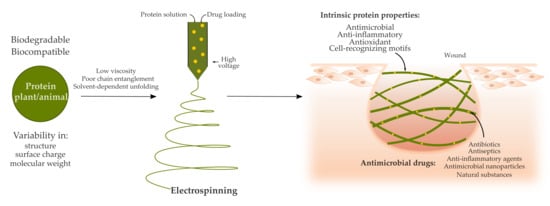
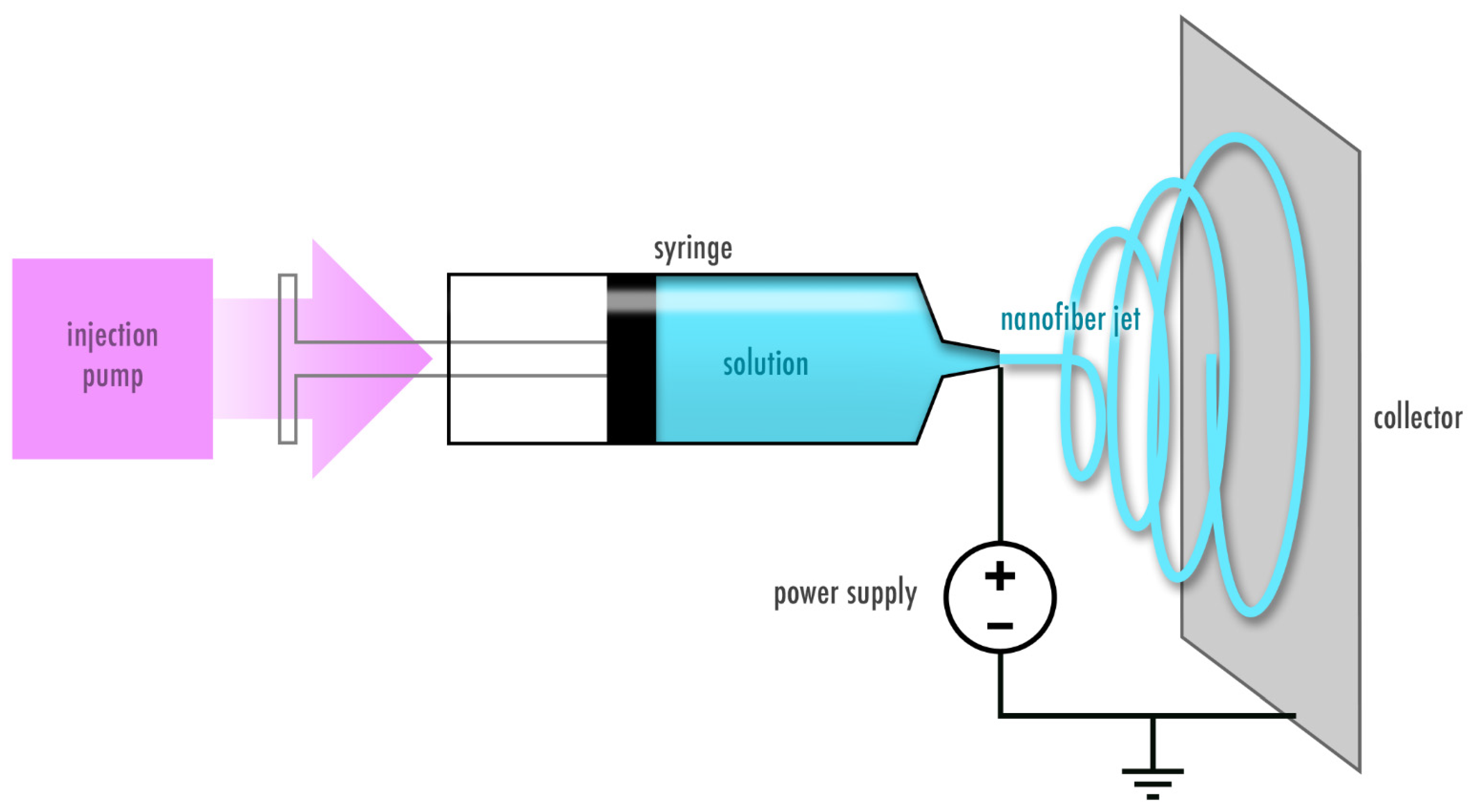
Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges
Abstract
1. Wound Healing and Electrospun Wound Dressings
2. Proteins as a Promising Starting Material for Electrospun Wound Dressings
3. Electrospinning of Plant-Derived Proteins for Wound Healing Purposes
3.1. Zein Protein
3.2. Soy Protein
3.3. Pea Protein
4. Electrospinning Animal-Derived Proteins for Wound Healing Purposes
4.1. Casein
4.2. Whey
4.2.1. Lactoglobulins
4.2.2. Lactoferrin
4.2.3. Lysozyme
4.3. Keratin
4.4. Silk Fibroin and Sericin
4.5. Collagen and Gelatin
4.6. Elastin, Tropoelastin and Elastin-Like Recombinamers
5. Opportunities and Challenges of Using Proteins for Electrospun Drug Delivery Systems for Wound Healing
Author Contributions
Funding
Conflicts of Interest
References
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Okur, N.Ü.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microb. 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Raeder, K.; Jachan, D.E.; Muller-Werdan, U.; Lahmann, N.A. Prevalence and risk factors of chronic wounds in nursing homes in germany: A cross-sectional study. Int. Wound J. 2020, 17, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Ruiz, M.J.; Llatas, F.P.; Jimenez, O.S. Assessment of chronic wounds in adults: An integrative review. Rev. Esc. Enferm. USP 2018, 52, e03315. [Google Scholar]
- Ashtikar, M.; Wacker, M.G. Nanopharmaceuticals for wound healing—Lost in translation? Adv. Drug Deliv. Rev. 2018, 129, 194–218. [Google Scholar] [CrossRef]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic delivery strategies to treat skin infections when innate antimicrobial defense fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Graves, J.L.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun nanofibrous materials for wound healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Park, M.; Park, S.-J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Steckl, A.J. Coaxial electrospinning formation of complex polymer fibers and their applications. ChemPlusChem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Kai, D.; Ramakrishna, S. Emulsion electrospun vascular endothelial growth factor encapsulated poly(l-lactic acid-co-ε-caprolactone) nanofibers for sustained release in cardiac tissue engineering. J. Mater. Sci. 2012, 47, 3272–3281. [Google Scholar] [CrossRef]
- Zong, H.X.; Xia, X.; Liang, Y.R.; Dai, S.Y.; Alsaedi, A.; Hayat, T.; Kong, F.T.; Pan, J.H. Designing function-oriented artificial nanomaterials and membranes via electrospinning and electrospraying techniques. Mater. Sci. Eng. C 2018, 92, 1075–1091. [Google Scholar] [CrossRef]
- Qi, H.Z.; Yang, L.J.; Shan, P.P.; Zhu, S.J.; Ding, H.; Xue, S.; Wang, Y.; Yuan, X.B.; Li, P.F. The stability maintenance of protein drugs in organic coatings based on nanogels. Pharmaceutics 2020, 12, 115. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Drug release kinetics of electrospun fibrous systems. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Focarete, M.L., Tampieri, A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 349–374. [Google Scholar]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Venkatraman, S. Electrospinning pure protein solutions in core–shell fibers. Polym. Int. 2012, 61, 1549–1555. [Google Scholar] [CrossRef]
- Datta, L.P.; Manchineella, S.; Govindaraju, T. Biomolecules-derived biomaterials. Biomaterials 2020, 230, 119633. [Google Scholar] [CrossRef] [PubMed]
- Hall Barrientos, I.J.; Paladino, E.; Brozio, S.; Passarelli, M.K.; Moug, S.; Black, R.A.; Wilson, C.G.; Lamprou, D.A. Fabrication and characterisation of drug-loaded electrospun polymeric nanofibers for controlled release in hernia repair. Int. J. Pharm. 2017, 517, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Gnanasekaran, G.; Sathishkumar, Y.; Lee, Y.S.; Kim, C.S. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr. Polym. 2014, 102, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, B.; Joshi, M.K.; Tiwari, A.P.; Park, C.H.; Kim, C.S. In-situ synthesis of agnps in the natural/synthetic hybrid nanofibrous scaffolds: Fabrication, characterization and antimicrobial activities. J. Mech. Behav. Biomed. Mater. 2017, 65, 66–76. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, X.; He, H.W.; Yu, M.; Ning, X.; Long, Y.Z. Solvent-free electrospinning: Opportunities and challenges. Polym. Chem. 2017, 8, 333–352. [Google Scholar] [CrossRef]
- Aguilar-Vázquez, G.; Ortiz-Frade, L.; Figueroa-Cárdenas, J.D.; López-Rubio, A.; Mendoza, S. Electrospinnability study of pea (Pisum sativum) and common bean (Phaseolus vulgaris L.) using the conformational and rheological behavior of their protein isolates. Polym. Test. 2020, 81, 106217. [Google Scholar] [CrossRef]
- Cho, D.; Nnadi, O.; Netravali, A.; Joo, Y.L. Electrospun hybrid soy protein/pva fibers. Macromol. Mater. Eng. 2010, 295, 763–773. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Woerdeman, D.L.; Shenoy, S.; Breger, D. Role of chain entanglements in the electrospinning of wheat protein-poly(vinyl alcohol) blends. J. Adhes. 2007, 83, 785–798. [Google Scholar] [CrossRef]
- Yildiz, A.; Kara, A.A.; Acarturk, F. Peptide-protein based nanofibers in pharmaceutical and biomedical applications. Int. J. Biol. Macromol. 2020, 148, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-based fiber materials in medicine: A review. Nanomaterials (Basel) 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, X.T.; Cuenca, A.A.; Jurado, A.T.; Corona, A.A.; Urista, C.R.M. Traditional methods for whey protein isolation and concentration: Effects on nutritional properties and biological activity. J. Mex. Chem. Soc. 2012, 56, 369–377. [Google Scholar]
- Boland, M. Whey proteins. In Handbook of Food Proteins; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 30–55. [Google Scholar]
- Silvetti, T.; Morandi, S.; Hintersteiner, M.; Brasca, M. Chapter 22—Use of hen egg white lysozyme in the food industry. In Egg Innovations and Strategies for Improvements; Hester, P.Y., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 233–242. [Google Scholar]
- Zheng, H.; Yan, G.; Marquez, S.; Andler, S.; Dersjant-Li, Y.; de Mejia, E.G. Molecular size and immunoreactivity of ethanol extracted soybean protein concentrate in comparison with other products. Process Biochem. 2020, 96, 122–130. [Google Scholar] [CrossRef]
- Anderson, T.J.; Lamsal, B.P. Review: Zein extraction from corn, corn products, and coproducts and modifications for various applications: A review. Cereal Chem. 2011, 88, 159–173. [Google Scholar] [CrossRef]
- Yeo, G.C.; Aghaei-Ghareh-Bolagh, B.; Brackenreg, E.P.; Hiob, M.A.; Lee, P.; Weiss, A.S. Fabricated elastin. Adv. Health. Mater. 2018, 7, e1801342. [Google Scholar] [CrossRef]
- Kanjanapongkul, K.; Wongsasulak, S.; Yoovidhya, T. Investigation and prevention of clogging during electrospinning of zein solution. J. Appl. Polym. Sci. 2010, 118, 1821–1829. [Google Scholar] [CrossRef]
- Vogt, L.; Liverani, L.; Roether, J.A.; Boccaccini, A.R. Electrospun zein fibers incorporating poly(glycerol sebacate) for soft tissue engineering. Nanomaterials 2018, 8, 150. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 393–402. [Google Scholar] [CrossRef]
- Thirugnanaselvam, M.; Gobi, N.; Arun Karthick, S. Spi/peo blended electrospun martrix for wound healing. Fibers Polym. 2013, 14, 965–969. [Google Scholar] [CrossRef]
- Li, J.J.; Feng, H.T.; He, J.M.; Li, C.; Mao, X.; Xie, D.M.; Ao, N.J.; Chu, B. Coaxial electrospun zein nanofibrous membrane for sustained release. J. Biomater. Sci. Polym. Ed. 2013, 24, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Pedram, R.Z.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of pcl/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Pedram, R.Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/pcl/zein/gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef]
- Alhusein, N.; Blagbrough, I.S.; Beeton, M.L.; Bolhuis, A.; De Bank, P.A. Electrospun zein/pcl fibrous matrices release tetracycline in a controlled manner, killing staphylococcus aureus both in biofilms and ex vivo on pig skin, and are compatible with human skin cells. Pharm. Res. 2016, 33, 237–246. [Google Scholar] [CrossRef]
- Dashdorj, U.; Reyes, M.K.; Unnithan, A.R.; Tiwari, A.P.; Tumurbaatar, B.; Park, C.H.; Kim, C.S. Fabrication and characterization of electrospun zein/ag nanocomposite mats for wound dressing applications. Int. J. Biol. Macromol. 2015, 80, 1–7. [Google Scholar] [CrossRef]
- Kimna, C.; Tamburaci, S.; Tihminlioglu, F. Novel zein-based multilayer wound dressing membranes with controlled release of gentamicin. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2057–2070. [Google Scholar] [CrossRef]
- Ullah, S.; Hashmi, M.; Khan, M.Q.; Kharaghani, D.; Saito, Y.; Yamamoto, T.; Kim, I.S. Silver sulfadiazine loaded zein nanofiber mats as a novel wound dressing. RSC Adv. 2019, 9, 268–277. [Google Scholar] [CrossRef]
- Yao, Z.C.; Chen, S.C.; Ahmad, Z.; Huang, J.; Chang, M.W.; Li, J.S. Essential oil bioactive fibrous membranes prepared via coaxial electrospinning. J. Food Sci. 2017, 82, 1412–1422. [Google Scholar] [CrossRef]
- Akhmetova, A.; Lanno, G.-M.; Kogermann, K.; Malmsten, M.; Rades, T.; Heinz, A. Highly elastic and water stable zein microfibers as a potential drug delivery system for wound healing. Pharmaceutics 2020, 12, 458. [Google Scholar] [CrossRef]
- Ghorbani, M.; Mahmoodzadeh, F.; Yavari Maroufi, L.; Nezhad-Mokhtari, P. Electrospun tetracycline hydrochloride loaded zein/gum tragacanth/poly lactic acid nanofibers for biomedical application. Int. J. Biol. Macromol. 2020, 165, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Labib, G. Overview on zein protein: A promising pharmaceutical excipient in drug delivery systems and tissue engineering. Expert Opin. Drug Deliv. 2018, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, Y.; Zheng, M.; Dunne, F.; Liu, T.; Fu, X.W.; Kong, L.; Pan, S.Y.; Zhong, W.H. Morphology engineering of protein fabrics for advanced and sustainable filtration. J. Mater. Chem. A 2018, 6, 21585–21595. [Google Scholar] [CrossRef]
- Ortiz-Sánchez, J.P.; Cabrera-Chávez, F.; de la Barca, A.M. Maize prolamins could induce a gluten-like cellular immune response in some celiac disease patients. Nutrients 2013, 5, 4174–4183. [Google Scholar] [CrossRef]
- Takagi, K.; Teshima, R.; Okunuki, H.; Sawada, J. Comparative study of in vitro digestibility of food proteins and effect of preheating on the digestion. Biol. Pharm. Bull. 2003, 26, 969–973. [Google Scholar] [CrossRef]
- Hurtado-López, P.; Murdan, S. An investigation into the adjuvanticity and immunogenicity of zein microspheres being researched as drug and vaccine carriers. J. Pharm. Pharmacol. 2006, 58, 769–774. [Google Scholar] [CrossRef]
- Argos, P.; Pedersen, K.; Marks, M.D.; Larkins, B.A. A structural model for maize zein proteins. J. Biol. Chem. 1982, 257, 9984–9990. [Google Scholar]
- Matsushima, N.; Danno, G.-I.; Takezawa, H.; Izumi, Y. Three-dimensional structure of maize α-zein proteins studied by small-angle x-ray scattering. BBA Protein Struct. Mol. Enzym. 1997, 1339, 14–22. [Google Scholar] [CrossRef]
- Momany, F.A.; Sessa, D.J.; Lawton, J.W.; Selling, G.W.; Hamaker, S.A.H.; Willett, J.L. Structural characterization of alpha-zein. J. Agric. Food Chem. 2006, 54, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Garratt, R.; Oliva, G.; Caracelli, I.; Leite, A.; Arruda, P. Studies of the zein-like alpha-prolamins based on an analysis of amino acid sequences: Implications for their evolution and three-dimensional structure. Proteins 1993, 15, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sun, Q.; Wang, J.-Y. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials 2004, 25, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Georget, D.M.R.; Belton, P.S.; Barker, S.A. Zein−iodine complex studied by ftir spectroscopy and dielectric and dynamic rheometry in films and precipitates. J. Agric. Food Chem. 2009, 57, 4334–4341. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Nanoscale characterization of zein self-assembly. Langmuir 2012, 28, 2429–2435. [Google Scholar] [CrossRef]
- Miyoshi, T.; Toyohara, K.; Minematsu, H. Preparation of ultrafine fibrous zein membranes via electrospinning. Polym. Int. 2005, 54, 1187–1190. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Q.; Shi, K.; Huang, Q. Scaling behaviors of α-zein in acetic acid solutions. J. Phys. Chem. B 2011, 115, 9695–9702. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wang, Y.; Liao, S.; Lallier, T.; Wen, Z.T.; Xu, X. Photo-cross-linked antibacterial zein nanofibers fabricated by reactive electrospinning and its effects against streptococcus mutans. Oral Health Dent. Stud. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Shi, C.; Xi, S.; Han, Y.; Zhang, H.; Liu, J.; Li, Y. Structure, rheology and electrospinning of zein and poly(ethylene oxide) in aqueous ethanol solutions. Chin. Chem. Lett. 2019, 30, 305–310. [Google Scholar] [CrossRef]
- Fang, Q.-Q.; Wang, X.-F.; Zhao, W.-Y.; Ding, S.-L.; Shi, B.-H.; Xia, Y.; Yang, H.; Wu, L.-H.; Li, C.-Y.; Tan, W.-Q. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical tgf-β1 pathways. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Ariyoshi, Y. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci. Technol. 1993, 4, 139–144. [Google Scholar] [CrossRef]
- Abadir, P.; Hosseini, S.; Faghih, M.; Ansari, A.; Lay, F.; Smith, B.; Beselman, A.; Vuong, D.; Berger, A.; Tian, J.; et al. Topical reformulation of valsartan for treatment of chronic diabetic wounds. J. Investig. Dermatol. 2018, 138, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.-T. Electrospun soy protein isolate-based fiber fortified with anthocyanin-rich red raspberry (rubus strigosus) extracts. Food Res. Int. 2013, 52, 467–472. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Göbel, J.; van der Goot, A.J.; Stefanidis, G.D. Production of structured soy-based meat analogues using simple shear and heat in a couette cell. J. Food Eng. 2015, 160, 34–41. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Nikiforidis, C.V.; van der Goot, A.J. Shear-induced fibrous structure formation from a pectin/spi blend. Innov. Food Sci. Emerg. 2016, 36, 193–200. [Google Scholar] [CrossRef]
- Cho, D.; Netravali, A.N.; Joo, Y.L. Mechanical properties and biodegradability of electrospun soy protein isolate/pva hybrid nanofibers. Polym. Degrad. Stab. 2012, 97, 747–754. [Google Scholar] [CrossRef]
- Sett, S.; Lee, M.W.; Weith, M.; Pourdeyhimi, B.; Yarin, A.L. Biodegradable and biocompatible soy protein/polymer/adhesive sticky nano-textured interfacial membranes for prevention of esca fungi invasion into pruning cuts and wounds of vines. J. Mater. Chem. B 2015, 3, 2147–2162. [Google Scholar] [CrossRef]
- Salas, C.; Ago, M.; Lucia, L.A.; Rojas, O.J. Synthesis of soy protein–lignin nanofibers by solution electrospinning. React. Funct. Polym. 2014, 85, 221–227. [Google Scholar] [CrossRef]
- Silva, F.T.D.; Cunha, K.F.D.; Fonseca, L.M.; Antunes, M.D.; Halal, S.; Fiorentini, A.M.; Zavareze, E.D.R.; Dias, A.R.G. Action of ginger essential oil (zingiber officinale) encapsulated in proteins ultrafine fibers on the antimicrobial control in situ. Int. J. Biol. Macromol. 2018, 118, 107–115. [Google Scholar] [CrossRef]
- Khabbaz, B.; Solouk, A.; Mirzadeh, H. Polyvinyl alcohol/soy protein isolate nanofibrous patch for wound-healing applications. Prog. Biomater. 2019, 8, 185–196. [Google Scholar] [CrossRef]
- Allur Subramaniyan, S.; Sheet, S.; Balasubramaniam, S.; Berwin Singh, S.V.; Rampa, D.R.; Shanmugam, S.; Kang, D.R.; Choe, H.S.; Shim, K.S. Fabrication of nanofiber coated with l-arginine via electrospinning technique: A novel nanomatrix to counter oxidative stress under crosstalk of co-cultured fibroblasts and satellite cells. Cell Commun. Adhes. 2018, 24, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Kandhare, A.; Zanwar, A.A.; Hegde, M.V.; Bodhankar, S.L.; Shinde, S.; Deshmukh, S.; Kharat, R. Oral l-glutamine administration attenuated cutaneous wound healing in wistar rats. Int. Wound J. 2016, 13, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Choi, S.-K.; Jeong, K.-S.; Lee, K.-M.; Jung, G.-S.; Park, B.-H.; Jeon, W.-B. Biomimetic rgd-engineered elastin-like extracellular matrix facilitates cutaneous wound healing in c57bl/6 mice by way of promoting the migration of epidermal keratinocytes and dermal fibroblasts. New Biotech. 2012, 29, S153. [Google Scholar] [CrossRef]
- Santin, M.; Ambrosio, L. Soybean-based biomaterials: Preparation, properties and tissue regeneration potential. Expert Rev. Med. Devices 2008, 5, 349–358. [Google Scholar] [CrossRef]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183, 406–412. [Google Scholar] [CrossRef]
- Taylor, S.L.; Remington, B.C.; Panda, R.; Goodman, R.E.; Baumert, J.L. 18-detection and control of soybeans as a food allergen. In Handbook of Food Allergen Detection and Control; Flanagan, S., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 341–366. [Google Scholar]
- Barac, M.; Pesic, M.; Stanojevic, S.; Kostic, A.; Cabrilo, S. Techno-functional properties of pea (pisum sativum) protein isolates: A review. Acta Period. Technol. 2015, 46, 1–18. [Google Scholar] [CrossRef]
- Jia, X.W.; Qin, Z.Y.; Xu, J.X.; Kong, B.H.; Liu, Q.; Wang, H. Preparation and characterization of pea protein isolate-pullulan blend electrospun nanofiber films. Int. J. Biol. Macrom. 2020, 157, 641–647. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef]
- Kennedy, K.; Cal, R.; Casey, R.; Lopez, C.; Adelfio, A.; Molloy, B.; Wall, A.M.; Holton, T.A.; Khaldi, N. The anti-ageing effects of a natural peptide discovered by artificial intelligence. Int. J. Cosmet. Sci. 2020, 42, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Ellison, R.T., 3rd; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.D.; Reiter, B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim. Biophys. Acta Gen. Subj. 1968, 170, 351–365. [Google Scholar] [CrossRef]
- Sarkar, S.; Gulati, K.; Mishra, A.; Poluri, K.M. Protein nanocomposites: Special inferences to lysozyme based nanomaterials. Int. J. Biol. Macromol. 2020, 151, 467–482. [Google Scholar] [CrossRef]
- Selvaraj, S.; Thangam, R.; Fathima, N.N. Electrospinning of casein nanofibers with silver nanoparticles for potential biomedical applications. Int. J. Biol. Macrom. 2018, 120, 1674–1681. [Google Scholar] [CrossRef]
- Tomasula, P.M.; Sousa, A.M.M.; Liou, S.C.; Li, R.; Bonnaillie, L.M.; Liu, L.S. Short communication: Electrospinning of casein/pullulan blends for food-grade applications1. J. Dairy Sci. 2016, 99, 1837–1845. [Google Scholar] [CrossRef]
- Horne, D.S. Casein structure, self-assembly and gelation. Curr. Opin. Colloid Interface Sci. 2002, 7, 456–461. [Google Scholar] [CrossRef]
- Song, N.; Chen, Y.; Luo, J.; Huang, L.; Tian, H.; Li, C.; Loor, J.J. Negative regulation of αs1-casein (csn1s1) improves β-casein content and reduces allergy potential in goat milk. J. Dairy Sci. 2020, 103, 9561–9572. [Google Scholar] [CrossRef]
- Biranje, S.; Madiwale, P.; Adivarekar, R.V. Porous electrospun casein/pva nanofibrous mat for its potential application as wound dressing material. J. Porous Mater. 2019, 26, 29–40. [Google Scholar] [CrossRef]
- Khan, M.U.; Pirzadeh, M.; Förster, C.Y.; Shityakov, S.; Shariati, M.A. Role of milk-derived antibacterial peptides in modern food biotechnology: Their synthesis, applications and future perspectives. Biomolecules 2018, 8, 110. [Google Scholar] [CrossRef]
- Adamowicz, M.; Kelley, P.M.; Nickerson, K.W. Detergent (sodium dodecyl sulfate) shock proteins in escherichia coli. J. Bacteriol. 1991, 173, 229–233. [Google Scholar] [CrossRef]
- Ishikawa, S.; Matsumura, Y.; Katoh-Kubo, K.; Tsuchido, T. Antibacterial activity of surfactants against escherichia coli cells is influenced by carbon source and anaerobiosis. J. Appl. Microbiol. 2002, 93, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.B.; Corezzi, M.; Juncos Bombin, A.D.; Ajalloueian, F.; Attrill, E.; Pagliara, S.; Jacobsen, J.; Chronakis, I.S.; Nielsen, H.M.; Foderà, V. Waterborne electrospinning of α-lactalbumin generates tunable and biocompatible nanofibers for drug delivery. ACS Appl. Nano Mater. 2020, 3, 1910–1921. [Google Scholar] [CrossRef]
- Padrão, J.; Machado, R.; Casal, M.; Lanceros-Méndez, S.; Rodrigues, L.R.; Dourado, F.; Sencadas, V. Antibacterial performance of bovine lactoferrin-fish gelatine electrospun membranes. Int. J. Biol. Macromol. 2015, 81, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, M.; Park, H.S.; Jang, A.; Min, J.; Kim, Y.H. Immobilization of lysozyme-clea onto electrospun chitosan nanofiber for effective antibacterial applications. Int. J. Biol. Macromol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun silver nanoparticles-embedded feather keratin/poly(vinyl alcohol)/poly(ethylene oxide) antibacterial composite nanofibers. Polymers 2020, 12, 305. [Google Scholar] [CrossRef]
- Singaravelu, S.; Ramanathan, G.; Muthukumar, T.; Raja, M.D.; Nagiah, N.; Thyagarajan, S.; Aravinthan, A.; Gunasekaran, P.; Natarajan, T.S.; Selva, G.V.; et al. Durable keratin-based bilayered electrospun mats for wound closure. J. Mater. Chem. B 2016, 4, 3982–3997. [Google Scholar] [CrossRef]
- Chen, D.W.; Hsu, Y.H.; Liao, J.Y.; Liu, S.J.; Chen, J.K.; Ueng, S.W.N. Sustainable release of vancomycin, gentamicin and lidocaine from novel electrospun sandwich-structured plga/collagen nanofibrous membranes. Int. J. Pharmaceut. 2012, 430, 335–341. [Google Scholar] [CrossRef]
- Chen, D.W.; Lee, F.Y.; Liao, J.Y.; Liu, S.J.; Hsiao, C.Y.; Chen, J.K. Preclinical experiments on the release behavior of biodegradable nanofibrous multipharmaceutical membranes in a model of four-wall intrabony defect. Antimicrob. Agents Chemother. 2013, 57, 9–14. [Google Scholar] [CrossRef][Green Version]
- Chen, D.W.; Liao, J.Y.; Liu, S.J.; Chan, E.C. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: An in vitro and in vivo study. Int. J. Nanomed. 2012, 7, 763–771. [Google Scholar]
- Cheng, W.; Zhang, Z.; Xu, R.; Cai, P.; Kristensen, P.; Chen, M.; Huang, Y. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Collagen nanofiber containing silver nanoparticles for improved wound-healing applications. J. Drug Target. 2016, 24, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Han, J.; Liu, Z.; Wei, S.; Su, X.; Zhang, G. The facile fabrication of wound compatible anti-microbial nanoparticles encapsulated collagenous chitosan matrices for effective inhibition of poly-microbial infections and wound repairing in burn injury care: Exhaustive in vivo evaluations. J. Photochem. Photobiol. B 2019, 197, 111539. [Google Scholar] [CrossRef] [PubMed]
- Tort, S.; Acarturk, F.; Besikci, A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Chen, M.; Zhang, H.; Li, X. Electrospun gelatin fibers with a multiple release of antibiotics accelerate dermal regeneration in infected deep burns. Macromol. Biosci. 2016, 16, 1368–1380. [Google Scholar] [CrossRef]
- Dhand, C.; Barathi, V.A.; Ong, S.T.; Venkatesh, M.; Harini, S.; Dwivedi, N.; Goh, E.T.; Nandhakumar, M.; Venugopal, J.R.; Diaz, S.M.; et al. Latent oxidative polymerization of catecholamines as potential cross-linkers for biocompatible and multifunctional biopolymer scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 32266–32281. [Google Scholar] [CrossRef]
- Inal, M.; Mulazimoglu, G. Production and characterization of bactericidal wound dressing material based on gelatin nanofiber. Int. J. Biol. Macromol. 2019, 137, 392–404. [Google Scholar] [CrossRef]
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-healing effect of electrospun gelatin nanofibres containing centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 2017, 11, 905–915. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.L.; Zhang, Y.; Zhang, D.W.; Tang, Z.H.; Chen, X.S.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.L.; Tong, Y.; Jiang, Z.Q.; Wang, C. Nitrofurazone-loaded electrospun plla/sericin-based dual-layer fiber mats for wound dressing applications. RSC Adv. 2015, 5, 16940–16949. [Google Scholar] [CrossRef]
- Sapru, S.; Das, S.; Mandal, M.; Ghosh, A.K.; Kundu, S.C. Prospects of nonmulberry silk protein sericin-based nanofibrous matrices for wound healing - in vitro and in vivo investigations. Acta Biomater. 2018, 78, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.M.; Ma, X.; Fan, L.; Gao, Y.Y.; Deng, H.B.; Wang, Y.N. Accelerating dermal wound healing and mitigating excessive scar formation using lbl modified nanofibrous mats. Mater. Des. 2020, 185, 108265. [Google Scholar] [CrossRef]
- Bayraktar, O.; Balta, A.B.; Bayraktar, G.B. Adsorption/desorption and biofunctional properties of oleuropein loaded on different types of silk fibroin matrices. Maced. J. Chem. Chem. Eng. 2017, 36, 153–165. [Google Scholar] [CrossRef]
- Cai, Z.X.; Mo, X.M.; Zhang, K.H.; Fan, L.P.; Yin, A.L.; He, C.L.; Wang, H.S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Calamak, S.; Erdogdu, C.; Ozalp, M.; Ulubayram, K. Silk fibroin based antibacterial bionanotextiles as wound dressing materials. Mat. Sci. Eng. C Mater. 2014, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Calamak, S.; Aksoy, E.A.; Erdogdu, C.; Sagiroglu, M.; Ulubayram, K. Silver nanoparticle containing silk fibroin bionanotextiles. J. Nanopart. Res. 2015, 17, 87. [Google Scholar] [CrossRef]
- Chouhan, D.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Role of non-mulberry silk fibroin in deposition and regulation of extracellular matrix towards accelerated wound healing. Acta Biomater. 2017, 48, 157–174. [Google Scholar] [CrossRef]
- Chouhan, D.; Janani, G.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Functionalized pva-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling. J. Tissue Eng. Regen. M. 2018, 12, E1559–E1570. [Google Scholar] [CrossRef]
- Chomachayi, M.D.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: Thyme essential oil and doxycycline monohydrate release study. J. Biomed. Mater. Res. A 2018, 106, 1092–1103. [Google Scholar] [CrossRef]
- Hu, W.K.; Wang, Z.J.; Xu, Y.; Wang, X.H.; Xiao, Y.; Zhang, S.M.; Wang, J.L. Remodeling of inherent antimicrobial nanofiber dressings with melamine-modified fibroin into neoskin. J. Mater. Chem. B 2019, 7, 3412–3423. [Google Scholar] [CrossRef]
- Jao, W.C.; Yang, M.C.; Lin, C.H.; Hsu, C.C. Fabrication and characterization of electrospun silk fibroin/tio2 nanofibrous mats for wound dressings. Polym. Adv. Technol. 2012, 23, 1066–1076. [Google Scholar] [CrossRef]
- Lian, Y.; Zhan, J.C.; Zhang, K.H.; Mo, X.M. Fabrication and characterization of curcumin-loaded silk fibroin/p(lla-cl) nanofibrous scaffold. Front. Mater. Sci. 2014, 8, 354–362. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simoes, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.X.; Xu, Z.P.; Wang, L.B.; Meng, K.; Wang, H.; Zhao, H.J. Silk fibroin/chitosan/halloysite composite medical dressing with antibacterial and rapid haemostatic properties. Mater. Res. Express 2019, 6, 125409. [Google Scholar] [CrossRef]
- Safdari, M.; Shakiba, E.; Kiaie, S.H.; Fattahi, A. Preparation and characterization of ceftazidime loaded electrospun silk fibroin/gelatin mat for wound dressing. Fiber Polym. 2016, 17, 744–750. [Google Scholar] [CrossRef]
- Chung, S.; Ercan, B.; Roy, A.K.; Webster, T.J. Addition of selenium nanoparticles to electrospun silk scaffold improves the mammalian cell activity while reducing bacterial growth. Front. Physiol. 2016, 7, 297. [Google Scholar] [CrossRef]
- Tu, H.; Wu, G.M.; Yi, Y.; Huang, M.T.; Liu, R.; Shi, X.W.; Deng, H.B. Layer-by-layer immobilization of amphoteric carboxymethyl chitosan onto biocompatible silk fibroin nanofibrous mats. Carbohydr. Polym. 2019, 210, 9–16. [Google Scholar] [CrossRef]
- Uttayarat, P.; Jetawattana, S.; Suwanmala, P.; Eamsiri, J.; Tangthong, T.; Pongpat, S. Antimicrobial electrospun silk fibroin mats with silver nanoparticles for wound dressing application. Fiber Polym. 2012, 13, 999–1006. [Google Scholar] [CrossRef]
- Wang, S.D.; Ma, Q.; Wang, K.; Chen, H.W. Improving antibacterial activity and biocompatibility of bioinspired electrospinning silk fibroin nanofibers modified by graphene oxide. ACS Omega 2018, 3, 406–413. [Google Scholar] [CrossRef]
- Yang, X.X.; Fan, L.P.; Ma, L.L.; Wang, Y.Y.; Lin, S.; Yu, F.; Pan, X.H.; Luo, G.J.; Zhang, D.D.; Wang, H.S. Green electrospun manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Zhang, K.H.; Wu, B.; Yang, H.Y.; Wang, M.Q.; Dong, T.H.; Zhang, J.Y.; He, Y. Green electrospun nanocuprous oxide-poly(ethylene oxide)-silk fibroin composite nanofibrous scaffolds for antibacterial dressings. J. Appl. Polym. Sci. 2019, 136, 47730. [Google Scholar] [CrossRef]
- Maciel, K.S.; Santos, L.S.; Bonomo, R.C.F.; Verissimo, L.A.A.; Minim, V.P.R.; Minim, L.A. Purification of lactoferrin from sweet whey using ultrafiltration followed by expanded bed chromatography. Sep. Purif. Technol. 2020, 251, 117324. [Google Scholar] [CrossRef]
- Falkowski, M.; Maciejczyk, M.; Koprowicz, T.; Mikołuć, B.; Milewska, A.; Zalewska, A.; Car, H. Whey protein concentrate wpc-80 improves antioxidant defense systems in the salivary glands of 14-month wistar rats. Nutrients 2018, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.T.; Tang, C.; Kennedy, A.; Talwar, S.; Khan, S.A. Electrospinning and heat treatment of whey protein nanofibers. Food Hydrocoll. 2014, 35, 36–50. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Ahmed, H.; Tian, C.; Tu, Q.; Guo, Y.; Wang, J. Whey protein concentrate doped electrospun poly(epsilon-caprolactone) fibers for antibiotic release improvement. Colloids Surf. B 2016, 143, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Van den Akker, C.C.; Schleeger, M.; Bonn, M.; Koenderink, G.H. Chapter 31—Structural basis for the polymorphism of β-lactoglobulin amyloid-like fibrils. In Bio-Nanoimaging; Uversky, V.N., Lyubchenko, Y.L., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 333–343. [Google Scholar]
- Erfan, N.A.; Barakat, N.A.M.; Muller-Borer, B.J. Preparation and characterization of ß-lactoglobulin/poly(ethylene oxide) magnetic nanofibers for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 63–72. [Google Scholar] [CrossRef]
- Ipsen, R.; Otte, J. Self-assembly of partially hydrolysed α-lactalbumin. Biotechnol. Adv. 2007, 25, 602–605. [Google Scholar] [CrossRef]
- Machado, R.; da Costa, A.; Silva, D.M.; Gomes, A.C.; Casal, M.; Sencadas, V. Antibacterial and antifungal activity of poly(lactic acid)-bovine lactoferrin nanofiber membranes. Macromol. Biosci. 2018, 18, 1700324. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020, 10, 456. [Google Scholar] [CrossRef]
- James, E.N.; Nair, L.S. Development and characterization of lactoferrin loaded poly(epsilon-caprolactone) nanofibers. J. Biomed. Nanotechnol. 2014, 10, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.P.; Rahman, M.; Welling, M.M. Discovery and development of a synthetic peptide derived from lactoferrin for clinical use. Peptides 2011, 32, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernández-Calderón, M.C.; Pérez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodríguez, D. Covalent immobilization of hlf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, V.; Vertuccio, L.; Viscusi, G.; Gorrasi, G. Antimicrobial membranes of bio-based pa 11 and hnts filled with lysozyme obtained by an electrospinning process. Nanomaterials (Basel) 2018, 8, 139. [Google Scholar] [CrossRef]
- Edmans, J.G.; Murdoch, C.; Santocildes-Romero, M.E.; Hatton, P.V.; Colley, H.E.; Spain, S.G. Incorporation of lysozyme into a mucoadhesive electrospun patch for rapid protein delivery to the oral mucosa. Mater. Sci. Eng. C 2020, 112, 110917. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Qi, M.; Zhou, S.; Weng, J. Release pattern and structural integrity of lysozyme encapsulated in core–sheath structured poly(dl-lactide) ultrafine fibers prepared by emulsion electrospinning. Eur. J. Pharm. Biopharm. 2008, 69, 106–116. [Google Scholar] [CrossRef]
- Liu, Y.; Vincent Edwards, J.; Prevost, N.; Huang, Y.; Chen, J.Y. Physico- and bio-activities of nanoscale regenerated cellulose nonwoven immobilized with lysozyme. Mater. Sci. Eng. C 2018, 91, 389–394. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Reichl, S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 2009, 30, 6854–6866. [Google Scholar] [CrossRef]
- Fearing, B.V.; Van Dyke, M.E. In vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater. 2014, 10, 3136–3144. [Google Scholar] [CrossRef]
- Yao, C.-H.; Lee, C.-Y.; Huang, C.-H.; Chen, Y.-S.; Chen, K.-Y. Novel bilayer wound dressing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater. Sci. Eng. C 2017, 79, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Esparza, Y.; Ullah, A.; Wu, J. Molecular mechanism and characterization of self-assembly of feather keratin gelation. Int. J. Biol. Macromol. 2018, 107, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, S.; Ramirez, D.O.S.; Carletto, R.A.; Varesano, A.; Vineis, C.; Zanzoni, S.; Molinari, H.; Ragona, L. Electrospun lipid binding proteins composite nanofibers with antibacterial properties. Macromol. Biosci. 2017, 17, 1600300. [Google Scholar] [CrossRef]
- Zarei, M.; Tanideh, N.; Zare, S.; Aslani, F.S.; Koohi-Hosseinabadi, O.; Rowshanghias, A.; Pourjavaheri, F.; Mehryar, P.; Muthuraj, R. Electrospun poly(3-hydroxybutyrate)/chicken feather-derived keratin scaffolds: Fabrication, in vitro and in vivo biocompatibility evaluation. J. Biomater. Appl. 2020, 34, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.H.; Le, T.H.; Huynh, V.Q.N.; Vo, D.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk fibroin-based biomaterials for biomedical applications: A review. Polymers (Basel) 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Siritientong, T.; Angspatt, A.; Ratanavaraporn, J.; Aramwit, P. Clinical potential of a silk sericin-releasing bioactive wound dressing for the treatment of split-thickness skin graft donor sites. Pharm. Res. 2014, 31, 104–116. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Fare, S.; Draghi, L. Cross-linking strategies for electrospun gelatin scaffolds. Materials (Basel) 2019, 12, 2476. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar] [PubMed]
- Lee, P.; Bax, D.V.; Bilek, M.M.; Weiss, A.S. A novel cell adhesion region in tropoelastin mediates attachment to integrin alphavbeta5. J. Biol. Chem. 2014, 289, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and elastin biomaterials for the fabrication of engineered living tissues. Acs. Biomater. Sci. Eng. 2017, 3, 694–711. [Google Scholar] [CrossRef]
- Wen, Q.Y.; Mithieux, S.M.; Weiss, A.S. Elastin biomaterials in dermal repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Machado, R.; da Costa, A.; Sencadas, V.; Garcia-Arevalo, C.; Costa, C.M.; Padrao, J.; Gomes, A.; Lanceros-Mendez, S.; Rodriguez-Cabello, J.C.; Casal, M. Electrospun silk-elastin-like fibre mats for tissue engineering applications. Biomed. Mater. 2013, 8, 065009. [Google Scholar] [CrossRef]
- Chong, C.; Wang, Y.W.; Fathi, A.; Parungao, R.; Maitz, P.K.; Li, Z. Skin wound repair: Results of a pre-clinical study to evaluate electropsun collagen-elastin-pcl scaffolds as dermal substitutes. Burns 2019, 45, 1639–1648. [Google Scholar] [CrossRef]
- De Torre, I.G.; Ibanez-Fonseca, A.; Quintanilla, L.; Alonso, M.; Rodriguez-Cabello, J.C. Random and oriented electrospun fibers based on a multicomponent, in situ clickable elastin-like recombinamer system for dermal tissue engineering. Acta Biomater. 2018, 72, 137–149. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous scaffolds with biomimetic composition for skin regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.M.; Young, C.J.; Wang, Y.W.; Weiss, A.S. Electrospun synthetic human elastin:Collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012, 8, 3714–3722. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Huang, W.W.; Zhang, Q.; Ling, S.J.; Chen, Y.; Kaplan, D.L. Aqueous-based coaxial electrospinning of genetically engineered silk elastin core-shell nanofibers. Materials 2016, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lu, C.; Zhu, L.; Lu, T. The biodegradation of zein in vitro and in vivo and its application in implants. AAPS PharmSciTech 2011, 12, 172–176. [Google Scholar] [CrossRef] [PubMed]


| Protein | Co-Polymer | Electrospinning Type | Solvent | Antimicrobial Agent | Tested Bacterial Strain | Reference |
|---|---|---|---|---|---|---|
| Pea | PVA, CA | Uniaxial | Water | CA | E. coli, L. monocytogenes | [43] |
| Soy | PEO | Uniaxial | NaOH | None | S. aureus, P. aeruginosa | [44] |
| Zein | None | Co-axial | AA | ATPPB | E. coli, S. aureus | [45] |
| Zein | PU/CA | Uniaxial | DMF, MEK | Streptomycin | E. coli, S. typhimurium, V. vulnificus, S. aureus, B. subtilis | [26] |
| Zein | HA | Uniaxial | TFE, AA | Salicylic acid | S. aureus | [46] |
| Zein | PU | Uniaxial | DMF, THF | Ag NPs | E. coli, S. aureus | [27] |
| Zein | PCL, GA | Uniaxial | FA, AA | GA | E. coli, S. aureus | [47] |
| Zein | PCL, GA | Uniaxial, multilayer | FA, AA | GA, C. officinalis | E. coli, S. aureus | [48] |
| Zein | PCL | Uniaxial | TFE, DCM | Tetracycline hydrochloride | MRSA | [49] |
| Zein | None | Uniaxial | EtOH, water | Ag NPs | E. coli, S. aureus | [50] |
| Zein | None | Uniaxial | EtOH, water | Gentamicin | E. coli, S. aureus | [51] |
| Zein | None | Uniaxial | EtOH, water | Ag NPs | E. coli, Bacillus | [52] |
| Zein | None | Co-axial | EtOH, water | OEO | E. coli | [53] |
| Zein | PEO | Co-axial | EtOH, water | Tetracycline hydrochloride | E. coli, S. aureus | [54] |
| Zein | GT, PLA | Uniaxial | EtOH, water, CHL | Tetracycline hydrochloride | S. aureus, P. aeruginosa | [55] |
| Protein | Co-Polymer | Electrospinning Type | Solvent | Antimicrobial Agent | Tested Bacterial Strain | Reference |
|---|---|---|---|---|---|---|
| Casein | PEO | Uniaxial | Water | Ampicillin | E. coli, S. aureus | [99] |
| α-lactoglobulin | PEO | Uniaxial | Water | Ampicillin | E. coli, P. aeruginosa, B. thailandensis | [107] |
| Lactoferrin | Gelatin | Uniaxial | FA, DMF | None | E. coli, S. aureus | [108] |
| Lysozyme | CS, PVA | Uniaxial | AA, water | CS | S. aureus, B. subtilis, S. flexnery, P. aeruginosa | [109] |
| Keratin | PVA, PEO | Uniaxial | NaOH | Ag NPs | E. coli, S. aureus | [110] |
| Keratin | CS, PHBA, gelatin | Uniaxial, multilayer | HFIP | Mupirocin | E. coli, S. aureus | [111] |
| Collagen | PLGA | Uniaxial, multilayer | HFIP | Vancomycin hydrochloride, gentamicin sulfate | E. coli, S. aureus | [112,113,114] |
| Collagen | PCL | Uniaxial | HFIP | Enterobacteria phage T4 | E. coli | [115] |
| Collagen | PLA | Uniaxial | HFIP | Levofloxacin | E. coli, S. aureus | [25] |
| Collagen | - | Uniaxial | HFIP | Ag NPs | S. aureus, P. aeruginosa | [116] |
| Collagen | CS | Uniaxial | 0.5 M AA | ZnO | S. aureus, E. coli | [117] |
| Collagen | PCL (core), PEO (shell) | Co-axial | HFIP, glacial AA | Doxycycline | n.a. | [118] |
| Gelatin | Alginate-dialde-hyde | Uniaxial | AA(40% w/w) | Ciprofloxacin, gentamicin | P. aeruginosa, S. epidermidis | [119] |
| Gelatin | - | Uniaxial | TFE | Vancomycin, caspofungin | MRSA, C. albicans | [120] |
| Gelatin | PMETAC | Uniaxial | FA, AA | PMETAC | S. aureus, E. coli, MRSA, A. baumannii | [121] |
| Gelatin | PVA | Uniaxial | FA | Centella asiatica extract | S. aureus, E. coli, P. aeruginosa | [122] |
| Silk sericin | CS | Uniaxial | TFA | CS | E. coli, B. subtilis | [123] |
| Silk sericin | PLLA | Uniaxial, multilayer | TFA | Nitrafurazone | E. coli, B. subtilis | [124] |
| Silk sericin | CS, PVA | Uniaxial | Water | Cephalexin hydrate | E. coli, B. subtilis | [125] |
| Silk fibroin | PCL | Uniaxial, multilayer | HFIP | CS | S. aureus, E. coli | [126] |
| Silk fibroin | - | Uniaxial | FA | Oleuropein | S. epidermidis, E. coli | [127] |
| Silk fibroin | CS | Uniaxial | HFIP, TFE | CS | S. aureus, E. coli | [128] |
| Silk fibroin, sulfated fibroin | PEI | Uniaxial | FA | PEI | S. aureus, P. aeruginosa | [129] |
| Silk fibroin | - | Uniaxial | FA | Ag NPs | S. aureus, P. aeruginosa | [130] |
| Silk fibroin | PVA | Uniaxial | Water | EGF, ciprofloxacin hydrochloride | S. aureus, S. epidermidis, E. coli, P. aeruginosa | [131] |
| Silk fibroin | PVA | Uniaxial | Water | LL-37 antimicrobial peptide, EGF | S. epidermidis, P. aeruginosa | [132] |
| Silk fibroin | Gelatin | Uniaxial | FA | Thyme essential oil, doxycycline monohydrate | S. aureus, K. pneumoniae | [133] |
| Melamine-modified silk fibroin | PCL | Uniaxial | HFIP | Melamine-modified silk fibroin | S. aureus, E. coli | [134] |
| Silk fibroin | PEO | Uniaxial | FA | TiO2 NPs | E. coli | [135] |
| Silk fibroin | P(LLA-CL) | Uniaxial | HFIP | Curcumin | S. aureus | [136] |
| Silk fibroin | PCL, HA, PEO | Uniaxial, multilayer | FA, TFE, water | Thymol | S. aureus, P. aeruginosa | [137] |
| Silk fibroin | CS, halloysite nanotubes, PEO | Uniaxial | FA, AA, water | Chlorhexidine digluconate | S. aureus, E. coli | [138] |
| Silk fibroin | Gelatin | Uniaxial | FA | Ceftazidime | P. aeruginosa | [139] |
| Silk fibroin | Uniaxial | FA | Selenium NP coating | S. aureus | [140] | |
| Silk fibroin | Carboxy-methyl CS coating | Uniaxial | HFIP, AA | Carboxymethyl CS coating | S. aureus, E. coli | [141] |
| Silk fibroin | Uniaxial | HFIP, FA | Ag NP coating | S. aureus, P. aeruginosa | [142] | |
| Silk fibroin | Uniaxial | FA, water | Graphene oxide coating | S. aureus, E. coli | [143] | |
| Silk fibroin | PEO | Uniaxial | Water | Manuka honey | MRSA, P. aeruginosa, E. coli, S. aureus | [144] |
| Silk fibroin | PEO | Uniaxial | Water | Cu2O NPs | S. aureus, E. coli | [145] |
| Protein | Co-Polymer | Electrospinning Type | Solvent | Potential Applications | Reference |
|---|---|---|---|---|---|
| Elastin | Collagen, PCL | Uniaxial | HFIP | Skin grafting, dermal substitute for burn wounds | [182] |
| ELR | - | Uniaxial | TFE | Wound dressings, skin tissue engineering | [183] |
| Elastin | Gelatin, CA | Uniaxial | AA | Skin injuries caused by trauma and diseases | [184] |
| SELPs | - | Uniaxial | Water, FA | Wound dressings, skin regeneration | [181] |
| Tropoelastin | - | Uniaxial | HFIP | Dermal tissue engineering | [185] |
| Tropoelastin | Collagen | Uniaxial | HFIP | Dermal tissue engineering | [186] |
| SELPs | Silk fibroin | Co-axial | Water | Biomedical applications, drug delivery | [187] |
| Protein | Water Soluble | Nutritional Value | Indu- strial By- Product | Aller- genic | Anti- microbial | Anti-oxi-dant | Anti-Inflamma-tory | Cell-Recog-nizing Motifs | Self-Assem-bly |
|---|---|---|---|---|---|---|---|---|---|
| Zein | ✓ | ✓ | ✓ | ||||||
| Soy | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Pea | ✓ | ✓ | ✓ | ✓ | |||||
| Casein | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| α-lacto-globulin | ✓ | ✓ | ✓ | ||||||
| β-lacto-globulin | ✓ | ✓ | ✓ | ||||||
| Lacto-ferrin | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Lysozyme | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Keratin | ✓ | ✓ | ✓ | ||||||
| Collagen | ✓ | (✓) | ✓ | ✓ | |||||
| Gelatin | ✓ | ✓ | (✓) | ✓ | ✓ | ||||
| Elastin | ✓ | (✓) | ✓ | ✓ | |||||
| Silk sericin | (✓) | ✓ | ✓ | ✓ | |||||
| Silk fibroin | ✓ | (✓) | (✓) | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetova, A.; Heinz, A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics 2021, 13, 4. https://doi.org/10.3390/pharmaceutics13010004
Akhmetova A, Heinz A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics. 2021; 13(1):4. https://doi.org/10.3390/pharmaceutics13010004
Chicago/Turabian StyleAkhmetova, Alma, and Andrea Heinz. 2021. "Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges" Pharmaceutics 13, no. 1: 4. https://doi.org/10.3390/pharmaceutics13010004
APA StyleAkhmetova, A., & Heinz, A. (2021). Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics, 13(1), 4. https://doi.org/10.3390/pharmaceutics13010004