Evaluation of an Intravitreal Rho-Associated Kinase Inhibitor Depot Formulation in a Rat Model of Diabetic Retinopathy
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Pharmacokinetic Analysis
2.3. Treatment
2.4. Evaluation of Retinal Hypoxia and Retinal Vessels
2.5. Retinal Pigment Epithelium (RPE) Flat Mounts and ROCK Immunohistochemistry
2.6. Electroretinography
2.7. Blood-Retinal Barrier Evaluation
2.8. Tolerability Evaluation
2.9. ROCK Activity
2.10. RNA Isolation and Quality Control
2.11. Transcriptome Profiling with Total RNA Sequencing
2.12. Data Processing
3. Results
3.1. Sustained Inhibition of ROCK Activity After Intravitreous Injection of BIRKI
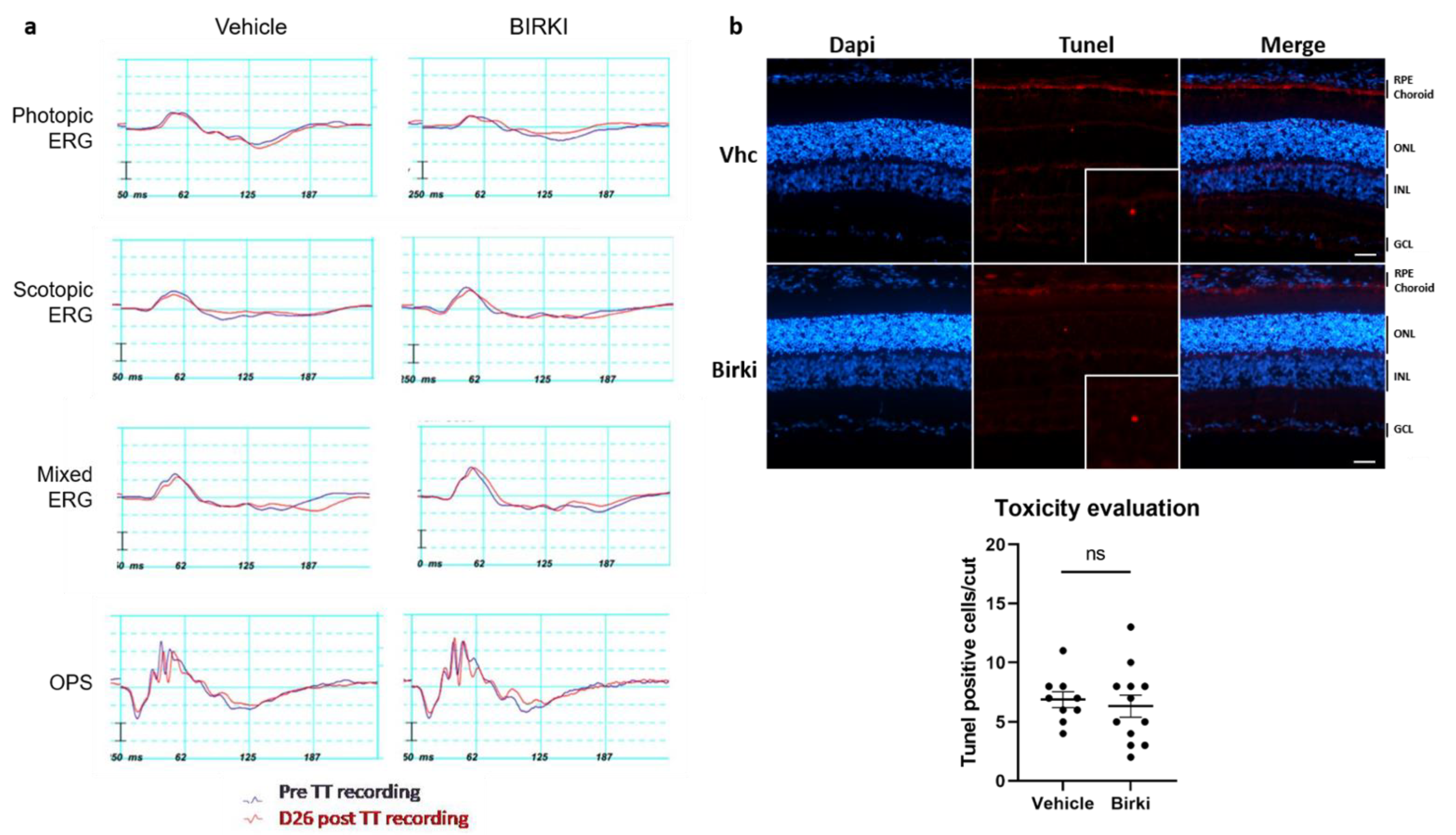
3.2. No Functional or Structural Damage Occurred Secondary to BIRKI Injection in the Rat Eye
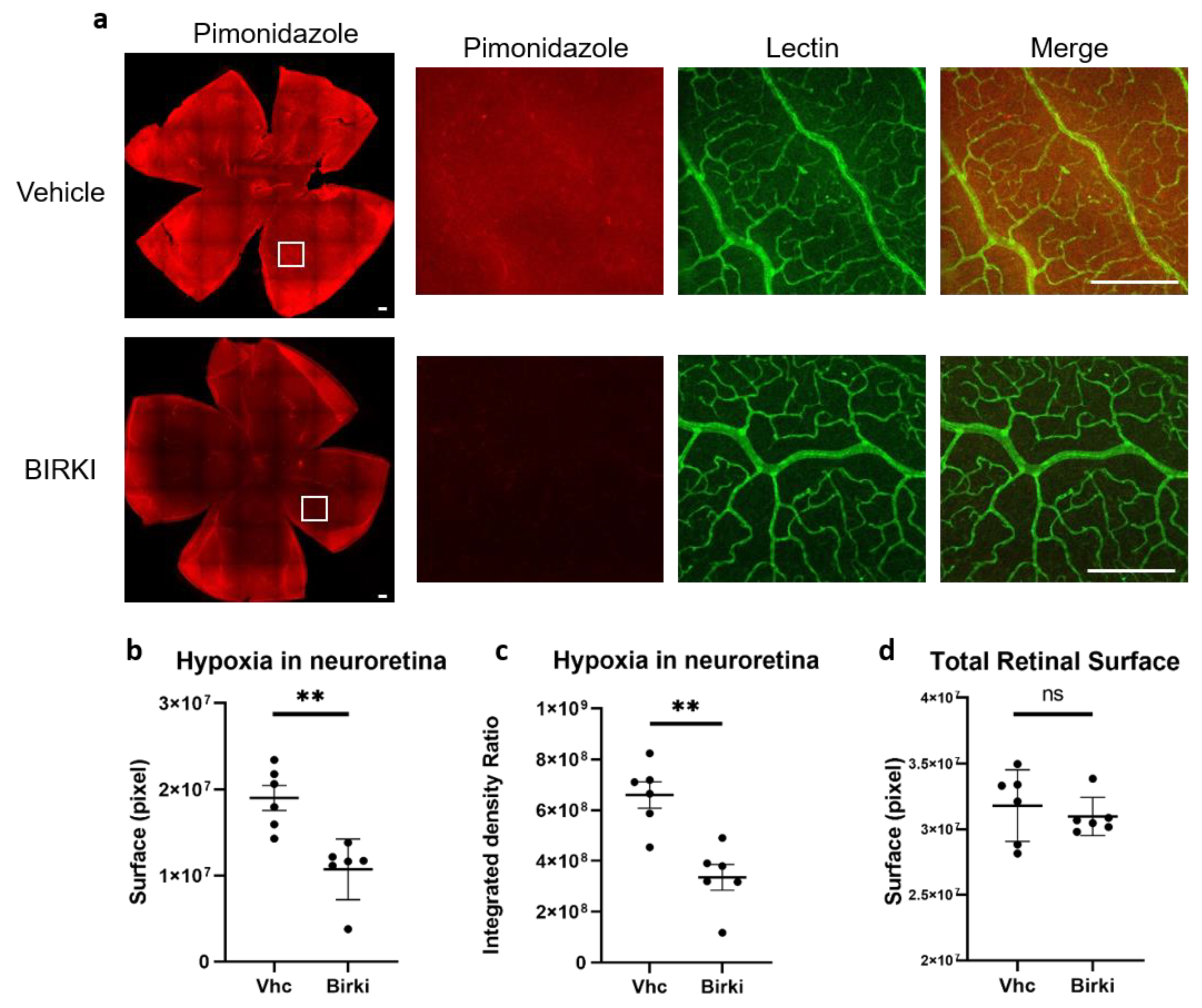
3.3. One Single Injection of BIRKI Significantly Reduced Retinal Hypoxia and Capillary Constriction in GK Rats at 28 Days
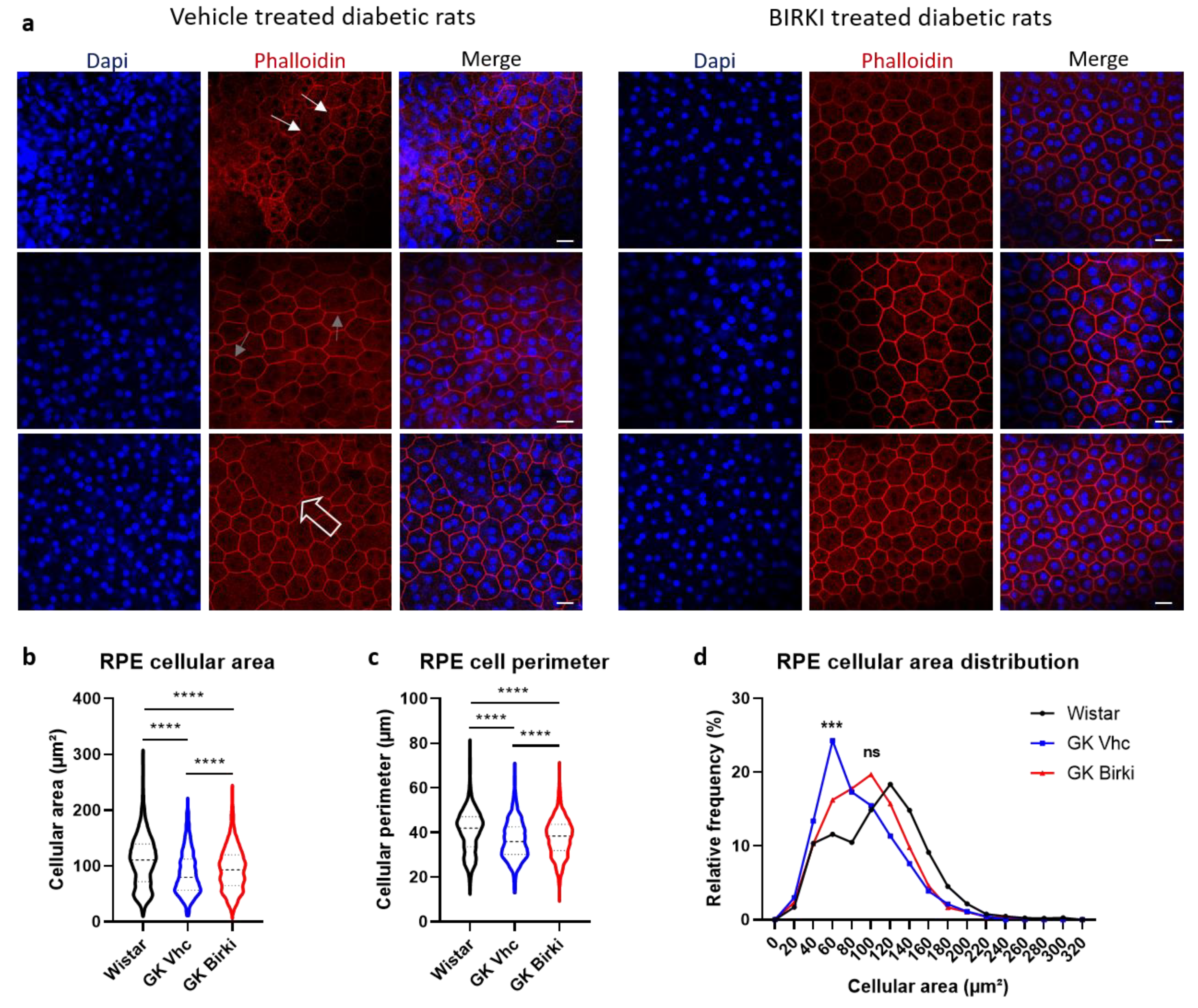
3.4. BIRKI Reduced Retinal Vascular Leakage and Improved the RPE Morphology at 28 Days
3.5. Transcriptomic Effects of BIRKI in Neuroretina and RPE/Choroid Complex at 28 Days After Injection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheloni, R.; Gandolfi, S.A.; Signorelli, C.; Odone, A. Global prevalence of diabetic retinopathy: Protocol for a systematic review and meta-analysis. BMJ Open 2019, 9, e022188. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Lobo, C.L.; Bernardes, R.C.; Cunha-Vaz, J.G. Alterations of the blood-retinal barrier and retinal thickness in preclinical retinopathy in subjects with type 2 diabetes. Arch. Ophthalmol. Chic. 2000, 118, 1364–1369. [Google Scholar] [CrossRef] [Green Version]
- Madonna, R.; Balistreri, C.R.; Geng, Y.-J.; De Caterina, R. Diabetic microangiopathy: Pathogenetic insights and novel therapeutic approaches. Vascul. Pharmacol. 2017, 90, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Simó, R.; Hernández, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.E.; Browning, D.J.; Wong, K.; Flynn, H.W., Jr.; Bhavsar, A.R. The Evolving Treatment of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 653–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, R.; Hata, Y.; Ishibashi, T. ROCK as a Therapeutic Target of Diabetic Retinopathy. J. Ophthalmol. 2010, 2010, 175163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wu, X.; Surma, M.; Vemula, S.; Zhang, L.; Yang, Y.; Kapur, R.; Wei, L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013, 4, e483. [Google Scholar] [CrossRef] [Green Version]
- Kutcher, M.E.; Kolyada, A.Y.; Surks, H.K.; Herman, I.M. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am. J. Pathol. 2007, 171, 693–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glyn, M.C.; Lawrenson, J.G.; Ward, B.J.; Clark, P. Rho Kinase-Mediated Reduction in Cardiac Capillary Endothelial Cell Dimensions, In Situ, Against Flow. Microcirculation 2008, 15, 175–190. [Google Scholar] [CrossRef]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 276. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Parker, L.; Birdsong, O.C.; Ronquillo, Y.C.; Hofstedt, D.; Shah, T.J.; Gomez, A.T.; Hoopes, P.C., Sr. Use of Rho kinase Inhibitors in Ophthalmology: A Review of the Literature. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2018, 7, 101–111. [Google Scholar]
- Mohammad, G.; AlSharif, H.M.; Siddiquei, M.M.; Ahmad, A.; Alam, K.; Abu El-Asrar, A.M. Rho-Associated Protein Kinase-1 Mediates the Regulation of Inflammatory Markers in Diabetic Retina and in Retinal Müller Cells. Ann. Clin. Lab. Sci. 2018, 48, 137–145. [Google Scholar] [PubMed]
- Zhang, X.-H.; Feng, Z.-H.; Wang, X.-Y. The ROCK pathway inhibitor Y-27632 mitigates hypoxia and oxidative stress-induced injury to retinal Müller cells. Neural Regen. Res. 2018, 13, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Hata, Y.; Nakao, S.; Kita, T.; Miura, M.; Kawahara, S.; Zandi, S.; Almulki, L.; Tayyari, F.; Shimokawa, H.; et al. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes 2009, 58, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollanders, K.; Hove, I.V.; Sergeys, J.; Bergen, T.V.; Lefevere, E.; Kindt, N.; Castermans, K.; Vandewalle, E.; van Pelt, J.; Moons, L.; et al. AMA0428, A Potent Rock Inhibitor, Attenuates Early and Late Experimental Diabetic Retinopathy. Curr. Eye Res. 2017, 42, 260–272. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Nourinia, R.; Hafezi-Moghadam, A. Intravitreal fasudil combined with bevacizumab for persistent diabetic macular edema: A novel treatment. JAMA Ophthalmol. 2013, 131, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, P.R.; Salah, S.; Berdugo, M.; Gelize, E.; Delaunay, K.; Naud, M.C.; Klein, C.; Moulin, A.; Savoldelli, M.; Bergin, C.; et al. ROCK-1 mediates diabetes-induced retinal pigment epithelial and endothelial cell blebbing: Contribution to diabetic retinopathy. Sci. Rep. 2017, 7, 8834. [Google Scholar] [CrossRef] [Green Version]
- Rimpelä, A.-K.; Garneau, M.; Baum-Kroker, K.S.; Schönberger, T.; Runge, F.; Sauer, A. Quantification of Drugs in Distinctly Separated Ocular Substructures of Albino and Pigmented Rats. Pharmaceutics 2020, 12, 1174. [Google Scholar] [CrossRef]
- Berdugo, M.; Delaunay, K.; Naud, M.-C.; Guegan, J.; Moulin, A.; Savoldelli, M.; Picard, E.; Radet, L.; Jonet, L.; Djerada, Z.; et al. The antidiabetic drug glibenclamide exerts direct retinal neuroprotection. Transl. Res. 2021, 229, 83–99. [Google Scholar] [CrossRef]
- Zhou, M.; Li, G.; Zhu, L.; Zhou, H.; Lu, L. Arctiin attenuates high glucose-induced human retinal capillary endothelial cell proliferation by regulating ROCK1/PTEN/PI3K/Akt/VEGF pathway in vitro. J. Cell. Mol. Med. 2020, 24, 5695–5706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.-Y.; Chen, W.; Lu, L.; Zheng, Z.; Xu, X. Involvement of RhoA/ROCK1 signaling pathway in hyperglycemia-induced microvascular endothelial dysfunction in diabetic retinopathy. Int. J. Clin. Exp. Pathol. 2014, 7, 7268–7277. [Google Scholar] [PubMed]
- Wang, J.; Yao, Y.; Liu, X.; Wang, K.; Zhou, Q.; Tang, Y. Protective effects of lycium barbarum polysaccharides on blood-retinal barrier via ROCK1 pathway in diabetic rats. Am. J. Transl. Res. 2019, 11, 6304–6315. [Google Scholar]
- Itoh, Y.; Kimoto, K.; Imaizumi, M.; Nakatsuka, K. Inhibition of RhoA/Rho-kinase pathway suppresses the expression of type I collagen induced by TGF-β2 in human retinal pigment epithelial cells. Exp. Eye Res. 2007, 84, 464–472. [Google Scholar] [CrossRef]
- Garnock-Jones, K.P. Ripasudil: First global approval. Drugs 2014, 74, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Hida, Y.; Nakamura, S.; Nishinaka, A.; Inoue, Y.; Shimazawa, M.; Hara, H. Effects of ripasudil, a ROCK inhibitor, on retinal edema and nonperfusion area in a retinal vein occlusion murine model. J. Pharmacol. Sci. 2018, 137, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; LoGrasso, P.V.; Defert, O.; Li, R. Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. J. Med. Chem. 2016, 59, 2269–2300. [Google Scholar] [CrossRef]
- Zimmerman, M.; Rolandsson Enes, S.; Skärstrand, H.; Pourhamidi, K.; Gottsäter, A.; Wollmer, P.; Rolandsson, O.; Westergren-Thorsson, G.; Dahlin, L.B. Temporal trend of autonomic nerve function and HSP27, MIF and PAI-1 in type 1 diabetes. J. Clin. Transl. Endocrinol. 2017, 8, 15–21. [Google Scholar] [CrossRef]
- Aso, Y. Intensive Risk Factor Management and Cardiovascular Autonomic Neuropathy in Type 2 Diabetes in the ACCORD Trial: A post-hoc analysis. J. Diabetes Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Malerbi, F.K.; Regatieri, C.V.; de Sa, J.R.; Morales, P.H.; Farah, M.E.; Dib, S.A. Microalbuminuria is associated with increased choroidal thickness in type 1 diabetes mellitus patients without diabetic retinopathy. Acta Ophthalmol. 2018, 96, e95–e97. [Google Scholar] [CrossRef]
- Shen, Z.-J.; Yang, X.-F.; Xu, J.; She, C.-Y.; Wei, W.-W.; Zhu, W.-L.; Liu, N.-P. Association of choroidal thickness with early stages of diabetic retinopathy in type 2 diabetes. Int. J. Ophthalmol. 2017, 10, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, M.; Alonso, N.; Castelblanco, E.; Real, J.; Ramírez-Morros, A.; Simó, R.; Hernández, C.; Jurjo, C.; Traveset, A.; Valldeperas, X.; et al. Assessment of Inner Retinal Layers and Choroidal Thickness in Type 1 Diabetes Mellitus: A Cross-Sectional Study. J. Clin. Med. 2019, 8, 1412. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, S.; Qiu, Z.; He, M.; Wang, L.; Li, Y.; Huang, W. Choroidal Thickness in Diabetes and Diabetic Retinopathy: A Swept Source OCT Study. Invest. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.R.; Muke, I.; Bobylev, I.; Lehmann, H.C. ROCK inhibition improves axonal regeneration in a preclinical model of amyotrophic lateral sclerosis. J. Comp. Neurol. 2019, 527, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Kalpachidou, T.; Spiecker, L.; Kress, M.; Quarta, S. Rho GTPases in the Physiology and Pathophysiology of Peripheral Sensory Neurons. Cells 2019, 8, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imanishi, A.; Ichise, H.; Fan, C.; Nakagawa, Y.; Kuwahara, K.; Sumiyama, K.; Matsuda, M.; Terai, K. Visualization of Spatially-Controlled Vasospasm by Sympathetic Nerve-Mediated ROCK Activation. Am. J. Pathol. 2021, 191, 194–203. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebon, C.; Neubauer, H.; Berdugo, M.; Delaunay, K.; Markert, E.; Becker, K.; Baum-Kroker, K.S.; Prestle, J.; Fuchs, H.; Bakker, R.A.; et al. Evaluation of an Intravitreal Rho-Associated Kinase Inhibitor Depot Formulation in a Rat Model of Diabetic Retinopathy. Pharmaceutics 2021, 13, 1105. https://doi.org/10.3390/pharmaceutics13081105
Lebon C, Neubauer H, Berdugo M, Delaunay K, Markert E, Becker K, Baum-Kroker KS, Prestle J, Fuchs H, Bakker RA, et al. Evaluation of an Intravitreal Rho-Associated Kinase Inhibitor Depot Formulation in a Rat Model of Diabetic Retinopathy. Pharmaceutics. 2021; 13(8):1105. https://doi.org/10.3390/pharmaceutics13081105
Chicago/Turabian StyleLebon, Cecile, Heike Neubauer, Marianne Berdugo, Kimberley Delaunay, Elke Markert, Kolja Becker, Katja S. Baum-Kroker, Jürgen Prestle, Holger Fuchs, Remko A. Bakker, and et al. 2021. "Evaluation of an Intravitreal Rho-Associated Kinase Inhibitor Depot Formulation in a Rat Model of Diabetic Retinopathy" Pharmaceutics 13, no. 8: 1105. https://doi.org/10.3390/pharmaceutics13081105
APA StyleLebon, C., Neubauer, H., Berdugo, M., Delaunay, K., Markert, E., Becker, K., Baum-Kroker, K. S., Prestle, J., Fuchs, H., Bakker, R. A., & Behar-Cohen, F. (2021). Evaluation of an Intravitreal Rho-Associated Kinase Inhibitor Depot Formulation in a Rat Model of Diabetic Retinopathy. Pharmaceutics, 13(8), 1105. https://doi.org/10.3390/pharmaceutics13081105





