Methacryloyl-GlcNAc Derivatives Copolymerized with Dimethacrylamide as a Novel Antibacterial and Biocompatible Coating
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical Synthesis and Characterization
2.1.1. General Methods
2.1.2. Synthesis of Azido Linkers 2, 4, 5, 6
2.1.3. General Tosylation Procedure
2.1.4. Chain Prolongment of Azido Linkers via Tosylate 5, 6
8-(2-{2-[2-(2-Azido-ethoxy)-ethoxy]-ethoxy}-ethoxy)-octan-1-ol 6
2.1.5. General Procedure for the Synthesis of Azidomethacrylates 7–10
2-Methyl-acrylic acid 2-(2-azido-ethoxy)-ethyl ester 7
2-Methyl-acrylic acid 2-{2-[2-(2-azido-ethoxy)-ethoxy]-ethoxy}-ethyl ester 8
2-Methyl-acrylic acid 2-[2-(2-{2-[2-(2-azido-ethoxy)-ethoxy]-ethoxy}-ethoxy)-ethoxy]-ethyl ester 9
2-Methyl-acrylic acid 8-(2-{2-[2-(2-azido-ethoxy)-ethoxy]-ethoxy}-ethoxy)-octyl ester 10
2.1.6. General Procedure for Click Reaction of Azidomethacrylates with 13
2-Methyl-acrylic acid 2-{2-[4-(3-acetylamino-4,5-dihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxymethyl)-[1,2,3]triazol-1-yl]-ethoxy}-ethyl ester 11a
2-Methyl-acrylic acid 2-[2-(2-{2-[4-(4,5-diacetoxy-6-acetoxymethyl-3-acetylamino-tetrahydro-pyran-2-yloxymethyl)-[1,2,3]triazol-1-yl]-ethoxy}-ethoxy)-ethoxy]-ethyl ester 11b
2-Methyl-acrylic acid 2-(2-{2-[2-(2-{2-[4-(3-acetylamino-4,5-dihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxymethyl)-[1,2,3]triazol-1-yl]-ethoxy}-ethoxy)-ethoxy]-ethoxy}-ethoxy)-ethyl ester 11c
2-Methyl-acrylic acid 8-{2-[2-(2-{2-[4-(4,5-diacetoxy-6-acetoxymethyl-3-acetylamino-tetrahydro-pyran-2-yloxymethyl)-[1,2,3]triazol-1-yl]-ethoxy}-ethoxy)-ethoxy]-ethoxy}-octyl ester 11d
2.1.7. General Procedure for Click Reaction of Azidomethacrylates with Propargyl Alcohol
2-Methyl-acrylic acid 2-[2-(4-hydroxymethyl-[1,2,3]triazol-1-yl)-ethoxy]-ethyl ester 12a
2-Methyl-acrylic acid 2-[2-(4-hydroxymethyl-[1,2,3]triazol-1-yl)-ethoxy]-ethyl ester 12b
2-Methyl-acrylic acid 2-{2-[2-(2-{2-[2-(4-hydroxymethyl-[1,2,3]triazol-1-yl)-ethoxy]-ethoxy}-ethoxy)-ethoxy]-ethoxy}-ethyl ester 12c
2.1.8. General Procedure for Free Radical Polymerization
2.1.9. General Deprotection Procedure of GlcNAc Polymers
2.2. Preparation of Polymer Coatings
2.3. Physicochemical Surface Characterization
2.4. Biological Evaluation
2.4.1. L-929 Mouse Fibroblast Cell Culture
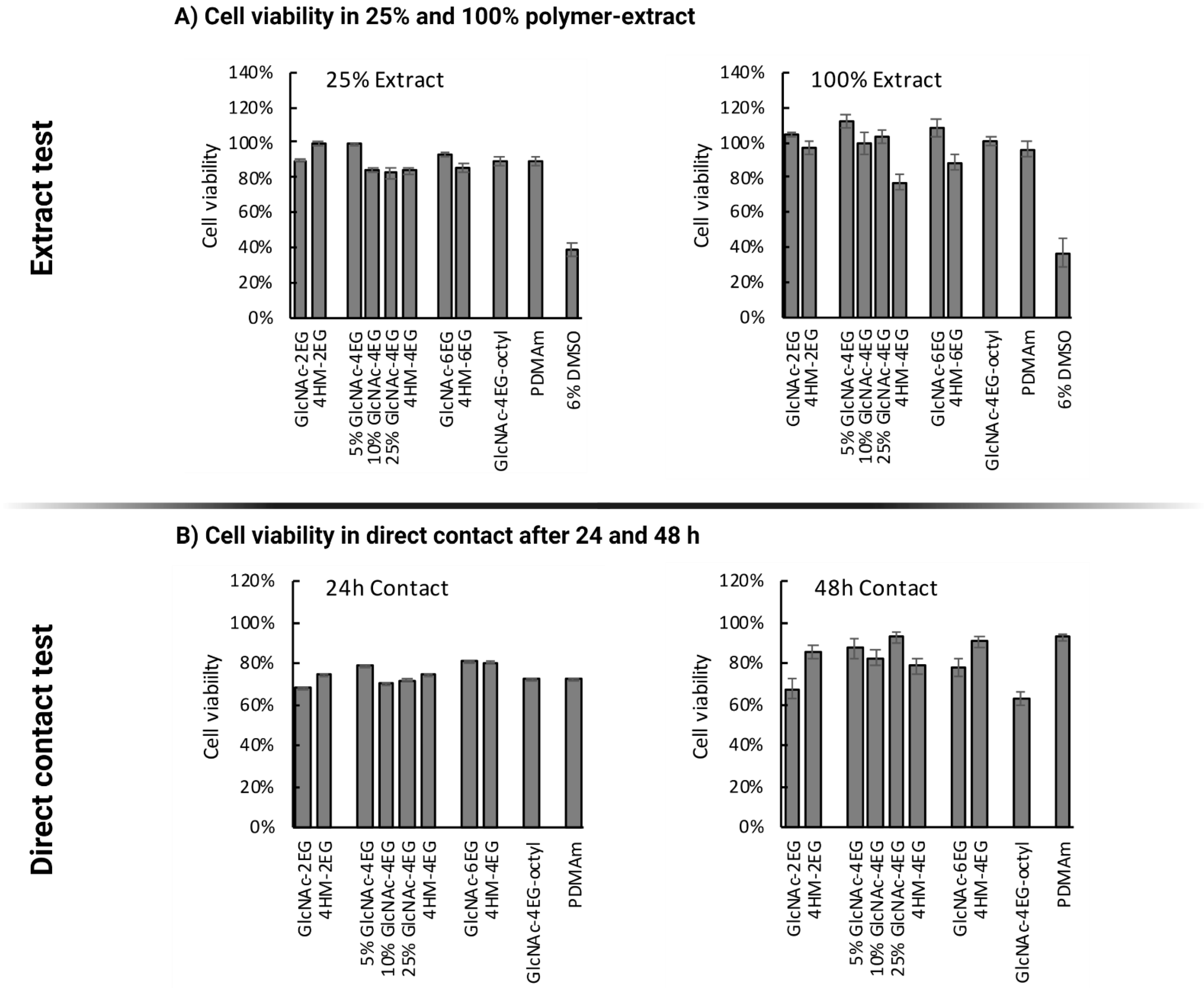
2.4.2. Extract Test Using the MTT Assay
2.4.3. Direct Contact Test Using the MTT Assay
2.4.4. Bacterial Cell Culture
2.4.5. Antibacterial Assay by Optical Density
2.4.6. Antibacterial Assay by Colony-Forming Units
2.4.7. Crystal Violet Assay for Biofilm Assessment
2.4.8. Extracellular Polymeric Substance (EPS) Assessment by Phenol-Sulfuric Acid Method
2.4.9. Live/Dead Staining
2.4.10. Statistical Analysis
3. Results and Discussion
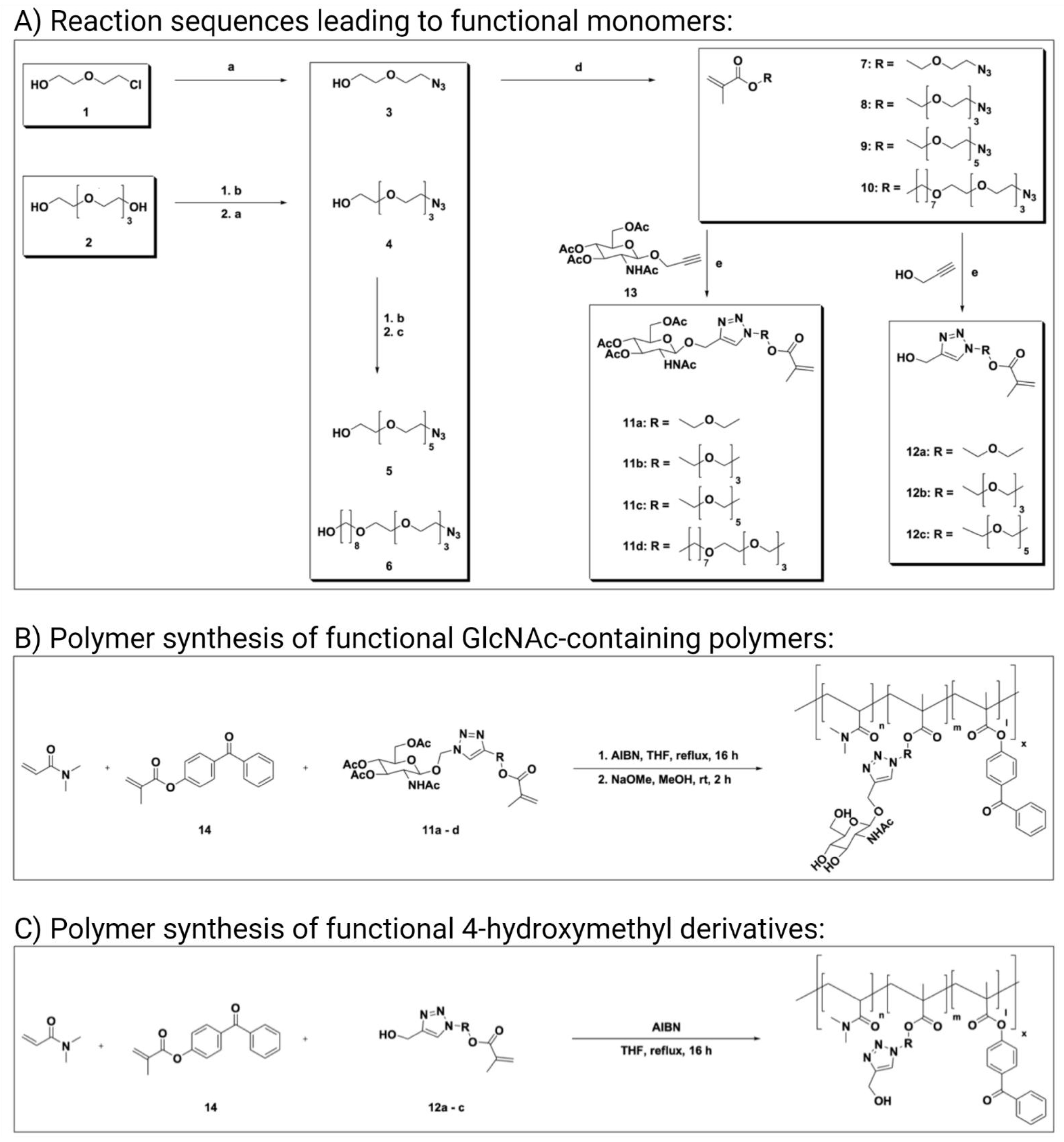
3.1. Monomer Synthesis
3.2. Polymer Synthesis
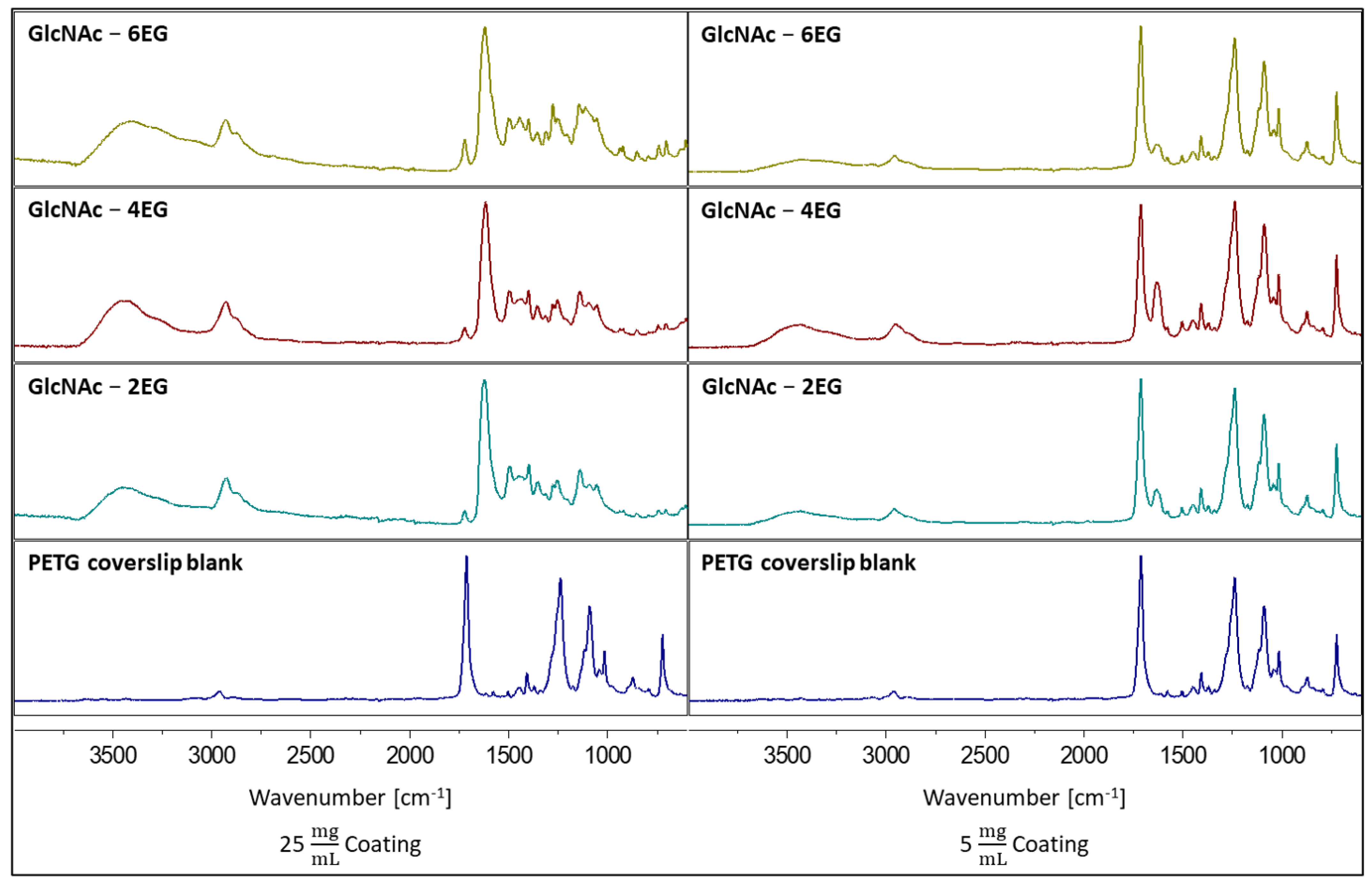
3.3. Coating of PETG Coverslips
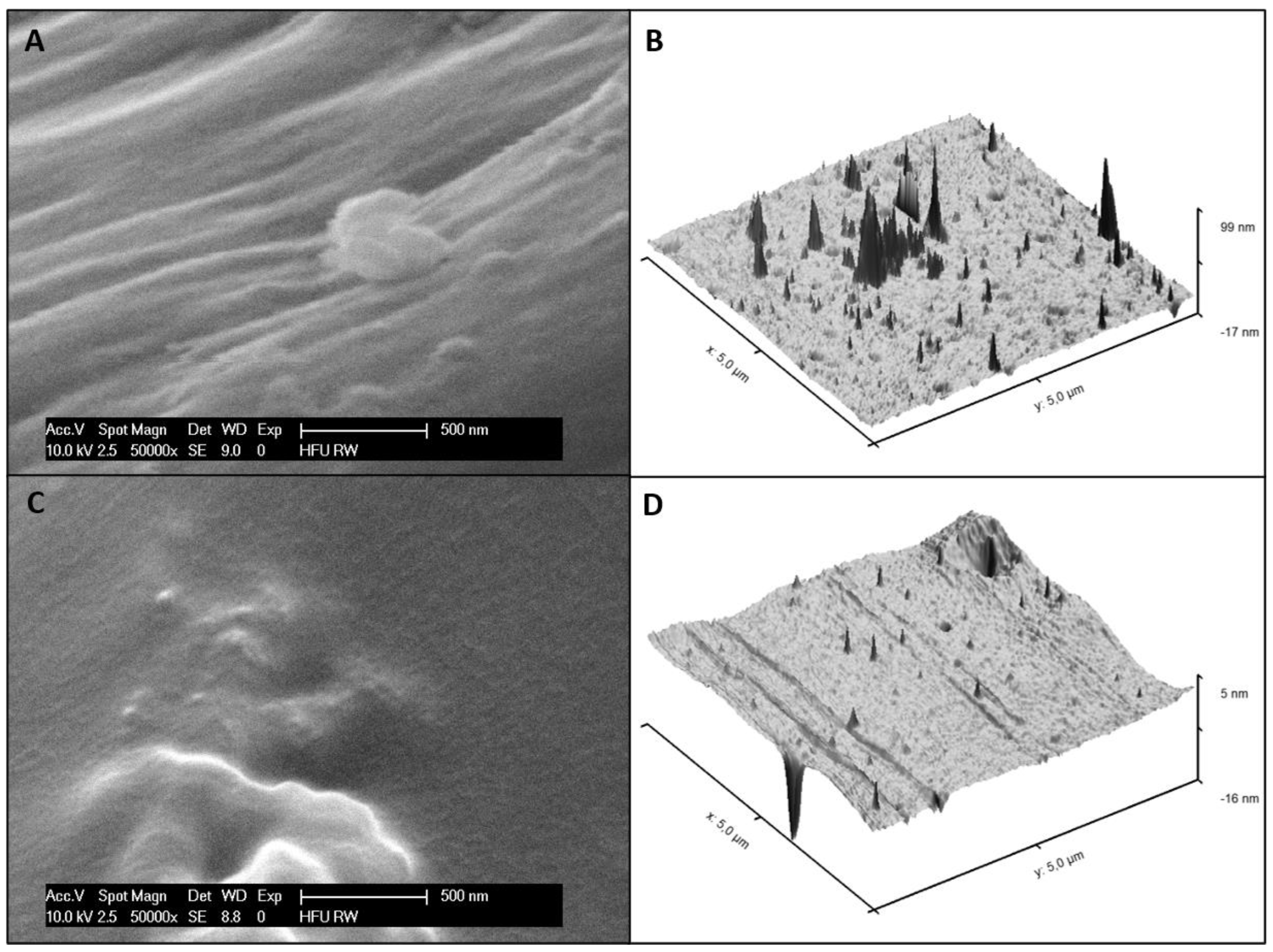
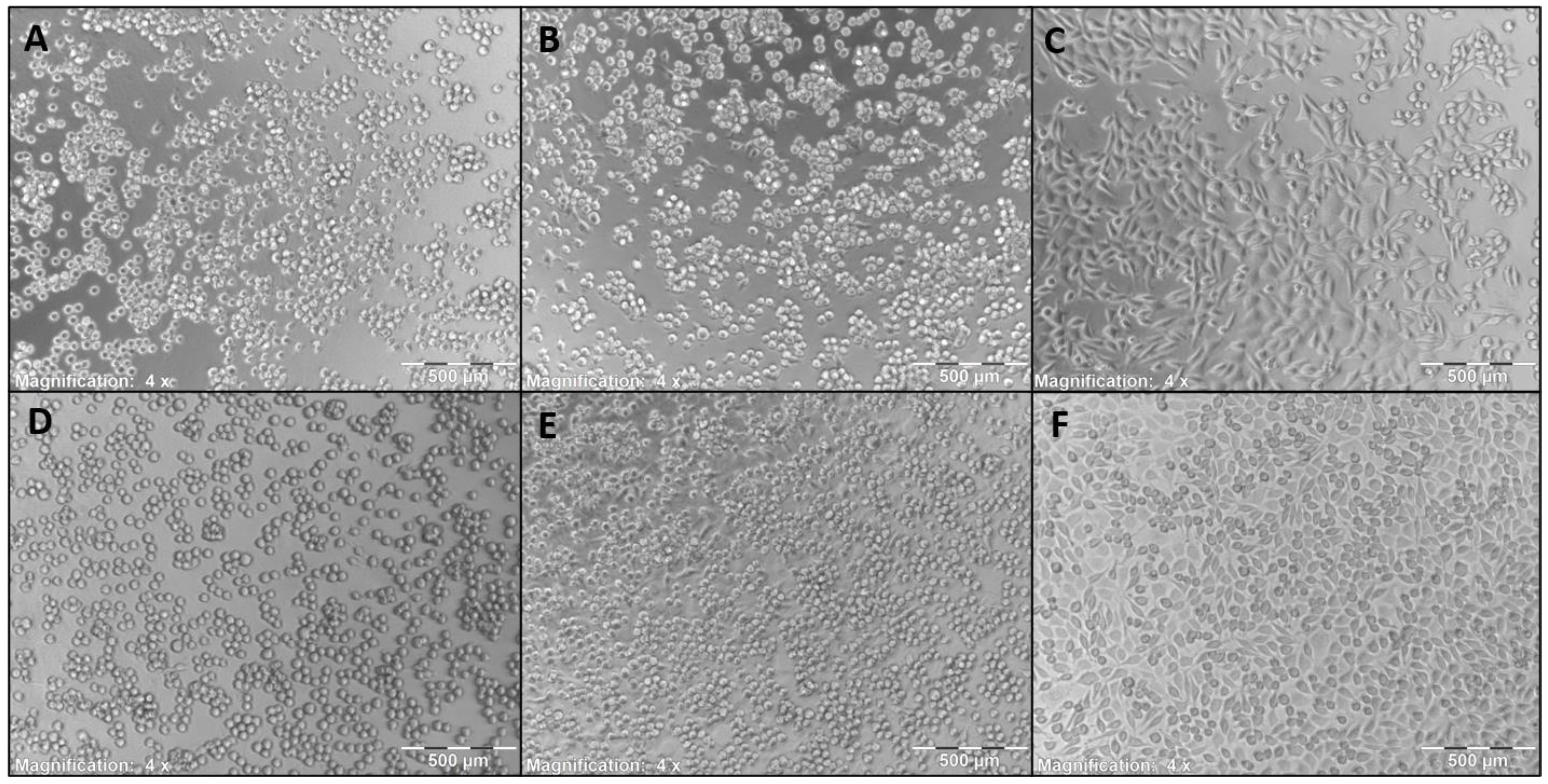
3.4. Surface Morphology
3.5. Coating Sterilization
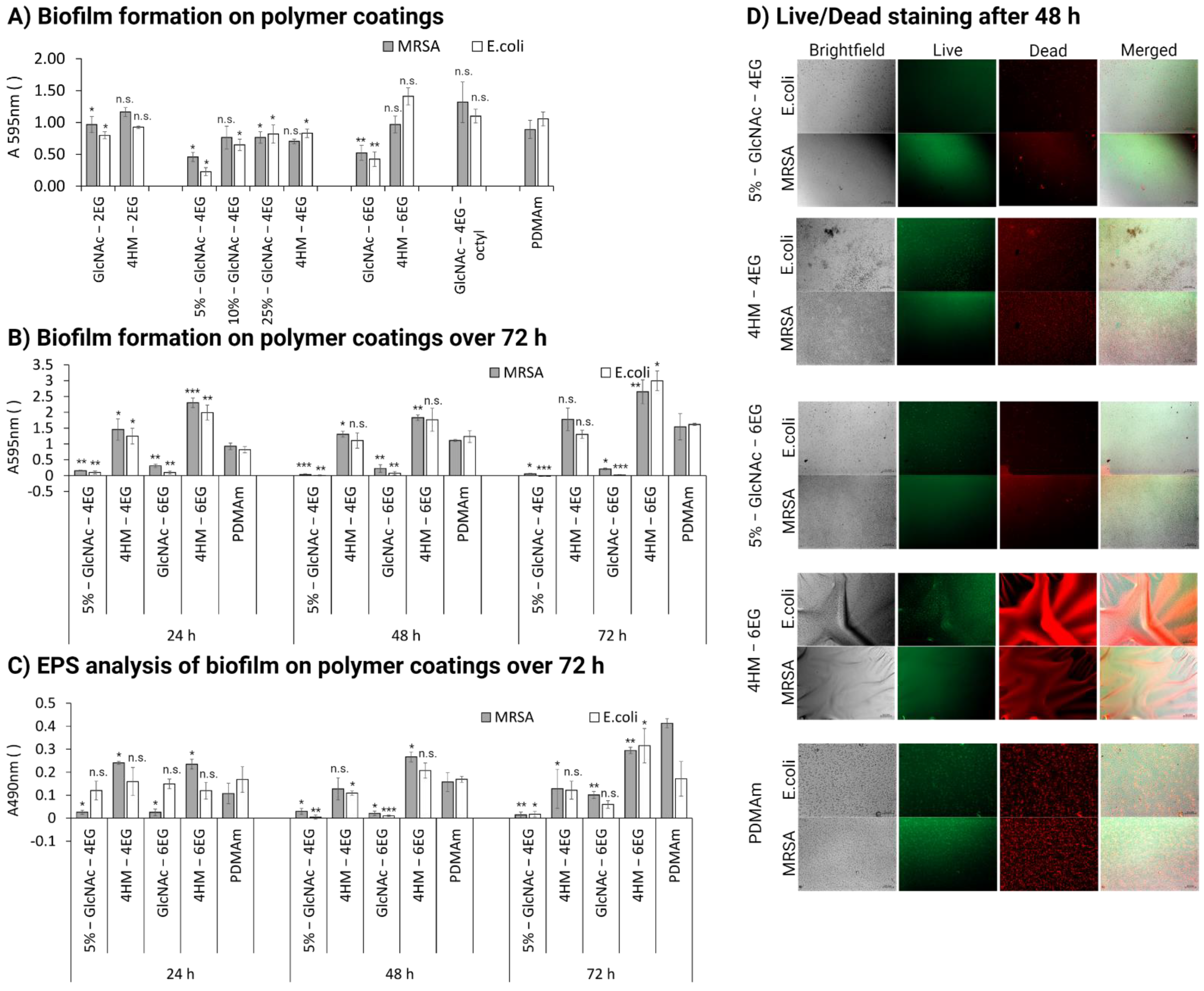
3.6. Antibacterial Activity
3.7. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darouiche, R.O. Device-Associated Infections: A Macroproblem That Starts with Microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, H.; Zhang, W.; Cui, G.; Zhu, J.; Duan, J. Chitosan Coupling Makes Microbial Biofilms Susceptible to Antibiotics. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef]
- Teerawattanapong, N.; Panich, P.; Kulpokin, D.; Na Ranong, S.; Kongpakwattana, K.; Saksinanon, A.; Goh, B.-H.; Lee, L.-H.; Apisarnthanarak, A.; Chaiyakunapruk, N. A Systematic Review of the Burden of Multidrug-Resistant Healthcare-Associated Infections among Intensive Care Unit Patients in Southeast Asia: The Rise of Multidrug-Resistant Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 2018, 39, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G. Biomaterial-Centered Infection: Microbial Adhesion versus Tissue Integration. Science 1987, 237, 1588. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-Associated Infection: Locating the Finish Line in the Race for the Surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poelstra, K.A.; Barekzi, N.A.; Rediske, A.M.; Felts, A.G.; Slunt, J.B.; Grainger, D.W. Prophylactic Treatment of Gram-Positive and Gram-Negative Abdominal Implant Infections Using Locally Delivered Polyclonal Antibodies. J. Biomed. Mater. Res. 2002, 60, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival Strategies of Infectious Biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Voyich, J.M.; Fischer, E.R.; Braughton, K.R.; Whitney, A.R.; DeLeo, F.R.; Otto, M. Polysaccharide Intercellular Adhesin (PIA) Protects Staphylococcus epidermidis against Major Components of the Human Innate Immune System. Cell. Microbiol. 2004, 6, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A common Cause of Persistent Infections. Science 1999, 284, 1318. [Google Scholar] [CrossRef] [Green Version]
- Zimmerli, W.; Widmer, A.F.; Blatter, M.; Frei, R.; Ochsner, P.E. Role of Rifampin for Treatment of Orthopedic Implant-Related Staphylococcal Infections: A Randomized Controlled Trial. J. Am. Med. Assoc. 1998, 279, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and Treatment of Implant-Related Infections by Antibiotic-Coated Implants: A Review. Injury 2006, 37, 105–112. [Google Scholar] [CrossRef]
- Ghafouri, H.B.; Bagheri-Behzad, B.; Yasinzadeh, M.R.; Modirian, E.; Divsalar, D.; Farahmand, S. Prophylactic Antibiotic Therapy in Contaminated Traumatic Wounds: Two Days versus Five Days Treatment. BioImpacts 2012, 2, 33–37. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nations, U.; Assembly, G.; York, N.; Humphreys, G.; Fleck, F. United Nations Meeting on Antimicrobial Resistance. Bull. World Health Organ. 2016, 94, 638–639. [Google Scholar]
- Kargupta, R.; Bok, S.; Darr, C.M.; Crist, B.D.; Gangopadhyay, K.; Gangopadhyay, S.; Sengupta, S. Coatings and Surface Modifications Imparting Antimicrobial Activity to Orthopedic Implants. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2014, 6, 475–495. [Google Scholar] [CrossRef]
- Brooks, B.D.; Brooks, A.E. Therapeutic Strategies to Combat Antibiotic Resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Rafie, M.H.; EL-Sheikh, M.A.; Seleem, A.A.; El-Naggar, M.E. Antimicrobial Wound Dressing and Anti-Inflammatory Efficacy of Silver Nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, Non-Leaching Antibacterial Surfaces-2: How High Density Cationic Surfaces Kill Bacterial Cells. Biomaterials 2007, 28, 4870–4879. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.-J.; Lewis, K.; Klibanov, A.M. Designing Surfaces That Kill Bacteria on Contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [Green Version]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic Polymers and Their Therapeutic Potential. Chem. Soc. Rev. 2012, 41, 7147. [Google Scholar] [CrossRef]
- Huang, J.; Murata, H.; Koepsel, R.R.; Russell, A.J.; Matyjaszewski, K. Antibacterial Polypropylene via Surface-Initiated Atom Transfer Radical Polymerization. Biomacromolecules 2007, 8, 1396–1399. [Google Scholar] [CrossRef]
- Lee, S.B.; Koepsel, R.R.; Morley, S.W.; Matyjaszewski, K.; Sun, Y.; Russell, A.J. Permanent, Nonleaching Antibacterial Surfaces, 1. Synthesis by Atom Transfer Radical Polymerization. Biomacromolecules 2004, 5, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Madkour, A.E.; Dabkowski, J.M.; Nüsslein, K.; Tew, G.N. Fast Disinfecting Antimicrobial Surfaces. Langmuir 2009, 25, 1060–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Weiss, T.M.; Lehrer, R.I.; Huang, H.W. Crystallization of Antimicrobial Pores in Membranes: Magainin and Protegrin. Biophys. J. 2000, 79, 2002–2009. [Google Scholar] [CrossRef] [Green Version]
- Shai, Y. Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by α-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochim. Biophys. Acta-Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K. Why and How are Peptide–Lipid Interactions Utilized for Self-Defense? Magainins and Tachyplesins as Archetypes. Biochim. Biophys. Acta-Biomembr. 1999, 1462, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Milović, N.M.; Wang, J.; Lewis, K.; Klibanov, A.M. Immobilized N-Alkylated Polyethylenimine Avidly Kills Bacteria by Rupturing Cell Membranes with No Resistance Developed. Biotechnol. Bioeng. 2005, 90, 715–722. [Google Scholar] [CrossRef]
- Lin, J.; Tiller, J.C.; Lee, S.B.; Lewis, K.; Klibanov, A.M. Insights into Bactericidal Action of Surface-Attached Poly(Vinyl-N-Hexylpyridinium) Chains. Biotechnol. Lett. 2002, 24, 801–805. [Google Scholar] [CrossRef]
- Lin, J.; Murthy, S.K.; Olsen, B.D.; Gleason, K.K.; Klibanov, A.M. Making Thin Polymeric Materials, Including Fabrics, Microbicidal and Also Water-Repellent. Biotechnol. Lett. 2003, 25, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic Biocides with Pendant Active Groups: Molecular Weight Dependence of Antibacterial Activity. Antimicrob. Agents Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. New Polymeric Biocides: Synthesis and Antibacterial Activities of Polycations with Pendant Biguanide Groups. Antimicrob. Agents Chemother. 1984, 26, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isquith, A.J.; Abbott, E.A.; Walters, P.A. Surface-Bonded Antimicrobial Activity of an Organosilicon Quaternary Ammonium Chloride. Appl. Microbiol. 1972, 24, 859–863. [Google Scholar] [CrossRef]
- Luz, G.M.; Boesel, L.; Campo, A.D.; Mano, J.F. Micropatterning of Bioactive Glass Nanoparticles on Chitosan Membranes for Spatial Controlled Biomineralization. Langmuir 2012, 28, 6970–6977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shi, R.; Gong, P.; Li, J.; Li, J.; Ao, D.; Wang, P.; Yang, Y.; Man, Y.; Qu, Y. Bioelectric Effect of a Chitosan Bioelectret Membrane on Bone Regeneration in Rabbit Cranial Defects. J. Bioact. Compat. Polym. 2012, 27, 122–132. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its Derivatives for Tissue Engineering Applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Prospects of Chitosan-Based Scaffolds for Growth Factor Release in Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Wang, Y.; Lin, Q.; Yao, A.; Cao, F.; Li, D.; Zhou, J.; Duan, C.; Du, Z.; et al. The Influence of Chitosan Hydrogel on Stem Cell Engraftment, Survival and Homing in the Ischemic Myocardial Microenvironment. Biomaterials 2012, 33, 3093–3106. [Google Scholar] [CrossRef]
- Shi, W.; Nie, D.; Jin, G.; Chen, W.; Xia, L.; Wu, X.; Su, X.; Xu, X.; Ni, L.; Zhang, X.; et al. BDNF Blended Chitosan Scaffolds for Human Umbilical Cord MSC Transplants in Traumatic Brain Injury Therapy. Biomaterials 2012, 33, 3119–3126. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel Carboxymethyl Derivatives of Chitin and Chitosan Materials and Their Biomedical Applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan Disrupts the Barrier Properties of the Outer Membrane of Gram-Negative Bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Je, J.Y.; Kim, S.K. Chitosan Derivatives Killed Bacteria by Disrupting the Outer and Inner Membrane. J. Agric. Food Chem. 2006, 54, 6629–6633. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial Properties of Chitosan Nanoparticles: Mode of Action and Factors Affecting Activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Izano, E.A.; Sadovskaya, I.; Vinogradov, E.; Mulks, M.H.; Velliyagounder, K.; Ragunath, C.; Kher, W.B.; Ramasubbu, N.; Jabbouri, S.; Perry, M.B.; et al. Poly-N-Acetylglucosamine Mediates Biofilm Formation and Antibiotic Resistance in Actinobacillus pleuropneumoniae. Microb. Pathog. 2007, 43, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicard, J.F.; Vogeleer, P.; Le Bihan, G.; Rodriguez Olivera, Y.; Beaudry, F.; Jacques, M.; Harel, J. N-Acetyl-Glucosamine Influences the Biofilm Formation of Escherichia coli. Gut Pathog. 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskikh, I.V.; Kulikov, S.N.; Vyshivannaya, O.V.; Bezrodnykh, E.A.; Yamskov, I.A.; Tikhonov, V.E. Influence of Glucosamine on Oligochitosan Solubility and Antibacterial Activity. Carbohydr. Res. 2013, 381, 28–32. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal Attributes of 1,2,3-Triazoles: Current Developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Shi, L.; Shi, Z.; Ren, L.; Wang, Y. The Promotion of Antimicrobial Activity on Silicon Substrates Using a “click” Immobilized Short Peptide. Chem. Commun. 2014, 50, 975–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Głowacka, I.E.; Grzonkowski, P.; Lisiecki, P.; Kalinowski, Ł.; Piotrowska, D.G. Synthesis and Antimicrobial Activity of Novel 1,2,3-Triazole-Conjugates of Quinazolin-4-Ones. Arch. Pharm. 2019, 352, 1800302. [Google Scholar] [CrossRef] [PubMed]
- Petrova, K.T.; Potewar, T.M.; Correia-Da-Silva, P.; Barros, M.T.; Calhelha, R.C.; Ćiric, A.; Soković, M.; Ferreira, I.C.F.R. Antimicrobial and Cytotoxic Activities of 1,2,3-Triazole-Sucrose Derivatives. Carbohydr. Res. 2015, 417, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Holla, B.S.; Mahalinga, M.; Karthikeyan, M.S.; Poojary, B.; Akberali, P.M.; Kumari, N.S. Synthesis, Characterization and Antimicrobial Activity of Some Substituted 1,2,3-Triazoles. Eur. J. Med. Chem. 2005, 40, 1173–1178. [Google Scholar] [CrossRef]
- Abdel-Wahab, F.B.; Mohamed, H.A.; Awad, E.A.G. Synthesis and Biological Activity of Some New 1,2,3-Triazole Hydrazone Derivatives. Eur. Chem. Bull. 2015, 4, 106–109. [Google Scholar] [CrossRef]
- Pandiyarajan, C.K.; Prucker, O.; Zieger, B.; Rühe, J. Influence of the Molecular Structure of Surface-Attached Poly(N -alkyl Acrylamide) Coatings on the Interaction of Surfaces with Proteins, Cells and Blood Platelets. Macromol. Biosci. 2013, 13, 873–884. [Google Scholar] [CrossRef]
- Prucker, O.; Brandstetter, T.; Rühe, J. Surface-Attached Hydrogel Coatings via C,H-Insertion Crosslinking for Biomedical and Bioanalytical Applications (Review). Biointerphases 2018, 13, 010801. [Google Scholar] [CrossRef] [Green Version]
- Mahou, R.; Wandrey, C. Versatile Route to Synthesize Heterobifunctional Poly(Ethylene Glycol) of Variable Functionality for Subsequent Pegylation. Polymers 2012, 4, 561–589. [Google Scholar] [CrossRef]
- Öztürk, T.; Meyvacı, E.; Arslan, T. Synthesis and Characterization of Poly(Vinyl Chloride-g-ε-Caprolactone) Brush Type Graft Copolymers by Ring-Opening Polymerization and “Click” Chemistry. J. Macromol. Sci. Part. A 2020, 57, 171–180. [Google Scholar] [CrossRef]
- Sumerlin, B.S.; Tsarevsky, N.V.; Louche, G.; Lee, R.Y.; Matyjaszewski, K. Highly Efficient “Click” Functionalization of Poly(3-azidopropyl methacrylate) Prepared by ATRP. Macromolecules 2005, 38, 7540–7545. [Google Scholar] [CrossRef]
- Schmidt, M.S.; Leitner, K.; Welter, M.; Wurmthaler, L.A.; Ringwald, M. Carbohydrate-Based Cu(I) Stabilizing Ligands and Their Use in the Synthesis of Carbohydrate–Ferrocene Conjugates. Carbohydr. Res. 2014, 387, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, S.R. Zemplén Deacetylation. In Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 2010, p. 892. [Google Scholar]
- 87: Biological Reactivity Tests, in Vitro. In The United States Pharmacopeia-The National Formulary; Pharmacopeial Convention, Inc.: Rockville, MD, USA, 1979; Volume 35, pp. 92–94.
- Japan Food Research Laboratories. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; ISO: London, UK, 2011; pp. 1–24. [Google Scholar]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate Analysis by a Phenol-Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783642019982. [Google Scholar]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet Irradiation and the Mechanisms Underlying its Inactivation of Infectious Agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Bak, J.; Begovic, T. A Prototype Catheter Designed for Ultraviolet C Disinfection. J. Hosp. Infect. 2013, 84, 173–177. [Google Scholar] [CrossRef]
- Yang, J.-H.; Wu, U.-I.; Tai, H.-M.; Sheng, W.-H. Effectiveness of an Ultraviolet-C Disinfection System for Reduction of Healthcare-Associated Pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Gora, S.L.; Rauch, K.D.; Ontiveros, C.C.; Stoddart, A.K.; Gagnon, G.A. Inactivation of Biofilm-Bound Pseudomonas Aeruginosa Bacteria Using UVC Light Emitting Diodes (UVC LEDs). Water Res. 2019, 151, 193–202. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Li, C.-S. Inactivation of Viruses on Surfaces by Ultraviolet Germicidal Irradiation. J. Occup. Environ. Hyg. 2007, 4, 400–405. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and Device-Associated Infections. In Proceedings of the Emerging Infectious Diseases; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2001; Volume 7, pp. 277–281. [Google Scholar]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K. De Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- LI, M.; CHEN, C.; XIA, X.; GARBA, B.; SHANG, L.; WANG, Y. Proteomic Analysis of the Inhibitory Effect of Chitosan on Penicillium expansum. Food Sci. Technol. 2020, 40, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Chitosan and Their Derivatives: Antibiofilm Drugs against Pathogenic Bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef] [PubMed]
- Tantala, J.; Thumanu, K.; Rachtanapun, C. An Assessment of Antibacterial Mode of Action of Chitosan on Listeria innocua Cells Using Real-Time HATR-FTIR Spectroscopy. Int. J. Biol. Macromol. 2019, 135, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Andres, Y.; Giraud, L.; Gerente, C.; Le Cloirec, P. Antibacterial Effects of Chitosan Powder: Mechanisms of Action. Environ. Technol. 2007, 28, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan Kills Bacteria through Cell Membrane Damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- ISO. Biological Evaluation of Medical Devices-Part. 5: Tests for In Vitro Cytotoxicity; ISO: London, UK, 2009. [Google Scholar]


| Polymer | Calculated Ratio | Found Ratio (via NMR) | |
|---|---|---|---|
| MBP 14 [%] (a) | 11 or 12 [%] (b) | MBP vs. 11/12 | |
| GlcNAc-2EG | 5% | 5% 11a | 30:1 |
| 5%-GlcNAc-4EG | 5% | 5% 11b | 2:1 |
| 10%-GlcNAc-4EG | 5% | 10% 11b | 1:2 |
| 25%-GlcNAc-4EG | 5% | 25% 11b | 1:5 |
| 50%-GlcNAc-4EG (c) | 5% | 50% 11b | - |
| GlcNAc-6EG | 5% | 5% 11c | 3:1 |
| GlcNAc-4EG-octyl | 5% | 5% 11d | 6:1 |
| HM-2EG | 5% | 5% 12a | 1:1 |
| HM-4EG | 5% | 5% 12b | 1:1 |
| HM-6EG | 5% | 5% 12c | 1:1 |
| PDMAm (d) | 5% | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgolte, M.; Riester, O.; Kacerova, T.; Rentschler, S.; Schmidt, M.S.; Jacksch, S.; Egert, M.; Laufer, S.; Csuk, R.; Deigner, H.-P. Methacryloyl-GlcNAc Derivatives Copolymerized with Dimethacrylamide as a Novel Antibacterial and Biocompatible Coating. Pharmaceutics 2021, 13, 1647. https://doi.org/10.3390/pharmaceutics13101647
Borgolte M, Riester O, Kacerova T, Rentschler S, Schmidt MS, Jacksch S, Egert M, Laufer S, Csuk R, Deigner H-P. Methacryloyl-GlcNAc Derivatives Copolymerized with Dimethacrylamide as a Novel Antibacterial and Biocompatible Coating. Pharmaceutics. 2021; 13(10):1647. https://doi.org/10.3390/pharmaceutics13101647
Chicago/Turabian StyleBorgolte, Max, Oliver Riester, Tereza Kacerova, Simone Rentschler, Magnus S. Schmidt, Susanne Jacksch, Markus Egert, Stefan Laufer, René Csuk, and Hans-Peter Deigner. 2021. "Methacryloyl-GlcNAc Derivatives Copolymerized with Dimethacrylamide as a Novel Antibacterial and Biocompatible Coating" Pharmaceutics 13, no. 10: 1647. https://doi.org/10.3390/pharmaceutics13101647
APA StyleBorgolte, M., Riester, O., Kacerova, T., Rentschler, S., Schmidt, M. S., Jacksch, S., Egert, M., Laufer, S., Csuk, R., & Deigner, H.-P. (2021). Methacryloyl-GlcNAc Derivatives Copolymerized with Dimethacrylamide as a Novel Antibacterial and Biocompatible Coating. Pharmaceutics, 13(10), 1647. https://doi.org/10.3390/pharmaceutics13101647








