7 August 2019).
- Park, S.-C.; Park, Y.; Hahm, K.-S. The Role of Antimicrobial Peptides in Preventing Multidrug-Resistant Bacterial Infections and Biofilm Formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V.; et al. Perspective of Use of Antiviral Peptides against Influenza Virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, H.; Li, Y.; Wang, G.; Tang, B.; Zhao, J.; Huang, Y.; Zheng, J. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway. Front. Immunol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Lupetti, A.; van Dissel, J.T.; Brouwer, C.P.J.M.; Nibbering, P.H. Human antimicrobial peptides’ antifungal activity against Aspergillus fumigatus. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1125–1129. [Google Scholar] [CrossRef]
- Kočendová, J.; Vaňková, E.; Volejníková, A.; Nešuta, O.; Buděšínský, M.; Socha, O.; Hájek, M.; Hadravová, R.; Čeřovský, V. Antifungal activity of analogues of antimicrobial peptides isolated from bee venoms against vulvovaginal Candida spp. FEMS Yeast Res. 2019, 19. [Google Scholar] [CrossRef]
- Vale, N.; Aguiar, L.; Gomes, P. Antimicrobial peptides: a new class of antimalarial drugs? Front. Pharmacol. 2014, 5, 275. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- AB Naafs, M. The Antimicrobial Peptides: Ready for Clinical Trials? Biomed. J. Sci. Tech. Res. 2018, 7. [Google Scholar] [CrossRef]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krötz, F.; Zahler, S.; Gloe, T.; Issbrücker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C.; et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E.W. The Human Antimicrobial Peptide LL-37 Is a Multifunctional Modulator of Innate Immune Responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Mardirossian, M.; Pompilio, A.; Degasperi, M.; Runti, G.; Pacor, S.; Di Bonaventura, G.; Scocchi, M. D-BMAP18 Antimicrobial Peptide Is Active In vitro, Resists to Pulmonary Proteases but Loses Its Activity in a Murine Model of Pseudomonas aeruginosa Lung Infection. Front. Chem. 2017, 5, 40. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Storm, G.; Verrijk, R.; de Leede, L.; Jiskoot, W.; Hennink, W.E. Shifting paradigms: biopharmaceuticals versus low molecular weight drugs. Int. J. Pharm. 2003, 266, 3–16. [Google Scholar] [CrossRef]
- Park, W.; Na, K. Advances in the synthesis and application of nanoparticles for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 494–508. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Skwarecki, A.S.; Milewski, S.; Schielmann, M.; Milewska, M.J. Antimicrobial molecular nanocarrier–drug conjugates. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2215–2240. [Google Scholar] [CrossRef] [PubMed]
- García-Gallego, S.; Franci, G.; Falanga, A.; Gómez, R.; Folliero, V.; Galdiero, S.; De La Mata, F.J.; Galdiero, M. Function oriented molecular design: Dendrimers as novel antimicrobials. Molecules 2017, 22, 1581. [Google Scholar] [CrossRef] [PubMed]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Advances in the fabrication of antimicrobial hydrogels for biomedical applications. Materials (Basel) 2017, 10, 232. [Google Scholar] [CrossRef]
- Yang, J.; Lu, H.; Li, M.; Liu, J.; Zhang, S.; Xiong, L.; Sun, Q. Development of chitosan-sodium phytate nanoparticles as a potent antibacterial agent. Carbohydr. Polym. 2017, 178, 311–321. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro Junior, J.A.; Chiavacci, L.A. Physicochemical characterization of drug nanocarriers. Int. J. Nanomedicine 2017, 12, 4991–5011. [Google Scholar] [CrossRef]
- Domingues, M.M.; Santiago, P.S.; Castanho, M.A.R.B.; Santos, N.C. What can light scattering spectroscopy do for membrane-active peptide studies? J. Pept. Sci. 2008, 14, 394–400. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Liu, D.; Huang, L. Size homogeneity of a liposome preparation is crucial for liposome biodistribution in vivo. J. Liposome Res. 1992, 2, 57–66. [Google Scholar] [CrossRef]
- Palchetti, S.; Colapicchioni, V.; Digiacomo, L.; Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; La Barbera, G.; Laganà, A. The protein corona of circulating PEGylated liposomes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 189–196. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Maurer, N.; Fenske, D.B.; Cullis, P.R. Developments in liposomal drug delivery systems. Expert Opin. Biol. Ther. 2005, 1, 923–947. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.J.; Hsu, S.H.; Tsai, C.L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chemie Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef]
- Phan, C.M.; Nguyen, H.M. Role of Capping Agent in Wet Synthesis of Nanoparticles. J. Phys. Chem. A 2017, 121, 3213–3219. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Tashkhourian, J.; Nami Ana, S.F. Topical delivery of chitosan-capped silver nanoparticles speeds up healing in burn wounds: A preclinical study. Carbohydr. Polym. 2018, 200, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles – A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Jamil, B.; Imran, M. Factors pivotal for designing of nanoantimicrobials: an exposition. Crit. Rev. Microbiol. 2018, 44, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination With Conventional Antibiotics—A Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef]
- McPhee, J.; Scott, M.; Hancock, R. Design of Host Defence Peptides for Antimicrobial and Immunity Enhancing Activities. Comb. Chem. High Throughput Screen. 2005, 8, 257–272. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Chang, L.W.; Lin, P. Metal-Based Nanoparticles and the Immune System: Activation, Inflammation, and Potential Applications. Biomed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Crist, R.M.; Grossman, J.H.; Patri, A.K.; Stern, S.T.; Dobrovolskaia, M.A.; Adiseshaiah, P.P.; Clogston, J.D.; McNeil, S.E. Common pitfalls in nanotechnology: lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integr. Biol. 2013, 5, 66–73. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: new perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- de Leeuw, J.; de Vijlder, H.; Bjerring, P.; Neumann, H. Liposomes in dermatology today. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal Administration of TAT-Conjugated Lipid Nanocarriers Loading GDNF for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Vaz, G.R.; Hädrich, G.; Bidone, J.; Rodrigues, J.L.; Falkembach, M.C.; Putaux, J.-L.; Hort, M.A.; Monserrat, J.M.; Varela Junior, A.S.; Teixeira, H.F.; et al. Development of Nasal Lipid Nanocarriers Containing Curcumin for Brain Targeting. J. Alzheimer’s Dis. 2017, 59, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. (Chezy) Doxil® — The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Madeira, C.; Mendes, R.D.; Ribeiro, S.C.; Boura, J.S.; Aires-Barros, M.R.; da Silva, C.L.; Cabral, J.M.S. Nonviral Gene Delivery to Mesenchymal Stem Cells Using Cationic Liposomes for Gene and Cell Therapy. J. Biomed. Biotechnol. 2010, 2010, 1–12. [Google Scholar] [CrossRef]
- Gregoriadis, G. The Carrier Potential of Liposomes in Biology and Medicine. N. Engl. J. Med. 1976, 295, 765–770. [Google Scholar] [CrossRef]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle interactions with the immune system: Clinical implications for liposome-based cancer chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Recent Progress in Liposome Production, Relevance to Drug Delivery and Nanomedicine. Recent Pat. Nanotechnol. 2015, 9, 86–93. [Google Scholar] [CrossRef]
- Alipour, M.; Halwani, M.; Omri, A.; Suntres, Z.E. Antimicrobial effectiveness of liposomal polymyxin B against resistant Gram-negative bacterial strains. Int. J. Pharm. 2008, 355, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Briuglia, M.-L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Were, L.M.; Bruce, B.D.; Davidson, P.M.; Weiss, J. Size, Stability, and Entrapment Efficiency of Phospholipid Nanocapsules Containing Polypeptide Antimicrobials. J. Agric. Food Chem. 2003, 51, 8073–8079. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.; Yatvin, M.B.; Huang, L. pH-sensitive liposomes: Acid-induced liposome fusion. Proc. Natl. Acad. Sci. USA 1984, 81, 1715–1718. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Barar, J.; Omidi, Y. Dysregulated pH in tumor microenvironment checkmates cancer therapy. BioImpacts 2013, 3, 149–162. [Google Scholar]
- Were, L.M.; Bruce, B.; Davidson, P.M.; Weiss, J. Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. J. Food Prot. 2004, 67, 922–927. [Google Scholar] [CrossRef]
- Wiedemann, I.; Breukink, E.; Van Kraaij, C.; Kuipers, O.P.; Bierbaum, G.; De Kruijff, B.; Sahl, H.G. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 2001, 276, 1772–1779. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Alipour, M.; Suntres, Z.E.; Halwani, M.; Azghani, A.O.; Omri, A. Activity and Interactions of Liposomal Antibiotics in Presence of Polyanions and Sputum of Patients with Cystic Fibrosis. PLoS ONE 2009, 4, e5724. [Google Scholar] [CrossRef]
- He, J.; Abdelraouf, K.; Ledesma, K.R.; Chow, D.S.-L.; Tam, V.H. Pharmacokinetics and efficacy of liposomal polymyxin B in a murine pneumonia model. Int. J. Antimicrob. Agents 2013, 42, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Huang, X.; Wang, X.; Liao, J.; Chen, Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int. J. Nanomed. 2013, 8, 1285. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J. Complement activation-related pseudoallergy: A stress reaction in blood triggered by nanomedicines and biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Price, J.V.; Vance, R.E. The Macrophage Paradox. Immunity 2014, 41, 685–693. [Google Scholar] [CrossRef]
- Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies used by bacteria to grow in macrophages. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Xie, S.; Tao, Y.; Pan, Y.; Qu, W.; Cheng, G.; Huang, L.; Chen, D.; Wang, X.; Liu, Z.; Yuan, Z. Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. J. Control. Release 2014, 187, 101–117. [Google Scholar] [CrossRef]
- Stevenson, M.; Baillie, A.J.; Richards, R.M.E. Enhanced activity of streptomycin and chloramphenicol against intracellular Escherichia coli in the J774 macrophage cell line mediated by liposome delivery. Antimicrob. Agents Chemother. 1983, 24, 742–749. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, J.-S.; Lee, D.G. Scolopendin, an antimicrobial peptide from centipede, attenuates mitochondrial functions and triggers apoptosis in Candida albicans. Biochem. J. 2017, 474, 635–645. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Raza, K.; Kumar, P.; Kumar, N.; Malik, R. Pharmacokinetics and biodistribution of the nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–186. [Google Scholar]
- Price, D.J.E.; Graham, D.I. Effects of Large Doses of Colistin Sulphomethate Sodium on Renal Function. BMJ 1970, 4, 525–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zavascki, A.P.; Nation, R.L. Nephrotoxicity of Polymyxins: Is There Any Difference between Colistimethate and Polymyxin B? Antimicrob. Agents Chemother. 2017, 61, e02319-16. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, S.; Alpar, H.; McAllister, S.; Brown, M. Liposomal (MLV) polymyxin b: Physicochemical characterization and effect of surface charge on drug association. J. Drug Target. 1993, 1, 303–310. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.M. Antimicrobial properties of liposomal polymyxin B. J. Antimicrob. Chemother. 1999, 43, 203–210. [Google Scholar] [CrossRef][Green Version]
- Degnan, A.J.; Buyong, N.; Luchansky, J.B. Antilisterial activity of pediocin AcH in model food systems in the presence of an emulsifier or encapsulated within liposomes. Int. J. Food Microbiol. 1993, 18, 127–138. [Google Scholar] [CrossRef]
- Benech, R.O.; Kheadr, E.E.; Laridi, R.; Lacroix, C.; Fliss, I. Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl. Environ. Microbiol. 2002, 68, 3683–3690. [Google Scholar] [CrossRef]
- Brown, S.P.; Inglis, R.F.; Taddei, F. Evolutionary ecology of microbial wars: Within-host competition and (incidental) virulence. Evol. Appl. 2009, 2, 32–39. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar]
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Laursen, M.F.; Bahl, M.I.; Licht, T.R.; Gram, L.; Knudsen, G.M. A single exposure to a sublethal pediocin concentration initiates a resistance-associated temporal cell envelope and general stress response in Listeria monocytogenes. Environ. Microbiol. 2015, 17, 1134–1151. [Google Scholar] [CrossRef]
- Malheiros, P.S.; Cuccovia, I.M.; Franco, B.D.G.M. Inhibition of Listeria monocytogenes in vitro and in goat milk by liposomal nanovesicles containing bacteriocins produced by Lactobacillus sakei subsp. sakei 2a. Food Control 2016, 63, 158–164. [Google Scholar] [CrossRef]
- Drlica, K.; Zhao, X. Mutant Selection Window Hypothesis Updated. Clin. Infect. Dis. 2007, 44, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Sosunov, V.; Mischenko, V.; Eruslanov, B.; Svetoch, E.; Shakina, Y.; Stern, N.; Majorov, K.; Sorokoumova, G.; Selishcheva, A.; Apt, A. Antimycobacterial activity of bacteriocins and their complexes with liposomes. J. Antimicrob. Chemother. 2007, 59, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, T.; Zhang, Y.; Huang, X.; Li, J.; Li, C.; Li, Y.; Su, T.; Zhang, Y.; Huang, X.; et al. Liposomal co-delivery of daptomycin and clarithromycin at an optimized ratio for treatment of methicillin-resistant Staphylococcus aureus infection. Drug Deliv. 2015, 22, 627–637. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Wang, X.; Chen, Y.; Wu, F.; Men, K.; Xu, T.; Luo, Y.; Yang, L. Novel antimicrobial peptide—modified azithromycin-loaded liposomes against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2016, 11, 6781–6794. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Qayyum, S.; Khan, A.U. Nanoparticles vs. biofilms: a battle against another paradigm of antibiotic resistance. Med. Chem. Commun. 2016, 7, 1479–1498. [Google Scholar] [CrossRef]
- Yamakami, K.; Tsumori, H.; Sakurai, Y.; Shimizu, Y.; Nagatoshi, K.; Sonomoto, K. Sustainable inhibition efficacy of liposome-encapsulated nisin on insoluble glucan-biofilm synthesis by Streptococcus mutans. Pharm. Biol. 2013, 51, 267–270. [Google Scholar] [CrossRef]
- Search of: Liposome Infection—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=liposome+infection&cntry=&state=&city=&dist= (accessed on 14 August 2019).
- Lo, Y.L.; Tu, W.C. Co-encapsulation of chrysophsin-1 and epirubicin in PEGylated liposomes circumvents multidrug resistance in HeLa cells. Chem. Biol. Interact. 2015, 242, 13–23. [Google Scholar] [CrossRef]
- Juang, V.; Lee, H.P.; Lin, A.M.Y.; Lo, Y.L. Cationic PEGylated liposomes incorporating an antimicrobial peptide tilapia hepcidin 2–3: An adjuvant of epirubicin to overcome multidrug resistance in cervical cancer cells. Int. J. Nanomed. 2016, 11, 6047–6064. [Google Scholar] [CrossRef] [PubMed]
- DeGrado, W.F.; Musso, G.F.; Lieber, M.; Kaiser, E.T.; Kézdy, F.J. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys. J. 1982, 37, 329–338. [Google Scholar] [CrossRef]
- Mao, J.; Liu, S.; Ai, M.; Wang, Z.; Wang, D.; Li, X.; Hu, K.; Gao, X.; Yang, Y. A novel melittin nano-liposome exerted excellent anti-hepatocellular carcinoma efficacy with better biological safety. J. Hematol. Oncol. 2017, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Yang, K.; Gitter, B.; Rüger, R.; Wieland, G.D.; Chen, M.; Liu, X.; Albrecht, V.; Fahr, A. Antimicrobial peptide-modified liposomes for bacteria targeted delivery of temoporfin in photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2011, 10, 1593–1601. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, L.; Zhang, L.; Shi, K.; Cun, X.; Yang, Y.; Liu, Y.; Gao, H.; He, Q. Dual-functionalized liposomal delivery system for solid tumors based on RGD and a pH-responsive antimicrobial peptide. Sci. Rep. 2016, 6, 19800. [Google Scholar] [CrossRef]
- Lipinski, K.A.; Barber, L.J.; Davies, M.N.; Ashenden, M.; Sottoriva, A.; Gerlinger, M. Cancer Evolution and the Limits of Predictability in Precision Cancer Medicine. Trends Cancer 2016, 2, 49–63. [Google Scholar] [CrossRef]
- Toh, M.-R.; Chiu, G.N.C. Liposomes as sterile preparations and limitations of sterilisation techniques in liposomal manufacturing. Asian J. Pharm. Sci. 2013, 8, 88–95. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Huang, Y.; Gui, S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018, 8, 6978–6987. [Google Scholar] [CrossRef]
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Amar-Yuli, I.; Wachtel, E.; Shoshan, E.B.; Danino, D.; Aserin, A.; Garti, N. Hexosome and hexagonal phases mediated by hydration and polymeric stabilizer. Langmuir 2007, 23, 3637–3645. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Bysell, H.; Ringstad, L.; Wennman, D.; Umerska, A.; Cassisa, V.; Eriksson, J.; Joly-Guillou, M.-L.; Edwards, K.; Andersson, M. Lipid-Based Liquid Crystals As Carriers for Antimicrobial Peptides: Phase Behavior and Antimicrobial Effect. Langmuir 2016, 32, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Delekta, S.S. Hexosomes as Drug Delivery Vehicles for Antimicrobial Peptides; KTH Royal Institute of Nanotechnology: Stockholm, Sweden, 2015. [Google Scholar]
- Gontsarik, M.; Buhmann, M.T.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. Antimicrobial peptide-driven colloidal transformations in liquid-crystalline nanocarriers. J. Phys. Chem. Lett. 2016, 7, 3482–3486. [Google Scholar] [CrossRef] [PubMed]
- Gontsarik, M.; Mohammadtaheri, M.; Yaghmur, A.; Salentinig, S. pH-triggered nanostructural transformations in antimicrobial peptide/oleic acid self- assemblies. Biomater. Sci. 2018, 6, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Bernegossi, J.; Calixto, G.M.F.; Da Silva Sanches, P.R.; Fontana, C.R.; Cilli, E.M.; Garrido, S.S.; Chorilli, M. Peptide KSL-W-loaded mucoadhesive liquid crystalline vehicle as an alternative treatment for multispecies oral biofilm. Molecules 2016, 21, 37. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine (NBM) 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Battaglia, L.; Ugazio, E. Lipid nano- and microparticles: An overview of patent-related research. J. Nanomater. 2019, 2019, 1–22. [Google Scholar] [CrossRef]
- Moreno-Sastre, M.; Pastor, M.; Esquisabel, A.; Sans, E.; Viñas, M.; Bachiller, D.; Pedraz, J.L. Stability study of sodium colistimethate-loaded lipid nanoparticles. J. Microencapsul. 2016, 33, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Wentzel, J.F.; Jordaan, A.; Bezuidenhout, C.; Du Plessis, L.H. Interactions of the antimicrobial peptide nisin Z with conventional antibiotics and the use of nanostructured lipid carriers to enhance antimicrobial activity. Int. J. Pharm. 2017, 526, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Sans-Serramitjana, E.; Fusté, E.; Martínez-Garriga, B.; Merlos, A.; Pastor, M.; Pedraz, J.L.; Esquisabel, A.; Bachiller, D.; Vinuesa, T.; Viñas, M. Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. J. Cyst. Fibros. 2016, 15, 611–618. [Google Scholar] [CrossRef]
- Becker Peres, L.; Becker Peres, L.; de Araújo, P.H.H.; Sayer, C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf. B Biointerfaces 2016, 140, 317–323. [Google Scholar] [CrossRef]
- Kasongo, K.W.; Mller, R.H.; Walker, R.B. The use of hot and cold high pressure homogenization to enhance the loading capacity and encapsulation efficiency of nanostructured lipid carriers for the hydrophilic antiretroviral drug, didanosine for potential administration to paediatric patients. Pharm. Dev. Technol. 2012, 17, 353–362. [Google Scholar] [CrossRef]
- Rajchakit, U.; Sarojini, V. Recent Developments in Antimicrobial-Peptide-Conjugated Gold Nanoparticles. Bioconjug. Chem. 2017, 28, 2673–2686. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Majdalawieh, A.; Kanan, M.C.; El-Kadri, O.; Kanan, S.M. Recent Advances in Gold and Silver Nanoparticles: Synthesis and Applications. J. Nanosci. Nanotechnol. 2014, 14, 4757–4780. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Petros, R.A.; Desimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal. Toxicol. 2016, 6. [Google Scholar] [CrossRef]
- Jeong, W.-J.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide–nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, T.; Shukla, D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomedicine (NBM) 2017, 13, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Veigas, B.; Pedrosa, P.; Carlos, F.F.; Mancio-Silva, L.; Grosso, A.R.; Fortunato, E.; Mota, M.M.; Baptista, P.V. One nanoprobe, two pathogens: gold nanoprobes multiplexing for point-of-care. J. Nanobiotechnol. 2015, 13, 48. [Google Scholar] [CrossRef]
- Kumar, A.; Mazinder Boruah, B.; Liang, X.-J. Gold Nanoparticles: Promising Nanomaterials for the Diagnosis of Cancer and HIV/AIDS. J. Nanomater. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Wang, G.; Jin, F.; Dai, N.; Zhong, Z.; Qing, Y.; Li, M.; Yuan, R.; Wang, D. Signal-enhanced electrochemiluminescence immunosensor based on synergistic catalysis of nicotinamide adenine dinucleotide hydride and silver nanoparticles. Anal. Biochem. 2012, 422, 7–13. [Google Scholar] [CrossRef]
- Bajaj, M.; Pandey, S.K.; Nain, T.; Brar, S.K.; Singh, P.; Singh, S.; Wangoo, N.; Sharma, R.K. Stabilized cationic dipeptide capped gold/silver nanohybrids: Towards enhanced antibacterial and antifungal efficacy. Colloids Surf. B Biointerfaces 2017, 158, 397–407. [Google Scholar] [CrossRef]
- Moyano, D.F.; Vincent, M. Rotello Nano meets biology: Structure and function at the nanoparticle interface. Langmuir 2011, 27, 10376–10385. [Google Scholar]
- Cao-Milán, R.; Liz-Marzán, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef]
- Zhang, X. Gold Nanoparticles: Recent Advances in the Biomedical Applications. Cell Biochem. Biophys. 2015, 72, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Ha, S.M.; Rammohan, A.; Radhakrishnan, R.; Ramakrishnan, N. Multivalent Binding of a Ligand-Coated Particle: Role of Shape, Size, and Ligand Heterogeneity. Biophys. J. 2018, 114, 1830–1846. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Talamini, L.; Violatto, M.B.; Cai, Q.; Monopoli, M.P.; Kantner, K.; Krpetić, Ž.; Perez-Potti, A.; Cookman, J.; Garry, D.; Silveira, C.P.; et al. Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles. ACS Nano 2017, 11, 5519–5529. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding nanoparticle cellular entry: A physicochemical perspective. Adv. Colloid Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Paredes-Gamero, E.J.; Martins, M.N.C.; Cappabianco, F.A.M.; Ide, J.S.; Miranda, A. Characterization of dual effects induced by antimicrobial peptides: Regulated cell death or membrane disruption. Biochim. Biophys. Acta 2012, 1820, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, J.; Landfester, K.; Mailänder, V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of shape on Cellular Uptake of Gold Nanoparticles in the forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef] [PubMed]
- Zewde, B.; Ambaye, A.; Stubbs Iii, J.; Raghavan, D. A review of stabilized silver nanoparticles – Synthesis, biological properties, characterization, and potential areas of applications. JSM Nanotechnol. Nanomed. 2016, 4, 1043. [Google Scholar]
- Venkatesh, N. Metallic Nanoparticle: A Review. Biomed. J. Sci. Tech. Res. 2018, 4. [Google Scholar] [CrossRef]
- Richards, R.; Bönnemann, H. Synthetic Approaches to Metallic Nanomaterials. In Nanofabrication towards Biomedical Applications: Techniques, Tools, Applications, and Impact; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 1–32. [Google Scholar]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- FRENS, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Xie, J.; Luo, Z.; Jiang, J.; Yang, Y.Y.; Liu, S. The potent antimicrobial properties of cell penetrating peptide-conjugated silver nanoparticles with excellent selectivity for Gram-positive bacteria over erythrocytes. Nanoscale 2013, 5, 3834. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and Applications of Noble Metal Nanoparticles: A Review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Sarkar, S.; Leo, B.F.; Carranza, C.; Chen, S.; Rivas-Santiago, C.; Porter, A.E.; Ryan, M.P.; Gow, A.; Chung, K.F.; Tetley, T.D.; et al. Modulation of human macrophage responses to mycobacterium tuberculosis by silver nanoparticles of different size and surface modification. PLoS ONE 2015, 10, e0143077. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver nanoparticle-induced autophagic-Lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol. Sci. 2016, 150, 473–487. [Google Scholar] [CrossRef]
- Shin, S.-H.; Ye, M.-K.; Kim, H.-S.; Kang, H.-S. The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int. Immunopharmacol. 2007, 7, 1813–1818. [Google Scholar] [CrossRef]
- Parnsamut, C.; Brimson, S. Effects of silver nanoparticles and gold nanoparticles on IL-2, IL-6, and TNF-α production via MAPK pathway in leukemic cell lines. Genet. Mol. Res. 2015, 14, 3650–3668. [Google Scholar] [CrossRef]
- Martínez-Gutierrez, F.; Thi, E.P.; Silverman, J.M.; de Oliveira, C.C.; Svensson, S.L.; Vanden Hoek, A.; Sánchez, E.M.; Reiner, N.E.; Gaynor, E.C.; Pryzdial, E.L.G.; et al. Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 328–336. [Google Scholar] [CrossRef]
- Taratummarat, S.; Sangphech, N.; Vu, C.T.B.; Palaga, T.; Ondee, T.; Surawut, S.; Sereemaspun, A.; Ritprajak, P.; Leelahavanichkul, A. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Volkov, A.A.; Mezhenny, P.V.; Domnitsky, I.Y.; Fomin, A.S.; Kozlov, S.V.; Dykman, L.A.; Guliy, O.I. Prospects for the use of spherical gold nanoparticles in immunization. Appl. Microbiol. Biotechnol. 2019, 103, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Dul, M.; Nikolic, T.; Stefanidou, M.; McAteer, M.A.; Williams, P.; Mous, J.; Roep, B.O.; Kochba, E.; Levin, Y.; Peakman, M.; et al. Conjugation of a peptide autoantigen to gold nanoparticles for intradermally administered antigen specific immunotherapy. Int. J. Pharm. 2019, 562, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Search of: Silver Nanoparticles—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=silver+nanoparticles&cntry=&state=&city=&dist= (accessed on 14 August 2019).
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef]
- Mei, L.; Lu, Z.; Zhang, W.; Wu, Z.; Zhang, X.; Wang, Y.; Luo, Y.; Li, C.; Jia, Y. Bioconjugated nanoparticles for attachment and penetration into pathogenic bacteria. Biomaterials 2013, 34, 10328–10337. [Google Scholar] [CrossRef]
- Mohanty, S.; Jena, P.; Mehta, R.; Pati, R.; Banerjee, B.; Patil, S.; Sonawane, A. Cationic antimicrobial peptides and biogenic silver nanoparticles kill mycobacteria without eliciting dna damage and cytotoxicity in mouse macrophages. Antimicrob. Agents Chemother. 2013, 57, 3688–3698. [Google Scholar] [CrossRef]
- Pal, I.; Brahmkhatri, V.P.; Bera, S.; Bhattacharyya, D.; Quirishi, Y.; Bhunia, A.; Atreya, H.S. Enhanced stability and activity of an antimicrobial peptide in conjugation with silver nanoparticle. J. Colloid Interface Sci. 2016, 483, 385–393. [Google Scholar] [CrossRef]
- Pal, I.; Bhattacharyya, D.; Kar, R.K.; Zarena, D.; Bhunia, A.; Atreya, H.S. A Peptide-Nanoparticle System with Improved Efficacy against Multidrug Resistant Bacteria. Sci. Rep. 2019, 9, 4485. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A. Dos Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar]
- Chiodo, F.; Marradi, M.; Calvo, J.; Yuste, E.; Penadés, S. Glycosystems in nanotechnology: Gold glyconanoparticles as carrier for anti-HIV prodrugs. Beilstein J. Org. Chem. 2014, 10, 1339–1346. [Google Scholar] [CrossRef]
- Madhusudhan, A.; Reddy, G.; Venkatesham, M.; Veerabhadram, G.; Kumar, D.; Natarajan, S.; Yang, M.-Y.; Hu, A.; Singh, S. Efficient pH Dependent Drug Delivery to Target Cancer Cells by Gold Nanoparticles Capped with Carboxymethyl Chitosan. Int. J. Mol. Sci. 2014, 15, 8216–8234. [Google Scholar] [CrossRef] [PubMed]
- Ock, K.-S.; Ganbold, E.O.; Park, J.; Cho, K.; Joo, S.-W.; Lee, S.Y. Label-free Raman spectroscopy for accessing intracellular anticancer drug release on gold nanoparticles. Analyst 2012, 137, 2852. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Park, J.; Ryu, M.; Kim, S.; Joo, M.; Yeom, J.H.; Kim, S.; Park, Y.; Lee, K.; Bae, J. Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci. Rep. 2017, 7, 13572. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Pinto, S.; Velho, T.R.; Ferreira, A.F.; Moita, C.; Trivedi, U.; Evangelista, M.; Comune, M.; Rumbaugh, K.P.; Simões, P.N.; et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 2016, 85, 99–110. [Google Scholar] [CrossRef]
- Casciaro, B.; Moros, M.; Rivera-Fernández, S.; Bellelli, A.; de la Fuente, J.M.; Mangoni, M.L. Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a(1-21)NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 2017, 47, 170–181. [Google Scholar] [CrossRef]
- de Miranda, J.L.; Oliveira, M.D.L.; Oliveira, I.S.; Frias, I.A.M.; Franco, O.L.; Andrade, C.A.S. A simple nanostructured biosensor based on clavanin A antimicrobial peptide for gram-negative bacteria detection. Biochem. Eng. J. 2017, 124, 108–114. [Google Scholar] [CrossRef]
- Navani, N.K.; Ramulu Lambadi, P.; Kumar Sharma, T.; Kumar, P.; Vasnani, P.; Mouli Thalluri, S.; Bisht, N.; Pathania, R. Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int. J. Nanomed. 2015, 10, 2155. [Google Scholar] [CrossRef]
- de Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018, 11, 915–925. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Leite, N.B.; Aufderhorst-Roberts, A.; Palma, M.S.; Connell, S.D.; Neto, J.R.; Beales, P.A. PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties. Biophys. J. 2015, 109, 936–947. [Google Scholar] [CrossRef]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef] [PubMed]
- Bosso, M.; Ständker, L.; Kirchhoff, F.; Münch, J. Exploiting the human peptidome for novel antimicrobial and anticancer agents. Bioorg. Med. Chem. 2018, 26, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Ravishankar Rai, V.; Umashankar, M. Effect of peptide-conjugated nanoparticles on cell lines. Prog. Biomater. 2019, 8, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Farkhani, S.M.; Valizadeh, A.; Karami, H.; Mohammadi, S.; Sohrabi, N.; Badrzadeh, F. Cell penetrating peptides: Efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides 2014, 57, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Pereira, F.; Alves de Matos, A.P.; Fernandes, M.; Baptista, P.V.; Fernandes, A.R. Smuggling gold nanoparticles across cell types – A new role for exosomes in gene silencing. Nanomedicine (NBM) 2017, 13, 1389–1398. [Google Scholar] [CrossRef]
- Pedrosa, P.; Mendes, R.; Cabral, R.; Martins, L.M.D.R.S.; Baptista, P.V.; Fernandes, A.R. Combination of chemotherapy and Au-nanoparticle photothermy in the visible light to tackle doxorubicin resistance in cancer cells. Sci. Rep. 2018, 8, 11429. [Google Scholar] [CrossRef]
- Piddock, L.; Garneau-Tsodikova, S.; Garner, C. Ask the experts: how to curb antibiotic resistance and plug the antibiotics gap? Future Med. Chem. 2016, 8, 1027–1032. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T 2017, 42, 742–755. [Google Scholar]
- Search of: nano | Recruiting, Not yet recruiting, Active, not recruiting Studies—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?term=nano&Search=Apply&recrs=b&recrs=a&recrs=d&age_v=&gndr=&type=&rslt= (accessed on 27 May 2019).
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Park, S.; Chibli, H.; Wong, J.; Nadeau, J.L. Antimicrobial activity and cellular toxicity of nanoparticle–polymyxin B conjugates. Nanotechnology 2011, 22, 185101. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.D.; Das, G.; Ramesh, A. Retention of nisin activity at elevated pH in an organic acid complex and gold nanoparticle composite. Chem. Commun. 2012, 48, 8928–8930. [Google Scholar] [CrossRef] [PubMed]
- Vignoni, M.; de Alwis Weerasekera, H.; Simpson, M.J.; Phopase, J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I.; Scaiano, J.C. LL37 peptide@silver nanoparticles: combining the best of the two worlds for skin infection control. Nanoscale 2014, 6, 5725–5728. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Co-spinning of Silver Nanoparticles with Nisin Increases the Antimicrobial Spectrum of PDLLA: PEO Nanofibers. Curr. Microbiol. 2015, 71, 24–30. [Google Scholar] [CrossRef]
- Patil, S.D.; Sharma, R.; Bhattacharyya, T.; Kumar, P.; Gupta, M.; Chaddha, B.S.; Navani, N.K.; Pathania, R. Antibacterial potential of a small peptide from Bacillus sp. RPT-0001 and its capping for green synthesis of silver nanoparticles. J. Microbiol. 2015, 53, 643–652. [Google Scholar] [CrossRef]
- Sur, A.; Pradhan, B.; Banerjee, A.; Aich, P. Immune Activation Efficacy of Indolicidin Is Enhanced upon Conjugation with Carbon Nanotubes and Gold Nanoparticles. PLoS ONE 2015, 10, e0123905. [Google Scholar] [CrossRef]
- Peng, L.H.; Huang, Y.F.; Zhang, C.Z.; Niu, J.; Chen, Y.; Chu, Y.; Jiang, Z.H.; Gao, J.Q.; Mao, Z.W. Integration of antimicrobial peptides with gold nanoparticles as unique non-viral vectors for gene delivery to mesenchymal stem cells with antibacterial activity. Biomaterials 2016, 103, 137–149. [Google Scholar] [CrossRef]
- Arakha, M.; Borah, S.M.; Saleem, M.; Jha, A.N.; Jha, S. Interfacial assembly at silver nanoparticle enhances the antibacterial efficacy of nisin. Free Radic. Biol. Med. 2016, 101, 434–445. [Google Scholar] [CrossRef]
- McLaughlin, S.; Ahumada, M.; Franco, W.; Mah, T.-F.; Seymour, R.; Suuronen, E.J.; Alarcon, E.I. Sprayable peptide-modified silver nanoparticles as a barrier against bacterial colonization. Nanoscale 2016, 8, 19200–19203. [Google Scholar] [CrossRef]
- Yeom, J.H.; Lee, B.; Kim, D.; Lee, J.K.; Kim, S.; Bae, J.; Park, Y.; Lee, K. Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar Typhimurium. Biomaterials 2016, 104, 43–51. [Google Scholar] [CrossRef]
- Akrami, M.; Balalaie, S.; Hosseinkhani, S.; Alipour, M.; Salehi, F.; Bahador, A.; Haririan, I. Tuning the anticancer activity of a novel pro-apoptotic peptide using gold nanoparticle platforms. Sci. Rep. 2016, 6, 31030. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Rai, A.; Pinto, S.; Evangelista, M.; Cardoso, R.M.S.; Paulo, C.; Carvalheiro, T.; Paiva, A.; Imani, M.; Simchi, A.; et al. High Antimicrobial Activity and Low Human Cell Cytotoxicity of Core–Shell Magnetic Nanoparticles Functionalized with an Antimicrobial Peptide. ACS Appl. Mater. Interfaces 2016, 8, 11366–11378. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Pinto, S.; Evangelista, M.B.; Gil, H.; Kallip, S.; Ferreira, M.G.S.; Ferreira, L. High-density antimicrobial peptide coating with broad activity and low cytotoxicity against human cells. Acta Biomater. 2016, 33, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.F.; Rai, A.; Ferreira, L.; Simões, P.N. Findings on the interaction of the antimicrobial peptide cecropin-melittin with a gold surface from molecular dynamics studies. Eur. Biophys. J. 2017, 46, 247–256. [Google Scholar] [CrossRef]
- Wang, R.H.; Bai, J.; Deng, J.; Fang, C.J.; Chen, X. TAT-Modified Gold Nanoparticle Carrier with Enhanced Anticancer Activity and Size Effect on Overcoming Multidrug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 5828–5837. [Google Scholar] [CrossRef]
- Morales-Avila, E.; Ferro-Flores, G.; Ocampo-Garciá, B.E.; López-Téllez, G.; López-Ortega, J.; Rogel-Ayala, D.G.; Sánchez-Padilla, D. Antibacterial Efficacy of Gold and Silver Nanoparticles Functionalized with the Ubiquicidin (29-41) Antimicrobial Peptide. J. Nanomater. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Comune, M.; Rai, A.; Chereddy, K.K.; Pinto, S.; Aday, S.; Ferreira, A.F.; Zonari, A.; Blersch, J.; Cunha, R.; Rodrigues, R.; et al. Antimicrobial peptide-gold nanoscale therapeutic formulation with high skin regenerative potential. J. Control. Release 2017, 262, 58–71. [Google Scholar] [CrossRef]
- Jasim, R.; Schneider, E.K.; Han, M.; Azad, M.A.K.; Hussein, M.; Nowell, C.; Baker, M.A.; Wang, J.; Li, J.; Velkov, T. A fresh shine on cystic fibrosis inhalation therapy: Antimicrobial synergy of polymyxin B in combination with silver nanoparticles. J. Biomed. Nanotechnol. 2017, 13, 447–457. [Google Scholar] [CrossRef]
- Wadhwani, P.; Heidenreich, N.; Podeyn, B.; Bürck, J.; Ulrich, A.S. Antibiotic gold: Tethering of antimicrobial peptides to gold nanoparticles maintains conformational flexibility of peptides and improves trypsin susceptibility. Biomater. Sci. 2017, 5, 817–827. [Google Scholar] [CrossRef]
- Tan, H.; Huang, Y.; Xu, J.; Chen, B.; Zhang, P.; Ye, Z.; Liang, S.; Xiao, L.; Liu, Z. Spider toxin peptide lycosin-I functionalized gold nanoparticles for in vivo tumor targeting and therapy. Theranostics 2017, 7, 3168–3178. [Google Scholar] [CrossRef]
- Chowdhury, R.; Ilyas, H.; Ghosh, A.; Ali, H.; Ghorai, A.; Midya, A.; Jana, N.R.; Das, S.; Bhunia, A. Multivalent gold nanoparticle-peptide conjugates for targeting intracellular bacterial infections. Nanoscale 2017, 9, 14074–14093. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.F.; Comune, M.; Rai, A.; Ferreira, L.; Simões, P.N. Atomistic-Level Investigation of a LL37-Conjugated Gold Nanoparticle by Well-Tempered Metadynamics. J. Phys. Chem. B 2018, 122, 8359–8366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Y.; Li, L.; Fu, H.; Yang, W.; Yan, F. Human β-defensin 3-combined gold nanoparticles for enhancement of osteogenic differentiation of human periodontal ligament cells in inflammatory microenvironments. Int. J. Nanomed. 2018, 13, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.; Imani Fooladi, A.A.; Mahmoodzadeh Hosseini, H. Determining the effects of green chemistry synthesized Ag-nisin nanoparticle on macrophage cells. Microb. Pathog. 2018, 114, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, C.; Zhang, X.; Shi, D.; Chi, L.; Luo, G.; Deng, J. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater. Sci. 2018, 6, 2757–2772. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Tatè, R.; Gogliettino, M.; Balestrieri, M.; Rea, I.; Terracciano, M.; Proroga, Y.T.; Capuano, F.; Anastasio, A.; De Stefano, L. Small Synthetic Peptides Bioconjugated to Hybrid Gold Nanoparticles Destroy Potentially Deadly Bacteria at Submicromolar Concentrations. Bioconjug. Chem. 2018, 29, 3877–3885. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Chen, Y.; Li, C.; Jiang, H.; Wang, X. Conjugating gold nanoclusters and antimicrobial peptides: From aggregation-induced emission to antibacterial synergy. J. Colloid Interface Sci. 2019, 546, 1–10. [Google Scholar] [CrossRef]
- Sharma, R.; Raghav, R.; Priyanka, K.; Rishi, P.; Sharma, S.; Srivastava, S.; Verma, I. Exploiting chitosan and gold nanoparticles for antimycobacterial activity of in silico identified antimicrobial motif of human neutrophil peptide-1. Sci. Rep. 2019, 9, 7866. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery. Pharmaceutics 2019, 11, 588. https://doi.org/10.3390/pharmaceutics11110588
Makowski M, Silva ÍC, Pais do Amaral C, Gonçalves S, Santos NC. Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery. Pharmaceutics. 2019; 11(11):588. https://doi.org/10.3390/pharmaceutics11110588
Chicago/Turabian StyleMakowski, Marcin, Ítala C. Silva, Constança Pais do Amaral, Sónia Gonçalves, and Nuno C. Santos. 2019. "Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery" Pharmaceutics 11, no. 11: 588. https://doi.org/10.3390/pharmaceutics11110588
APA StyleMakowski, M., Silva, Í. C., Pais do Amaral, C., Gonçalves, S., & Santos, N. C. (2019). Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery. Pharmaceutics, 11(11), 588. https://doi.org/10.3390/pharmaceutics11110588