Ultrasound-Triggerable Coatings for Foley Catheter Balloons for Local Release of Anti-Inflammatory Drugs during Bladder Neck Dilation
Abstract
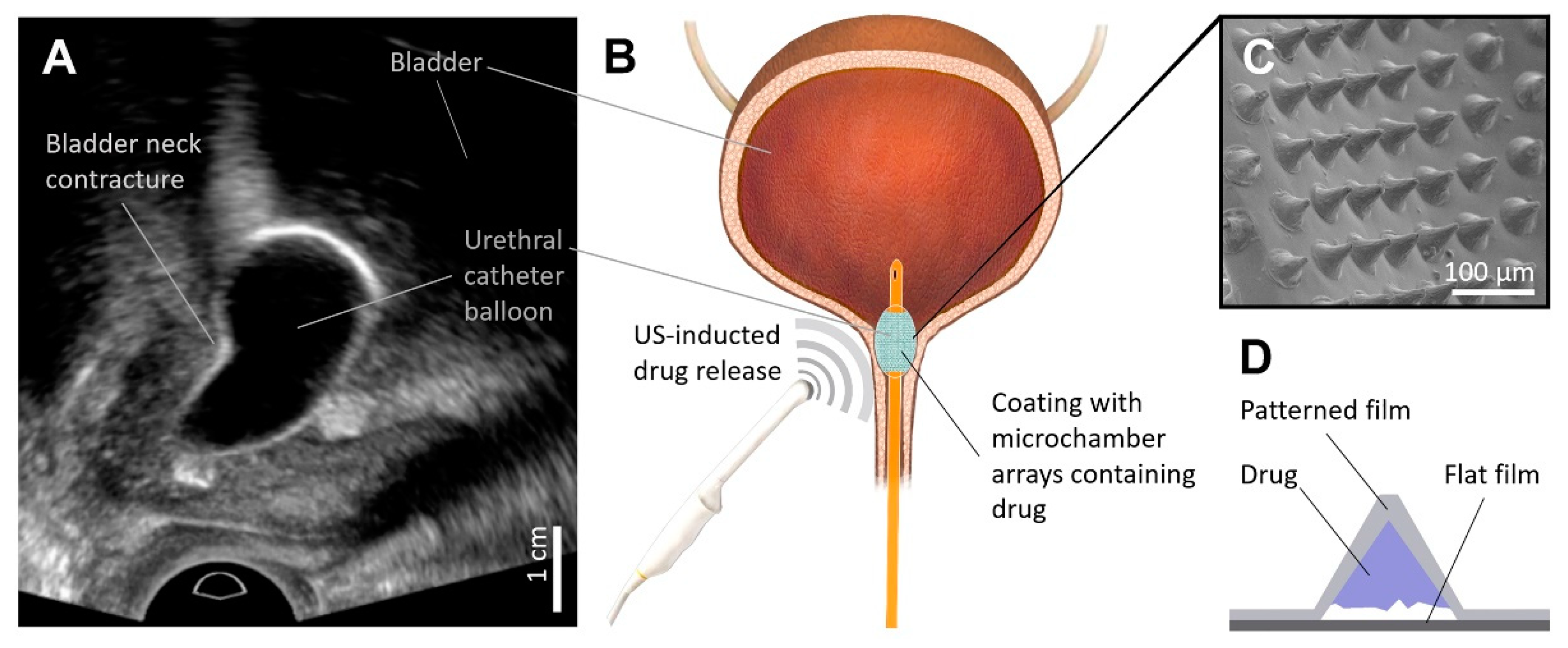
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
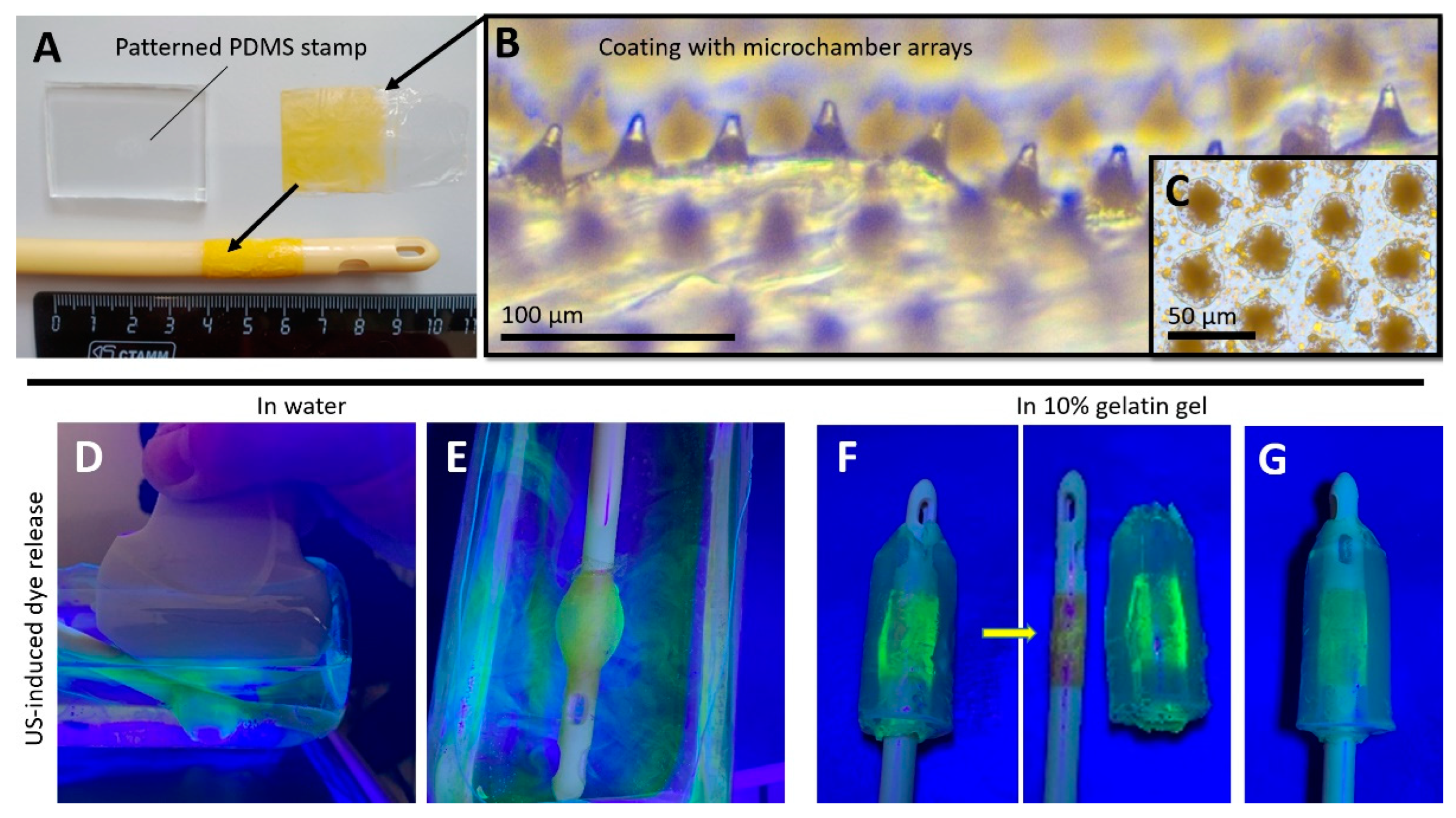
2.3. Fabrication of Patterned PDMS Stamp
2.4. Preparation of PLA Coating with Fluorescent or Biologically Active Cargo
2.5. Coating Procedure of Foley Catheter Balloon
2.6. Ultrasound-Induced Release of 5(6)-Carboxyfluorescein in Water and Gelatin Gel from a Foley Catheter Modified with PLA-Based Coatings with Microchamber Arrays Using a Diagnostic Ultrasound Device
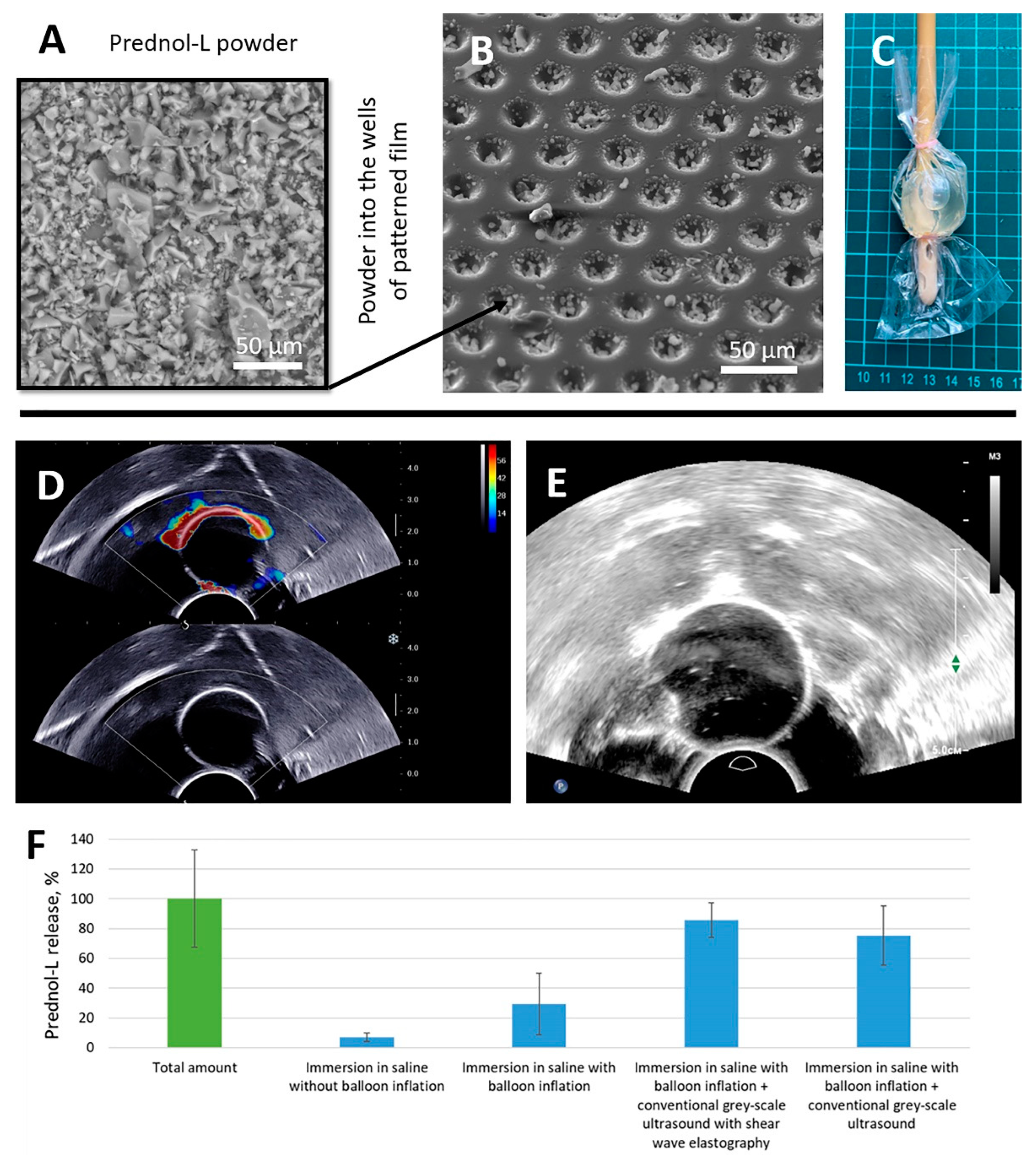
2.7. Ultrasound-Induced Release of Prednol-L in Saline from a Foley Catheter Modified with PLA-Based Coatings with Microchamber Arrays Using Different Modalities of Diagnostic Ultrasound
2.8. Quantification of the Ultrasound-Induced Release of Prednol-L
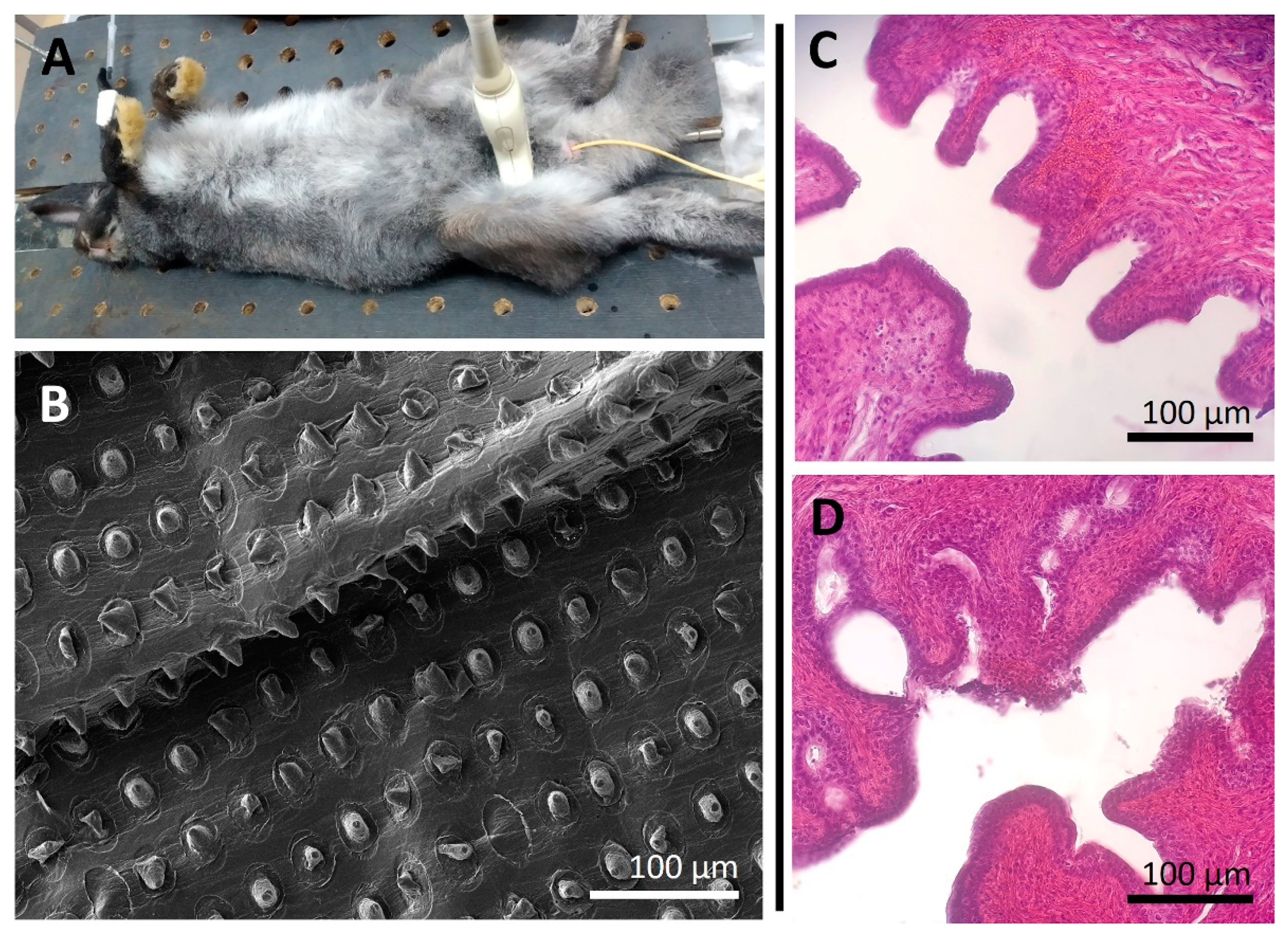
2.9. In Vivo Study
3. Results and Discussion
3.1. Ultrasound-Inducted Release of 5(6)-Carboxyfluorescein in Water and Gelatin Gel from a Foley Catheter Modified with PLA-Based Coatings with Microchamber Arrays Using a Diagnostic Ultrasound Device
3.2. Ultrasound-Inducted Release of Prednol-L in Saline from a Foley Catheter Modified with PLA-Based Coatings with Microchamber Arrays Using Different Modalities of Diagnostic Ultrasound
3.3. In Vivo Safety Assessment of Using a Foley Catheter Modified with an Ultrasound-Sensitive Coating Eluting Prednol-L
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simhan, J.; Ramirez, D.; Hudak, S.J.; Morey, A.F. Bladder neck contracture. Transl. Androl. Urol. 2014, 3, 214–220. [Google Scholar] [PubMed]
- Cindolo, L.; Marchioni, M.; Emiliani, E.; De Francesco, P.; Primiceri, G.; Castellan, P.; Schips, L. Bladder neck contracture after surgery for benign prostatic obstruction. Minerva Urol. Nephrol. 2017, 69, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Abbosov, S.A.; Sorokin, N.I.; Kadrev, A.V.; Shomarufov, A.B.; Strigunov, A.A.; Kabanova, O.O.; Nesterova, O.Y.; Shaparov, B.M.; Kamalov, A.A. Bladder neck sclerosis: Alternative methods of treatment and prospects for their development. Exp. Clinical Urol. 2021, 14, 94–99. [Google Scholar] [CrossRef]
- Abbosov, S.; Sorokin, N.; Shomarufov, A.; Kadrev, A.; Ugli Nuriddinov, K.; Mukhtarov, S.; Akilov, F.; Kamalov, A. Bladder neck contracture as a complication of prostate surgery: Alternative treatment methods and prospects (literature review). Urol. Sci. 2022, 33, 49–55. [Google Scholar] [CrossRef]
- Abbosov, S.A.; Sorokin, N.I.; Shomarufov, A.B.; Kadrev, A.V. Assessment of the Balloon Dilation Efficiency in Bladder Neck Contracture after Transurethral Interventions on the Prostate. Urol. Sci. 2022, 33, 130. [Google Scholar] [CrossRef]
- Lubahn, J.D.; Zhao, L.C.; Scott, J.F.; Hudak, S.J.; Chee, J.; Terlecki, R.; Breyer, B.; Morey, A.F. Poor Quality of Life in Patients with Urethral Stricture Treated with Intermittent Self-Dilation. J. Urol. 2014, 191, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Besarani, D.; Amoroso, P.; Kirby, R. Bladder neck contracture after radical retropubic prostatectomy. BJU Int. 2004, 94, 1245–1247. [Google Scholar] [CrossRef]
- Park, R.; Martin, S.; Goldberg, J.D.; Lepor, H. Anastomotic strictures following radical prostatectomy: Insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology 2001, 57, 742–746. [Google Scholar] [CrossRef]
- Abbosov, S.A.; Okhobotov, D.A.; Sorokin, N.I.; Shomarufov, A.B.; Shaparov, B.M.; Nadzhimitdinov, Y.S.; Mukhtarov, S.T.; Akilov, F.A.; Kamalov, A.A. Balloon dilatation of cicatricial bladder neck contracture: Evaluation of the efficacy after transurethral prostate interventions (preliminary results). Urol. Her. 2021, 9, 5–13. [Google Scholar] [CrossRef]
- Eltahawy, E.; Gur, U.; Virasoro, R.; Schlossberg, S.M.; Jordan, G.H. Management of recurrent anastomotic stenosis following radical prostatectomy using holmium laser and steroid injection. BJU Int. 2008, 102, 796–798. [Google Scholar] [CrossRef]
- Mazdak, H.; Meshki, I.; Ghassami, F. Effect of Mitomycin C on Anterior Urethral Stricture Recurrence after Internal Urethrotomy. Eur. Urol. 2007, 51, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Antipina, M.N.; Kiryukhin, M.V.; Chong, K.; Low, H.Y.; Sukhorukov, G.B. Patterned microcontainers as novel functional elements for µTAS and LOC. Lab Chip 2009, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Kiryukhin, M.V.; Man, S.M.; Gorelik, S.R.; Subramanian, G.S.; Low, H.Y.; Sukhorukov, G.B. Fabrication and mechanical properties of microchambers made of polyelectrolyte multilayers. Soft Matter 2011, 7, 6550–6556. [Google Scholar] [CrossRef]
- Kiryukhin, M.V.; Gorelik, S.R.; Man, S.M.; Subramanian, G.S.; Antipina, M.N.; Low, H.Y.; Sukhorukov, G.B. Individually Addressable Patterned Multilayer Microchambers for Site-Specific Release-On-Demand. Macromol. Rapid Commun. 2013, 34, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zykova, Y.; Kudryavtseva, V.; Gai, M.; Kozelskaya, A.; Frueh, J.; Sukhorukov, G.; Tverdokhlebov, S. Free-standing microchamber arrays as a biodegradable drug depot system for implant coatings. Eur. Polym. J. 2019, 114, 72–80. [Google Scholar] [CrossRef]
- Sindeeva, O.A.; Prikhozhdenko, E.S.; Schurov, I.; Sedykh, N.; Goriainov, S.; Karamyan, A.; Mordovina, E.A.; Inozemtseva, O.A.; Kudryavtseva, V.; Shchesnyak, L.E.; et al. Patterned Drug-Eluting Coatings for Tracheal Stents Based on PLA, PLGA, and PCL for the Granulation Formation Reduction: In Vivo Studies. Pharmaceutics 2021, 13, 1437. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Kudryavtseva, V.L.; Yashchenok, A.M.; Sukhorukov, G.B. Polylactic Acid Sealed Polyelectrolyte Multilayer Microchambers for Entrapment of Salts and Small Hydrophilic Molecules Precipitates. ACS Appl. Mater. Interfaces 2017, 9, 16536–16545. [Google Scholar] [CrossRef]
- Mu, L.; Rutkowski, S.; Gai, M.; Frueh, J. Alginate Microparticle Arrays as Self-Polishing Bio-Fouling Release Coatings. J. Nanosci. Nanotechnol. 2019, 19, 8052–8062. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Tao, T.; Petrov, A.V.; Petrov, V.V.; Shesterikov, E.V.; Tverdokhlebov, S.I.; Sukhorukov, G.B. Polylactic acid nano- and microchamber arrays for encapsulation of small hydrophilic molecules featuring drug release via high intensity focused ultrasound. Nanoscale 2017, 9, 7063–7070. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gai, M.; Sukvanitvichai, D.; Frueh, J.; Sukhorukov, G.B. pH dependent degradation properties of lactide based 3D microchamber arrays for sustained cargo release. Colloids Surf. B Biointerfaces 2020, 188, 110826. [Google Scholar] [CrossRef]
- Sindeeva, O.A.; Gusliakova, O.I.; Inozemtseva, O.A.; Abdurashitov, A.S.; Brodovskaya, E.P.; Gai, M.; Tuchin, V.V.; Gorin, D.A.; Sukhorukov, G.B. Effect of a Controlled Release of Epinephrine Hydrochloride from PLGA Microchamber Array: In Vivo Studies. ACS Appl. Mater. Interfaces 2018, 10, 37855–37864. [Google Scholar] [CrossRef]
- Kopach, O.; Zheng, K.; Sindeeva, O.A.; Gai, M.; Sukhorukov, G.B.; Rusakov, D.A. Polymer microchamber arrays for geometry-controlled drug release: A functional study in human cells of neuronal phenotype. Biomater. Sci. 2019, 7, 2358–2371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindeeva, O.A.; Kopach, O.; Kurochkin, M.A.; Sapelkin, A.; Gould, D.J.; Rusakov, D.A.; Sukhorukov, G.B. Polylactic Acid-Based Patterned Matrixes for Site-Specific Delivery of Neuropeptides On-Demand: Functional NGF Effects on Human Neuronal Cells. Front. Bioeng. Biotechnol. 2020, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Mordovina, E.A.; Sindeeva, O.A.; Abramova, A.M.; Tsyupka, D.V.; Atkin, V.S.; Bratashov, D.N.; Goryacheva, I.Y.; Sukhorukov, G.B. Controlled release of α-amylase from microchamber arrays containing carbon nanoparticle aggregates. Mendeleev Commun. 2021, 31, 869–871. [Google Scholar] [CrossRef]
- Gai, M.; Kurochkin, M.A.; Li, D.; Khlebtsov, B.N.; Dong, L.; Tarakina, N.; Poston, R.; Gould, D.J.; Frueh, J.; Sukhorukov, G.B. In-situ NIR-laser mediated bioactive substance delivery to single cell for EGFP expression based on biocompatible microchamber-arrays. J. Control. Release 2018, 276, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Mordovina, E.A.; Plastun, V.O.; Abdurashitov, A.S.; Proshin, P.I.; Raikova, S.V.; Bratashov, D.N.; Inozemtseva, O.A.; Goryacheva, I.Y.; Sukhorukov, G.B.; Sindeeva, O.A. “Smart” Polylactic Acid Films with Ceftriaxone Loaded Microchamber Arrays for Personalized Antibiotic Therapy. Pharmaceutics 2022, 14, 42. [Google Scholar] [CrossRef]
- Kurochkin, M.A.; Sindeeva, O.A.; Brodovskaya, E.P.; Gai, M.; Frueh, J.; Su, L.; Sapelkin, A.; Tuchin, V.V.; Sukhorukov, G.B. Materials Science & Engineering C Laser-triggered drug release from polymeric 3-D micro-structured fi lms via optical fi bers. Mater. Sci. Eng. C 2020, 110, 110664. [Google Scholar]
- Sindeeva, O.A.; Prikhozhdenko, E.S.; Bratashov, D.N.; Vostrikova, A.M.; Atkin, V.S.; Ermakov, A.V.; Khlebtsov, B.N.; Sapelkin, A.V.; Goryacheva, I.Y.; Sukhorukov, G.B. Carbon dot aggregates as an alternative to gold nanoparticles for the laser-induced opening of microchamber arrays. Soft Matter 2018, 14, 9012–9019. [Google Scholar] [CrossRef]
- Ermakov, A.; Lim, S.H.; Gorelik, S.; Kauling, A.P.; De Oliveira, R.V.B.; Neto, A.H.C.; Glukhovskoy, E.; Gorin, D.A.; Sukhorukov, G.B.; Kiryukhin, M.V. Polyelectrolyte—Graphene Oxide Multilayer Composites for Array of Microchambers which are Mechanically Robust and Responsive to NIR Light. Macromol. Rapid Commun. 2019, 40, 1700868. [Google Scholar] [CrossRef]
- Maleki, A.; Ueberroth, J.A.; Walsh, M.; Foster, F.; Chang, P.Y.; Anesi, S.D.; Foster, C.S. Combination of Intravenous Methotrexate and Methylprednisolone Therapy in the Treatment of Severe Ocular Inflammatory Diseases. Ocul. Immunol. Inflamm. 2021, 29, 1559–1563. [Google Scholar] [CrossRef]
- Hong, S.; Wang, H.; Zhang, Z.; Qiao, L. The roles of methylprednisolone treatment in patients with COVID-19: A systematic review and meta-analysis. Steroids 2022, 183, 109022. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Song, G.; Jo, Y.; Won, J.; Son, T.; Cha, O.; Kim, J.; Jung, B.; Park, H.; Kim, C.-W.; et al. Sonophoresis Using Ultrasound Contrast Agents: Dependence on Concentration. PLoS ONE 2016, 11, e0157707. [Google Scholar] [CrossRef] [PubMed]
- Vranić, E. Sonophoresis-mechanisms and application. Bosn. J. Basic Med. Sci. 2008, 4, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Oberli, M.A.; Schoellhammer, C.M.; Langer, R.; Blankschtein, D. Ultrasound-enhanced transdermal delivery: Recent advances and future challenges. Ther. Deliv. 2014, 5, 843–857. [Google Scholar] [CrossRef] [Green Version]
- Gusliakova, O.; Verkhovskii, R.; Abalymov, A.; Lengert, E.; Kozlova, A.; Atkin, V.; Nechaeva, O.; Morrison, A.; Tuchin, V.; Svenskaya, Y. Transdermal platform for the delivery of the antifungal drug naftifine hydrochloride based on porous vaterite particles. Mater. Sci. Eng. C 2021, 119, 111428. [Google Scholar] [CrossRef]
- Svenskaya, Y.I.; Genina, E.A.; Parakhonskiy, B.V.; Lengert, E.V.; Talnikova, E.E.; Terentyuk, G.S.; Utz, S.R.; Gorin, D.A.; Tuchin, V.V.; Sukhorukov, G.B. A Simple Non-Invasive Approach toward Efficient Transdermal Drug Delivery Based on Biodegradable Particulate System. ACS Appl. Mater. Interfaces 2019, 11, 17270–17282. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Lengert, E.V.; Verkhovskii, R.A.; Abalymov, A.A.; Pavlov, A.M.; Ermakov, A.V.; Prikhozhdenko, E.S.; Shtykov, S.N.; Svenskaya, Y.I. CaCO3-based carriers with prolonged release properties for antifungal drug delivery to hair follicles. Biomater. Sci. 2022, 10, 3323–3345. [Google Scholar] [CrossRef]
- Lengert, E.V.; Talnikova, E.E.; Tuchin, V.V.; Svenskaya, Y.I. Prospective Nanotechnology-Based Strategies for Enhanced Intra- and Transdermal Delivery of Antifungal Drugs. Skin Pharmacol. Physiol. 2020, 33, 261–269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindeeva, O.A.; Abdurashitov, A.S.; Proshin, P.I.; Kadrev, A.V.; Kulikov, O.A.; Shaparov, B.M.; Sorokin, N.I.; Ageev, V.P.; Pyataev, N.A.; Kritskiy, A.; et al. Ultrasound-Triggerable Coatings for Foley Catheter Balloons for Local Release of Anti-Inflammatory Drugs during Bladder Neck Dilation. Pharmaceutics 2022, 14, 2186. https://doi.org/10.3390/pharmaceutics14102186
Sindeeva OA, Abdurashitov AS, Proshin PI, Kadrev AV, Kulikov OA, Shaparov BM, Sorokin NI, Ageev VP, Pyataev NA, Kritskiy A, et al. Ultrasound-Triggerable Coatings for Foley Catheter Balloons for Local Release of Anti-Inflammatory Drugs during Bladder Neck Dilation. Pharmaceutics. 2022; 14(10):2186. https://doi.org/10.3390/pharmaceutics14102186
Chicago/Turabian StyleSindeeva, Olga A., Arkady S. Abdurashitov, Pavel I. Proshin, Alexey V. Kadrev, Oleg A. Kulikov, Boris M. Shaparov, Nikolay I. Sorokin, Valentin P. Ageev, Nikolay A. Pyataev, Aleksandr Kritskiy, and et al. 2022. "Ultrasound-Triggerable Coatings for Foley Catheter Balloons for Local Release of Anti-Inflammatory Drugs during Bladder Neck Dilation" Pharmaceutics 14, no. 10: 2186. https://doi.org/10.3390/pharmaceutics14102186





