Multicenter Observational/Exploratory Study Addressed to the Evaluation of the Effectiveness and Safety of Pharmacological Therapy in Opioid-Dependent Patients in Maintenance Therapy in Southern Italy
Abstract
:1. Introduction
- Tolerance: A phenomenon for which it is necessary to intensify the behavior of use (for example, by increasing the amount of drug to be used or frequency of intakes), in order to achieve the same effects on the body.
- Withdrawal: characterized by the presence of emotional or physical symptoms that occur when the subject cannot implement the behavior of intake.
- Interruption or reduction of social, work, or recreational activities: The use of drugs and onset of the disorder cause a series of damage to the functioning of the person who uses it (conflicts with affectively important people, work problems, influences on self-esteem, etc.) that increase in intensity, progressively harming the patient.
- Unsuccessful attempts to reduce and control use: it is frequent that the patient, before formally seeking help from the psychologist or services, has tried on his own to reduce the use or “control” it. Generally, a phase is observed, in which the patient is firmly convinced that he can limit his conduct on his own by creating a mode of use that can be reconciled (but only ideally) with the rest of his life, commitments, and duties.
- Expenditure of time: when the disorder is established, or being established, a criterion to look at is that of the time that the patient devotes to research, use, or recovery from the effects of the substance. The more the addiction is over, the greater the time that is dedicated to the substance in a day, until it becomes the only activity present in the most serious cases.
- Loss of control over use: The pathological behavior of use of the substance tends to occur, despite the negative consequences that it has brought over time and person’s awareness of it (the behavior of use becomes “compulsive”).
- Continuous use despite the awareness that the drug is a problem: many patients do not stop, even in the face of the onset of serious health risks or clear family crises.
- Recurrent use with the inability to fulfill their duties: many patients lose their jobs due to drug intake, interrupt the course of study, or become unable to perform their family or parental duties.
- Use in situations at risk: over time, the ability to estimate the risk associated with hiring is progressively reduced; becoming compulsive assumptions, it can happen to feel “forced” to make abuse, despite having to drive or perform precision tasks that cannot be “rationally” reconciled with the state of alteration given by the substances of abuse.
- Recurrent use despite this causes social or interpersonal problems: As previously stated, drug use becomes salient, even to the detriment of one’s effective relationships.
- Craving: urgent desire for the substance.
- a higher relapse rate;
- more frequent hospitalizations;
- more likely to commit crimes and end up in prison;
- higher risk of contracting infectious diseases, such as HIV and hepatitis.
2. Materials and Methods
2.1. Ethical Consideration
2.2. Protocol
2.3. Statistical Analysis
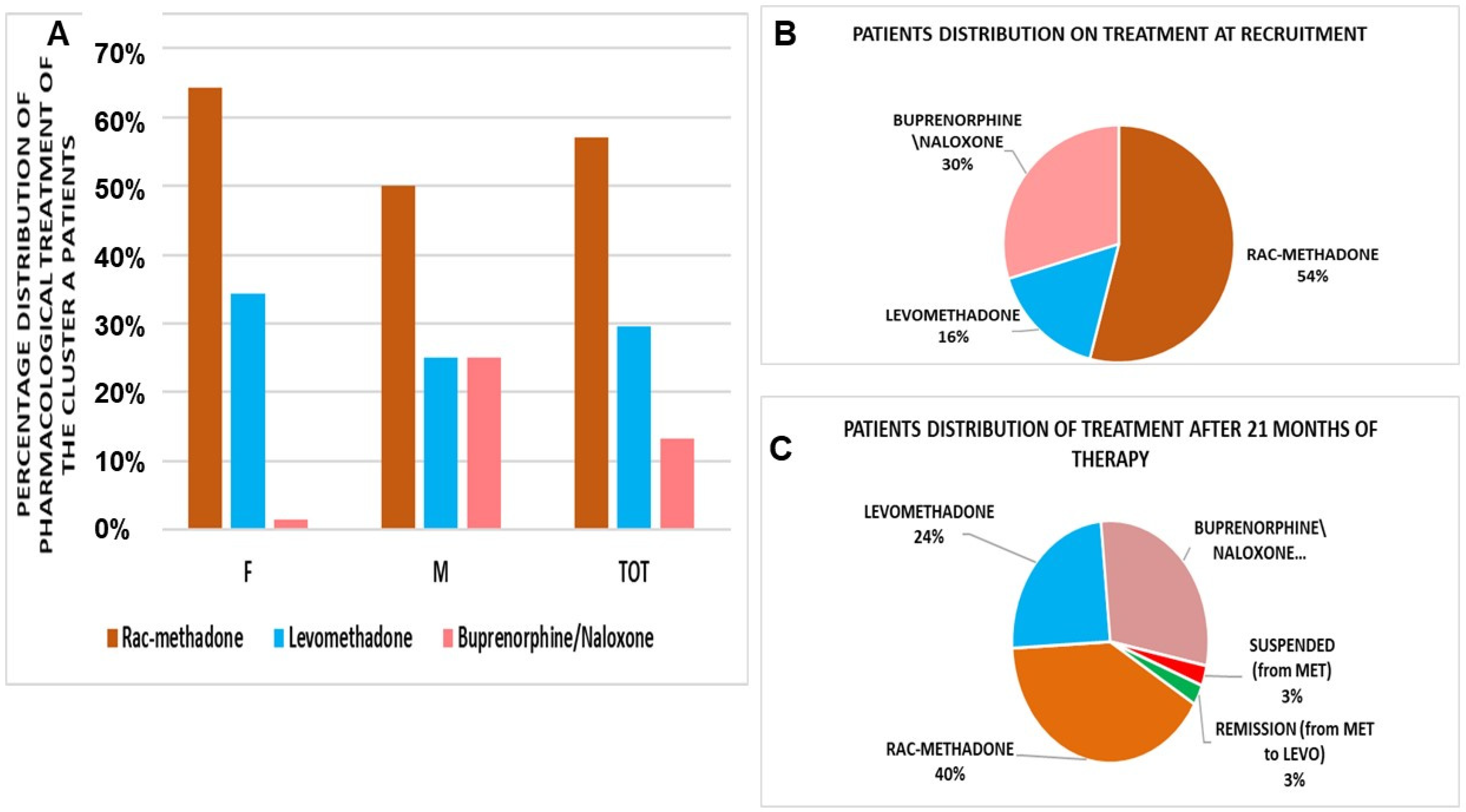
3. Results
- One patient (woman) switched positively from rac-methadone to levomethadone, until remission;
- One patient (woman) receiving rac-methadone discontinued therapy;
- Four patients (men) switched from rac-methadone to levomethadone;
- One patient (men) switched from levomethadone to rac-methadone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- World Drug Report 2021 (Sales No. E.21.XI.8). Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html (accessed on 7 December 2021).
- Osservatorio Europeo Delle Droghe e Delle Tossicodipendenze. 2021. Available online: https://www.emcdda.europa.eu/system/files/publications/13838/2021.2256_IT_02_.pdf (accessed on 7 December 2021).
- Relazione Annuale al Parlamento sul Fenomeno Delle Tossicodipendenze in Italia, Presidenza Consiglio dei Ministri, Curata dal Dipartimento Politiche Antidroga con il Supporto Tecnico dell’Istituto di Fisiologia Clinica del CNR. Available online: https://www.iss.it/dipendenze/-/asset_publisher/zwfXwoiZC6zu/content/id/5609921 (accessed on 7 December 2021).
- Regier, D.A.; Farmer, M.E.; Rae, D.S.; Locke, B.Z.; Keith, S.J.; Judd, L.L.; Goodwin, F.K. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990, 264, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Bakken, K.; Landheim, A.S.; Vaglum, P. Primary and secondary substance misusers: Do they differ in substance-induced and substance-independent mental disorders? Alcohol Alcohol. 2003, 38, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, D.; Kaczor, A.A.; Matosiuk, D. Interplay between Two Allosteric Sites and Their Influence on Agonist Binding in Human μ Opioid Receptor. J. Chem. Inf. Model. 2016, 56, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Livingston, K.E.; Traynor, J.R. Disruption of the Na+ ion binding site as a mechanism for positive allosteric modulation of the mu-opioid receptor. Proc. Natl. Acad. Sci. USA 2014, 111, 18369–18374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, R.J.; Au, J.D.; Paul, M.; Liu, C.; Yost, C.S. Functional inhibition by methadone of N-methyl-D-aspartate receptors expressed in Xenopus oocytes: Stereospecific and subunit effects. Anesth. Analg. 2004, 98, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Williams, J.T. Activation of µ-opioid receptors and block of Kir3 potassium channels and NMDA receptor conductance by L- and D-methadone in rat locus coeruleus. Br. J. Pharmacol. 2010, 161, 1403–1413. [Google Scholar] [CrossRef]
- Ferrari, A.; Coccia, C.P.; Bertolini, A.; Sternieri, E. Methadone--metabolism, pharmacokinetics and interactions. Pharmacol. Res. 2004, 50, 551–559. [Google Scholar] [CrossRef]
- Fonseca, F.; Torrens, M. Pharmacogenetics of Methadone Response. Mol. Diagn. Ther. 2018, 22, 57–78. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-M.; Chang, C.-C.; Wang, J.D.; Chang, K.C.; Ting, S.-Y.; Lin, C.-Y. Negative impacts of self-stigma on the quality of life of patients in methadone maintenance treatment: The mediated roles of psychological distress and social functioning. Int. J. Environ. Res. Public Health 2019, 16, 1299. [Google Scholar] [CrossRef] [Green Version]
- United Nations. United Nations Office on Drugs and Crime; World Drug Report; United Nations: San Francisco, CA, USA, 2018; Volume 2, pp. 85–86. Available online: https://www.unodc.org/wdr2018/ (accessed on 28 January 2022).
- Guillery, S.P.E.; Hellweg, R.; Kronenberg, G.; Bohr, U.; Kunte, H.; Enge, S. Quality of Life in Opioid Replacement Therapy: A Naturalistic Cross-Sectional Comparison of Methadone/Levomethadone, Buprenorphine, and Diamorphine Patients. Eur. Addict. Res. 2021, 27, 371–380. [Google Scholar] [CrossRef]
- United Nation. United Nations Office for Drug Control and Crime Prevention; United Nation Publication: New York, NY, USA, 2000; Available online: https://www.unodc.org/ (accessed on 28 January 2022).
- Dyer, K.R.; White, J.M. Patterns of symptom complaints in methadone maintenance patients. Addiction 1997, 92, 1445–1455. [Google Scholar] [CrossRef]
- De Vos, J.W.; Ufkes, J.G.; Kaplan, C.D.; Tursch, M.; Krause, J.K.; van Wilgenburg, H.; Woodcock, B.G.; Staib, A.H. L-Methadone and D,L-methadone in methadone maintenance treatment: A comparison of therapeutic effectiveness and plasma concentrations. Eur. Addict. Res. 1998, 4, 134–141. [Google Scholar] [CrossRef]
- Cowan, A.; Lewis, J.W.; Macfarlane, I.R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br. J. Pharmacol. 1977, 60, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Tejwani, G.A.; Rattan, A.K. The role of spinal opioid receptors in antinociceptive effects produced by intrathecal administration of hydromorphone and buprenorphine in the rat. Anesth. Analg. 2002, 94, 1542–1546. [Google Scholar] [CrossRef]
- Pfeiffer, A.; Brantl, V.; Herz, A.; Emrich, H.M. Psychotomimesis mediated by kappa opiate receptors. Science 1986, 233, 774–776. [Google Scholar] [CrossRef]
- Leander, J.D. Buprenorphine has potent kappa opioid receptor antagonist activity. Neuropharmacology 1987, 26, 1445–1447. [Google Scholar] [CrossRef]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014, 2, CD002207. [Google Scholar] [CrossRef]
- Maremmani, I.; Rolland, B.; Somaini, L.; Roncero, C.; Reimer, J.; Wright, N.; Littlewood, R.; Krajci, P.; Alho, H.; D’Agnone, O.; et al. Buprenorphine dosing choices in specific populations: Review of expert opinion. Expert. Opin. Pharmacother. 2016, 17, 1727–1731. [Google Scholar] [CrossRef]
- Mitchell, T.B.; Dyer, K.R.; Newcombe, D.; Salter, A.; Somogyi, A.A.; Bochner, F.; White, J.M. Subjective and physiological responses among racemic-methadone maintenance patients in relation to relative (S)- vs. (R)-methadone exposure. Br. J. Clin. Pharmacol. 2004, 58, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Ansermot, N.; Albayrak, O.; Schläpfer, J.; Crettol, S.; Croquette-Krokar, M.; Bourquin, M.; Déglon, J.J.; Faouzi, M.; Scherbaum, N.; Eap, C.B. Substitution of (R,S)-methadone by (R)-methadone: Impact on QTc interval. Arch. Intern. Med. 2010, 170, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Askari, M.S.; Martins, S.S.; Mauro, P.M. Medication for opioid use disorder treatment and specialty outpatient substance use treatment outcomes: Differences in retention and completion among opioid-related discharges in 2016. J. Subst. Abuse Treat. 2020, 114, 108028. [Google Scholar] [CrossRef]
- Dydyk, A.M.; Jain, N.K.; Gupta, M. Opioid Use Disorder. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553166/ (accessed on 12 July 2021).
- Taranto, A.; Brizzi, G.; Fabio, G.; Dellabona, T.; Cuzzola, D.; Casto, G.; Mammana, G.; Catalucci, F.; De Fazio, S.; Ariano, V.; et al. Interazioni Farmaco-Recettore Stereo-Selettive del Metadone e Cinetica Stereo-Selettiva di Eliminazione: Possibile Ruolo Nella Riduzione delle Reazioni Avverse nella Terapia di Mantenimento Metadonico; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Frank, F.; Mateu-Gelabert, P.; Guarino, H. “It’s like ‘liquid handcuffs”: The effects of take-home dosing policies on Methadone Maintenance Treatment (MMT) patients’ lives. Harm Reduct. J. 2021, 18, 88. [Google Scholar] [CrossRef]
- Figgatt, M.C.; Salazar, Z.; Day, E.; Vincent, L.; Dasgupta, N. Take-home dosing experiences among persons receiving methadone maintenance treatment during COVID-19. J. Subst. Abuse Treat. 2021, 123, 108276. [Google Scholar] [CrossRef]
- Amram, O.; Amiri, S.; Panwala, V.; Lutz, R.; Joudrey, P.J.; Socias, E. The impact of relaxation of methadone take-home protocols on treatment outcomes in the COVID-19 era. Am. J. Drug Alcohol. Abuse 2021, 47, 722–729. [Google Scholar] [CrossRef]
- Brothers, S.; Viera, A.; Heimer, R. Changes in methadone program practices and fatal methadone overdose rates in Connecticut during COVID-19. J. Subst. Abuse Treat. 2021, 131, 108449. [Google Scholar] [CrossRef]
- Soyka, M. Transition From Full Mu Opioid Agonists to Buprenorphine in Opioid Dependent Patients—A Critical Review. Front. Pharmacol. 2021, 12, 718811. [Google Scholar] [CrossRef]
- Mahon, D. Improving retention in opioid treatment. J. Addict. Addictv. Disord. 2020, 7, 47. [Google Scholar] [CrossRef]
- Beetham, T.; Saloner, B.; Gaye, M.; Wakeman, S.E.; Frank, R.G.; Barnett, M.L. Therapies offered at residential addiction treatment programs in the united states. JAMA 2020, 324, 804–806. [Google Scholar] [CrossRef]
- Guerrero, E.; Amaro, H.; Kong, Y.; Khachikian, T.; Marsh, J.C. Gender disparities in opioid treatment progress in methadone versus counseling. Subst. Abuse Treat Prev. Policy 2021, 16, 52. [Google Scholar] [CrossRef]
- Deutsch-Link, S.; Belcher, A.M.; Massey, E.; Cole, T.O.; Wagner, M.A.; Billing, A.S.; Greenblatt, A.D.; Eric Weintraub, E.; Wish, E.D. Race-based differences in drug use prior to onset of opioid use disorder. J. Ethn. Subst. Abuse 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.; Burr, N.E.; Brenner, D.M.; Ford, A.C. Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: Systematic review and network meta-analysis. Gut 2019, 68, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Carroll, K.M.; Petrakis, I.L.; Sofuoglu, M. Buprenorphine treatment for opioid use disorder: Recent progress. Expert. Rev. Clin. Pharmacol. 2019, 12, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Lintzeris, N.; Dunlop, A.J.; Haber, P.S.; Lubman, D.I.; Graham, R.; Hutchinson, S.; Arunogiri, S.; Hayes, V.; Hjelmström, P.; Svedberg, A.; et al. Patient-Reported Outcomes of Treatment of Opioid Dependence With Weekly and Monthly Subcutaneous Depot vs Daily Sublingual Buprenorphine: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e219041. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Viswanath, O.; Saadabadi, A. Buprenorphine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA.
- Dunlop, A.; White, B.; Roberts, J.; Cretikos, M.; Attalla, D.; Ling, R.; Searles, A.; Mackson, J.; Doyle, M.; McEntyre, E.; et al. Treatment of opioid dependence with depot buprenorphine (CAM2038) in custodial settings. Addiction 2021. [Google Scholar] [CrossRef]
- Elman, I.; Howard, M.; Borodovsky, J.T.; Mysels, D.; Rott, D.; Borsook, D.; Albanese, A. Metabolic and Addiction Indices in Patients on Opioid Agonist Medication-Assisted Treatment: A Comparison of Buprenorphine and Methadone. Sci. Rep. 2020, 10, 5617. [Google Scholar] [CrossRef]






| Patients | Number of Patients | Male | Female | Age | Body Max Index (BMI) |
|---|---|---|---|---|---|
| Nationality: Italian Ethnicity: Caucasian Status: Living alone (32%), living with parents or partner (68%), employed (30%), un-employed (35%), temporary job (35%) | 266 | 212 | 54 | 44.80 ± 5.65 | 26.21 ± 3.83 |
| Cluster A | 211 | 171 | 40 | 44.71 ± 4.79 | 26.41 ± 3.00 |
| Cluster B | 37 | 27 | 10 | 43.54 ± 8.73 | 25.18 ± 3.16 |
| Cluster C | 18 | 14 | 4 | 48.44 ± 9.41 | 26.03 ± 4.94 |
| Patients | N Patients | Percentage of Patients | Age | BMI |
|---|---|---|---|---|
| Psychiatric: 20 ± 1.3% Infectious: 12 ± 5.9% Cardiovascular: 7 ± 5% Metabolic: 5 ± 0.9% | 211 | 100% | 44.17 ± 4.79 | 26.41 ± 3.00 |
| Male VAS = 32/100 | 171 | 81% | 44.41 ± 5.24 | 27.09 ± 4 |
| Female VAS = 31/100 | 40 | 19% | 43.16 ± 2.89 | 23.51 ± 5 |
| Gender | Treatments | Daily Dose (mg/day) |
|---|---|---|
| Female (19%) | Rac-methadone | 55 ± 10 mg/day |
| Levomethadone | 45.11 ± 6.5 mg/day | |
| Buprenorphine/naloxone | 4 ± 2.62 mg/day | |
| Male (81%) | Rac-methadone | 57.21 ± 10.88 mg/day |
| Levomethadone | 28.75 ± 7.5 mg/day *° | |
| Buprenorphine/naloxone | 8 ± 3.62 mg/day |
| Levomethadone N Patients = 62 | Rac-Methadone N patients = 121 | Buprenorphine/Naloxone N Patients = 28 | |
|---|---|---|---|
| Heroin | −53 ± 9% * | −41 ± 8% * | −42 ± 3.5% * |
| Cannabinoids | −48 ± 8% * | −32 ± 5% * | −49 ± 6% * |
| Cocaine | −37 ± 6% * | −35 ± 8% * | −36 ± 9% * |
| Recruitment | Levomethadone N Patients = 35 | Rac-Methadone N Patients = 60 | Buprenorphine/ Naloxone N Patients = 28 | |
|---|---|---|---|---|
| QTcF (ms) | 430.2 ± 12.4 | 425.1 ± 7.4 | 444.5 ± 9.2 | 428.1 ± 9.4 |
| Heart rate (bpm) | 75.23 ± 5.3 | 76.13 ± 7.3 | 77.13 ± 6.3 | 76.13 ± 7.3 |
| Systolic blood pressure (mmHg) | 122.5 ± 14 | 129.5 ± 13 | 127.5 ± 11 | 124.5 ± 12 |
| Diastolic blood pressure (mmHg) | 81.4 ± 3.3 | 79.4 ± 8.3 | 81.1 ± 8 | 79.1 ± 9 |
| After 180 Days | Levomethadone N Patients = 35 | Rac-Methadone N Patients = 60 | Buprenorphine/ Naloxone N Patients = 28 | |
| QTcF (ms) | 431.77 ± 8.83 | 426 ± 8.4 | 439.1 ± 7.1 | 430.2 ± 23 |
| Heart rate (bpm) | 78.17 ± 6.53 | 79.1 ± 9 | 78.1 ± 4.3 | 77.3 ± 6.3 |
| Systolic blood pressure (mmHg) | 124.9 ± 11 | 123.1 ± 15 | 128.1 ± 13 | 123.5 ± 9 |
| Diastolic blood pressure (mmHg) | 78.07 ± 10 | 77.1 ± 10 | 78.1 ± 9 | 79 ± 11 |
| Number of Patients | Percentage | Age± | BMI± | |
|---|---|---|---|---|
| Total number of patients | 37 | 100% | 43.54 ± 4 | 25.18 ± 3.16 |
| Male | 27 | 72.97% | 44.93 ± 6 | 25.25 ± 3.06 |
| Female | 10 | 27.03% | 39.80 ± 8 | 24.98 ± 3.27 |
| Residence conditions: | ||||
| Family of origin (with parents or high relatives) | 15 | |||
| Newly formed family (with spouse, partner, children, etc.) | 17 | |||
| Alone | 5 | |||
| Previous: | ||||
| Criminal record (reports, arrest, house arrest, prison, etc.) | 10 | |||
| Therapeutic communities | 10 | |||
| Both | 8 | |||
| None | 9 | |||
| Drugs at Recruitment | Medium Dose (mg/day) | Male Dose (mg/day) | Female Dose (mg/day) |
|---|---|---|---|
| Rac-methadone | 66.25 ± 30.82 | 71.15 ± 31.27 | 57.14 ± 27.76 |
| Levomethadone | 58.33 ± 31.58 | 58.33 ± 31.58 | No patients |
| Buprenorphine\naloxone | 8.27 ± 4.94 | 8.88 ± 5.18 | 6.67 ± 3.77 |
| Drugs after 21 Months | Male Dose (mg/day) | Female Dose (mg/day) | Medium Dose (mg/day) |
| Rac-methadone | 59.00 ± 31.42 | 55.50 ± 34.89 | 66.00 ± 21.31 |
| Levomethadone | 62.22 ± 24.28 | 62.22 ± 24.28 | No patients |
| Buprenorphine\naloxone | 7.00 ± 3.79 | 7.13 ± 3.79 | 6.61 ± 2.17 |
| Patients (N = 18) Age= 48.4 ± 10; BMI =26.0 ± 6.41 Dose | Rac-Methadone Dose (mg/day) (Number of Patients = 3) 97.5 ± 8 | Levomethadone Dose (mg/day) (Number of Patients = 10) 51.8 ± 5 | Buprenorphine/Naloxone Dose (mg/day) (Number of Patients = 3) 2.5 ± 1 | Buprenorphine Dose (mg/day) (Number of Patients = 2) 3 |
|---|---|---|---|---|
| Female (N = 4) Age = 49.75 ± 12 BMI = 25.09 ± 9 | 60 ± 4 | 65 ± 3 | 1 ± 0.4 | / |
| Male (N = 14) Age = 48.07 ± 8.4; BMI = 26.96 ± 3.7 | 135 ± 11 | 38.6 ± 4 | 4 ± 1 | 3 |
| Drugs | SCL90 Global Severity Index (GSI) ± | Positive Symptom Total (PST) | SCL90 Positive Symptom Distress Index (PSDI) |
|---|---|---|---|
| Rac-Methadone | 1.61 ± 0.3 | 64.5 ± 4 | 2.27 ± 0.1 |
| Levomethadone | 1.59 ± 0.7 | 61 ± 9 | 2.26 ± 1 |
| Buprenorphine/Naloxone | 0.67 ± 0.09 | 35.5 ± 8 | 1.7 ± 1 |
| Buprenorphine | 2.74 ± 0.9 | 78 ± 11 | 3.17 ± 0.8 |
| Levomethadone Detox | 0.81 ± 0.2 | 33 ± 8 | 2.21 ± 0.9 |
| Medical Conditions | Number of Patients | Maintenance Treatments | Concomitant Drugs |
|---|---|---|---|
| Total patients Female (N = 4) Age = 49.75 ± 12.84 BMI = 25.09 ± 9.05 Male (N = 14) Age= 48.07 ± 8.43 BMI = 26.96 ± 3.77 | 18 | Antidepressant, antipsycotic | |
| Blood pressure (BP) (systolic/diastolic) | |||
| 133.33 ± 21/83 ± 9 mmHg | 3 | Rac-methadone | |
| 115.1 ± 18/80 ± 5 | 10 | Levomethadone | |
| 128.2 ± 18/78 ± 6 | 3 | Buprenorphine/naloxone | |
| 130.6 ± 15/90 ± 4 | 2 | Buprenorphine | |
| 130.1 ± 11/80 ± 6 | 3 | Levomethadone detox | |
| HR/RR | |||
| 67.67 ± 10 bpm/ms | 3 | Rac-methadone | |
| 64.67 ± 6 | 10 | Levomethadone | |
| 80 ± 7 | 3 | Buprenorphine/naloxone | |
| 89.5 ± 8 | 2 | Buprenorphine | |
| 76 ± 9 | 3 | Levomethadone detox | |
| QTcF | |||
| 438.67 ± 7.57 ms | 3 | Rac-methadone | |
| 437.71 ± 40.6 | 10 | Levomethadone | |
| 432.33 ± 2.31 | 3 | Buprenorphine/naloxone | |
| 438 ± 43.84 | 2 | Buprenorphine | |
| 383 ± 35.53 | 3 | Levomethadone detox | |
| bipolar disorder Substance use disorder (DUS) BMI = 28.56 ± 4.92 BP= 120.00 ± 11/75 ± 8 mmHg QTcF = 418.40 ± 18.93 HR/RR = 69.60 ± 9 bpm/ms | 5 (4 of type 2 and 1 of type 1) | ||
| QTcF = 433 ± 12 ms, HR/RR = 74.5 ± 13 bpm/ms BP = 120 ± 11/75 ± 8 mmHg urine heroin, cocaine, amphetamine negative, one case of cannabinoid positivity; GSI 0.59, PST 30, PSDI 1.77, urine negative | 2 | Buprenorphine/naloxone 3.5 ± 1 mg/day | Sertalin, litium, quetiapine, flurazepam, bupropione, sodium valproate, alipiprazol |
| QTcF = 430 ms, HR/RR = 76 bpm/ms BP = 130/80 mmHg urine heroin, cocaine, amphetamine negative; GSI 1.63, PST 59, PSDI 2.49 | 1 | Rac-methadone 60 mg/day | Vortioxetine, aripripazole, prazepam, zolpidem |
| QTcF = 394 ms, HR/RR = 58 bpm/ms, HCV+, cannabinoids and cocaine+, cannabis and bzd abused; urine cannabis, cocaine+; GSI 0.93, PST 45, PSDI 1.87 | 1 | Levomethadone 80 mg/day | Aripiprazole, asenapine |
| QTcF = 402 ms, HR/RR = 65 bpm/ms, HCV+, urine heroin, cocaine, amphetamine negative; GSI/, PST/, PSDI/ | 1 | Levomethadone switched from rac-methadone to levomethadone 15 mg/day and after one year to low dose 3 mg/day of levomethadone | Aripiprazole, valproate, clorpromazine |
| Major depressive disorder Substance Use Disorder (DUS) BMI = 23.32 ± 8 BP = 134 ± 21/84.86 ± 12 mmHg QTcF = 426.80 ± 17.51 ms HR/RR = 71.20 ± 19 bpm/ms | 7 | ||
| QTcF = 427.5 ± 18 ms, HR/RR= 65 ± 9 bpm/ms, BP = 130 ± 11/94.96 ± 10 mmHg HCV + 1a urine heroin, cocaine, amphetamine, cannabinoids negatives, one case of codeine positivity GSI 1,92 ± 0.1, PST 69 ± 8, PSDI 2.46 ± 0.3 | 2 | Levomethadone 90 ± 9 mg/die | Duloxetine, valproate, levosulpiride, clonazepam, pregabalin |
| QTcF = 406 ms, HR/RR = 62 bpm/ms, BP = 130/94.96 mmHg HCV + , urine heroin, cocaine, amphetamine, cannabinoids negatives | 1 | Levomethadone starting dose 35 mg/day and then 3 mg/day until completed detox | Valproate, fluoxetine, olanzapine, levomepromazine, estazolam |
| QTcF = 443 ± 2 ms, HR/RR = 63.5 ± 9 bpm/ms, BP = 131 ± 13/91.86 ± 10 mmHg (N patient =1 HIV+, HCV +) urine heroin, cocaine, amphetamine, cannabinoids negatives One case of severe hepatopathies One case of positivity to benzodiazepine One case of GSI 1.59, PST 70, PSDI 2.04 | 2 | Rac-methadone 105 ± 12 mg/die | Valproate, sertalin, zolpidem, lithium, duloxetine, pregabalin, clonazepam ritonavir, atazanavir, emtricitabine, tenofovir, disoproxil |
| QTcF = 469 ms, HR/RR = 104 bpm/ms, BP = 129/90.81 mmHg, urine heroin, cocaine, amphetamine, cannabinoids negatives, GSI 2.74, PST 78, PSDI 3.17 | 1 | Buprenorfine 2 mg/die | Litium, olanzepine, levomepromazin, clonazepam |
| QTcF = 431 ms, HR/RR = 91 bpm/ms, BP = 128/89 mmHg, urine heroin, cocaine, amphetamine, cannabinoids negatives GSI 0.74, PST 41, PSDI 1.63 | 1 | Buprenorphine/naloxone 8 mg/die | Aripiprazole, valproate, topiramate, trazodone (ER) |
| Psychoses BMI 23.99 QTcF = 413 ms, HR/RR = 91 bpm/ms, BP = 110/70 mmHg urine heroin, amphetamine, cannabinoids negatives cocaine positive | 1 | Levomethadone 60 mg/day until completed detox | Duloxetina, trazodone, clonazepam, quetiapina, flurazepam |
| Schizophrenia BMI = 26.79 ± 6 | 3 | ||
| QTcF = 471.00 ± 36.51 ms, HR/RR = 67.5 ± 12 bpm/ms, BP = 116.5 ± 11/83.34 ± 8 mmHg One case of congenital LQT = 500 ms urine heroin, cocaine, amphetamine, cannabinoids negative, and two cases of cannabinoid positive and one case of cocaine positive GSI/, PST/, PSDI/ | 3 | Levomethadone 50 ± 4 mg/day and one case of rac-methadone 120 mg/die switch to levomethadone 80 mg/die | Risperidone, lurasidone, promazine, clonazepam, aloperidol, flurazepam, aripirazolo, sertralin |
| Schizo-affective disorder BMI = 34.68 BP= 140/90 mmHg QTcF = 360 ms, HR/RR = 90 bpm/ms, urine heroin, cocaine, amphetamine, cannabinoids negative, GSI 0.81, PST 33, PSDI 2.21. | 1 | Rac-methadone 60 mg/die and switch to levomethadone starting dose 30 mg/day and then 3 mg/day until completed detox | Valproate, levomepromazine, diazepam, biberidene, quetiapine, flurazepam |
| DUS cocaine BMI = 32.66 BP = 130/10 mmHg QTcF = 407 ms, HR/RR = 75 bpm/ms urine/ GSI/, PST/, PSDI/ | 1 | Buprenorphine 4 mg/die | Lithium carbonate, aripiprazole, clonazepam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqoud, F.; Fabio, G.; Ciliero, N.; Antonacci, M.; Mastrangelo, F.; Sammarruco, G.; Cataldini, R.; Schirosi, G.; De Fazio, S.; Tricarico, D. Multicenter Observational/Exploratory Study Addressed to the Evaluation of the Effectiveness and Safety of Pharmacological Therapy in Opioid-Dependent Patients in Maintenance Therapy in Southern Italy. Pharmaceutics 2022, 14, 461. https://doi.org/10.3390/pharmaceutics14020461
Maqoud F, Fabio G, Ciliero N, Antonacci M, Mastrangelo F, Sammarruco G, Cataldini R, Schirosi G, De Fazio S, Tricarico D. Multicenter Observational/Exploratory Study Addressed to the Evaluation of the Effectiveness and Safety of Pharmacological Therapy in Opioid-Dependent Patients in Maintenance Therapy in Southern Italy. Pharmaceutics. 2022; 14(2):461. https://doi.org/10.3390/pharmaceutics14020461
Chicago/Turabian StyleMaqoud, Fatima, Giada Fabio, Nunzio Ciliero, Marina Antonacci, Francesca Mastrangelo, Giorgio Sammarruco, Roberto Cataldini, Gabriella Schirosi, Salvatore De Fazio, and Domenico Tricarico. 2022. "Multicenter Observational/Exploratory Study Addressed to the Evaluation of the Effectiveness and Safety of Pharmacological Therapy in Opioid-Dependent Patients in Maintenance Therapy in Southern Italy" Pharmaceutics 14, no. 2: 461. https://doi.org/10.3390/pharmaceutics14020461
APA StyleMaqoud, F., Fabio, G., Ciliero, N., Antonacci, M., Mastrangelo, F., Sammarruco, G., Cataldini, R., Schirosi, G., De Fazio, S., & Tricarico, D. (2022). Multicenter Observational/Exploratory Study Addressed to the Evaluation of the Effectiveness and Safety of Pharmacological Therapy in Opioid-Dependent Patients in Maintenance Therapy in Southern Italy. Pharmaceutics, 14(2), 461. https://doi.org/10.3390/pharmaceutics14020461







