Iontophoresis of Biological Macromolecular Drugs
Abstract
:1. Introduction
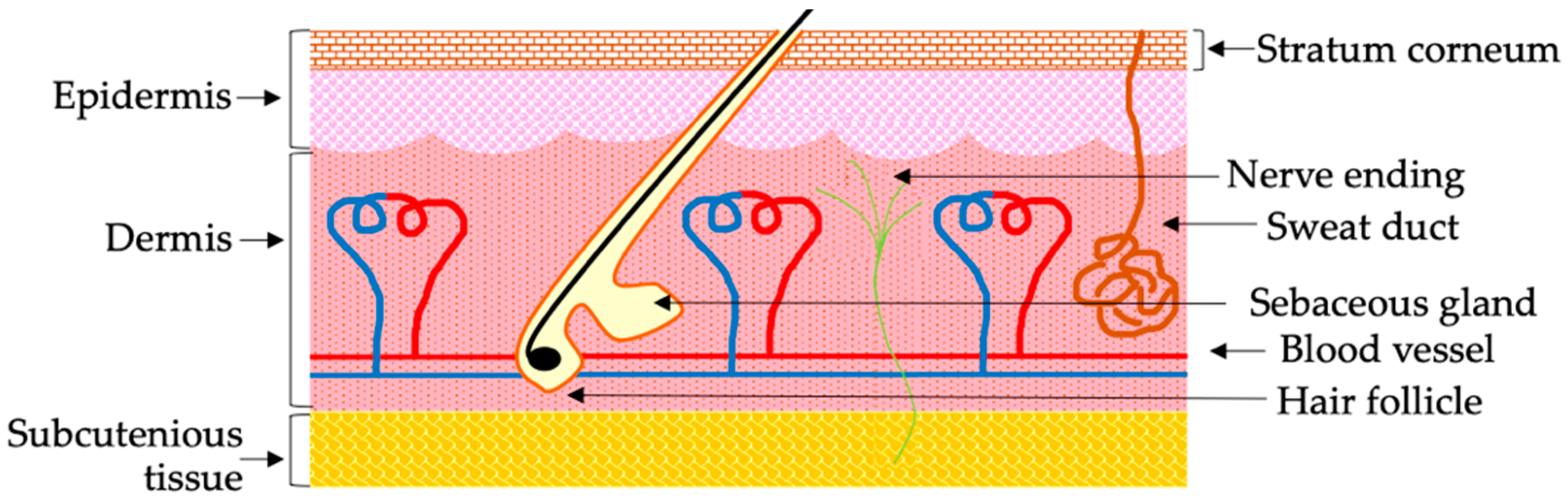
2. Challenges of the Noninvasive Transdermal Delivery of Biological Macromolecular Drugs
3. Prospects of IP for the Noninvasive Transdermal Delivery of Biological Macromolecules
4. Recent Advances in the IP-Mediated Transdermal Delivery of Biological Macromolecular Drugs
4.1. IP-Mediated Intradermal Delivery of siRNA in Skin with Atopic Dermatitis
4.2. IP-Mediated Transdermal Delivery of Biological Macromolecules for Cancer Immunotherapy
4.3. Targeting Psoriasis by the IP-Mediated Transdermal Delivery of Biological Macromolecular Drugs
4.4. IP-Mediated Transdermal Delivery of Cetuximab
4.5. IP-Mediated Transdermal Delivery of Biologically Active Human Basic Fibroblast Growth Factor (hbFGF)
4.6. Application of IP onto Internal Organs
5. Delivery of Biological Macromolecules by the Combined Application of IP and Other Permeation Techniques
6. Limitations of the IP-mediated Delivery of Biological Macromolecular Drugs
7. Clinical Status and Commercialization of the IP of Biological Macromolecular Drugs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA. Center for Biologics Evaluation and Research (CBER). What Are “biologics” Questions and Answers. 2018. Available online: https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers (accessed on 30 January 2022).
- Guo, Q.; Jiang, C. Delivery strategies for macromolecular drugs in cancer therapy. Acta Pharm. Sin. B 2020, 10, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Mullad, A. 2020 FDA drug approvals. Nat. Rev. Drug Discov. 2021, 20, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2018, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Jlinaro, R. Challenges to macromolecular drug delivery. Biochem. Soc. Trans. 2007, 35, 41–43. [Google Scholar]
- Belting, M.; Wittrup, A. Macromolecular drug delivery: Basic principles and therapeutic applications. Mol. Biotechnol. 2009, 43, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N.; Healy, C.E.A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar] [CrossRef]
- Smith, P.L.; Wall, D.A.; Gochoco, C.H.; Wilson, G. (D) Routes of delivery: Case studies: (5) Oral absorption of peptides and proteins. Adv. Drug Deliv. Rev. 1992, 8, 253–290. [Google Scholar] [CrossRef]
- Chung, S.W.; Hil-Lal, T.A.; Byun, Y. Strategies for non-invasive delivery of biologics. J. Drug Target. 2012, 20, 481–501. [Google Scholar] [CrossRef]
- Miller, M.A.; Pisani, E. The cost of unsafe injections. Bull. World Health Organ. 1999, 77, 808–811. [Google Scholar]
- Gill, H.S.; Prausnitz, M.R. Does needle size matter? J. Diabetes Sci. Technol. 2007, 1, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Norman, J.J.; Prausnitz, M.R. Improving patient acceptance of insulin therapy by improving needle design. J. Diabetes Sci. Technol. 2012, 6, 336–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrouk, M.; Nakamura, M.; Zhu, T.H.; Farahnik, B.; Singh, R.K.; Lee, K.M.; Jose, V.M.; Koo, J.; Bhutani, T.; Liao, W. The patient’s guide to psoriasis treatment. Part 3: Biologic injectables. Dermatol. Ther. 2016, 6, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrer, J.; Lupo, N.; Bernkop-Schnürch, A. Advanced formulations for intranasal delivery of biologics. Int. J. Pharm. 2018, 553, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Nicolini, M.; Morales, J.O. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech 2016, 18, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Senel, S.; Mremer, M.; Nagy, K.; Squire, C. Delivery of bioactive peptides and proteins across oral (buccal) mucosa. Curr. Pharm. Biotechnol. 2001, 2, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Pan, H.W.; Vllasaliu, D.; Lam, J.K.W. Pulmonary delivery of biological drugs. Pharmaceutics 2020, 12, 1025. [Google Scholar] [CrossRef]
- Morales, J.O.; Fathe, K.R.; Brunaugh, A.; Rerrati, S.; Li, S.; Montenegro-Nicolini, M.; Mousavikhamene, Z.; McConville, J.T.; Prausnitz, M.R.; Smyth, H.D.C. Challenges and future prospects for the delivery of biologics: Oral mucosal, pulmonary, and transdermal routes. AAPS J. 2017, 19, 652–668. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Bakshi, P.; Vora, D.; Hemmady, K.; Banga, A. Iontophoretic skin delivery systems: Success and failures. Int. J. Pharm. 2020, 586, 119584. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Vengurlekar, S.; Rakesh, B.; Jain, S.; Srikarti, G. Transdermal delivery by iontophoresis. Indian J. Pharm. Sci. 2008, 70, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocki, A.B. Skin anatomy, physiology, and pathophysiology. Nurs. Clin. N. Am. 1999, 34, 777–797. [Google Scholar]
- Matsui, T.; Amagai, M. Dissecting the formation, structure, and barrier function of stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed]
- Toll, R.; Jacobi, U.; Richter, H.; Laddermann, J.; Schaefer, H.; Blume-Peytavi, U. Penetration profile of microspheres in follic-ular targeting of terminal hair follicles. J. Investig. Dermatol. 2004, 123, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Kajimoto, K.; Yamamoto, M.; Watanabe, M.; Kigasawa, K.; Kanamura, K.; Harashima, H.; Kogure, K. Noninvasive and per-sistence transfollicular drug delivery system using a combination of liposome and iontophoresis. Int. J. Pharm. 2011, 403, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Peña-Juárez, M.C.; Guadarrama-Escobar, O.R.; Escobar-Chávez, J.J. Transdermal Delivery Systems for Biomolecules. J. Pharm. Innov. 2021, 1–14. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Bozena, M.K. Effects of solvents and penetration enhancers on transdermal delivery of thymoquinone: Permeation and skin deposition study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as permeation enhancers in transdermal drug delivery. J. Pharm. Bioallied. Sci. 2012, 4, 2–9. [Google Scholar]
- Mandal, A.; Kumbhojkar, N.; Reilly, C.; Dharamdasani, V.; Ukidve, A.; Ingber, D.E.; Mitragotri, S. Treatment of psoriasis with NFKBIZ siRNA using topical ionic liquid formulations. Sci. Adv. 2020, 6, eabb6049. [Google Scholar] [CrossRef]
- Ashtikar, M.; Nagarsekar, K.; Fahr, A. Transdermal delivery from liposomes formulations: Evaluation of technology over the last three decades. J. Control. Release 2016, 242, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Venuganti, V.V.K.; Perumal, O.P. Effect of poly(amidoamine) (PAMAM) dendrimer on skin permeation of 5-fluorouracil. Int. J. Pharm. 2008, 361, 230–238. [Google Scholar] [CrossRef]
- Wang, Y.; Su, W.; Li, Q.; Li, C.; Wang, H.; Li, Y.; Cao, Y.; Chang, J.; Zhang, L. Preparation and evaluation of lidocaine hy-drochloride-loaded TAT-conjugated polymeric liposomes for transdermal delivery. Int. J. Pharm. 2013, 441, 748–756. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Lasch, J.; Laub, R.; Wohlrab, W. How deep do intact liposomes penetrate into human skin? J. Control. Release 1992, 18, 55–58. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Ita, K. Transdermal iontophoretic drug delivery: Advances and challenges. J. Drug Target. 2015, 24, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Perspectives on Transdermal Electroporation. Pharmaceutics 2016, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daradhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systemic review. Drug Dev. Ind. Pharm. 2018, 45, 188–201. [Google Scholar] [CrossRef]
- Simmons, J.A.; Davis, J.; Thomas, J.; Lopez, J.; Le Blanc, A.; Allison, H.; Slook, H.; Lewis, P.; Holtz, J.; Fisher, P.; et al. Characterization of skin blebs from intradermal jet injection: Ex-vivo studies. J. Control. Release 2019, 307, 200–210. [Google Scholar] [CrossRef]
- Szunerists, S.; Boukherroub, R. Heat: A highly efficient skin enhancer for transdermal drug delivery. Front. Bioeng. Biotechnol. 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Fukuta, T.; Oshima, Y.; Michiue, K.; Tanaka, D.; Kogure, K. Non-invasive delivery of biological macromolecular drugs into the skin by iontophoresis and its application to psoriasis treatment. J. Control. Release 2020, 323, 323–332. [Google Scholar] [CrossRef]
- Rinat, L.Z.; Galya, L.; Einar, E.; Tal, M.; Aviv, B.; Moamen, M.; Ramesh, C.; Etili, H.; Riki, G.; Tamar, T.; et al. Ultrasound targeting of Q-starch/miR-197 complexes for topical treatment of psoriasis. J. Control. Release 2018, 284, 103–111. [Google Scholar]
- Lee, W.R.; Lin, Y.K.; Alalaiwe, A.; Wang, P.W.; Liu, P.Y.; Fang, J.Y. Fractional laser-mediated siRNA delivery for mitigating psoriasis-like lesions via IL-6 silencing. Nucleic Acids 2020, 19, 240–251. [Google Scholar] [CrossRef]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in der-mal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Kalia, Y.N.; Naik, A.; Garrison, J.; Guy, R. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 619–658. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Khatun, A.; Fukuta, T.; Kogure, K. Noninvasive transdermal delivery of liposomes by weak electric current. Adv. Drug Deliv. Rev. 2020, 154-155, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Subramony, J.A.; Sharma, A.; Phipps, J. Microprocessor controlled transdermal drug delivery. Int. J. Pharm. 2006, 317, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Alberti, I.; Lapteva, M.; Kalia, Y.N. Next generation intra- and transdermal therapeutic systems: Using non- and minimally-invasive technologies to increase drug delivery into and across the skin. Eur. J. Pharm. Sci. 2013, 50, 609–622. [Google Scholar] [CrossRef]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.W. Electrical, magnetic photomechanical and cavitational waves to overcome skin barrier for transdermal drug de-livery. J. Control. Release 2014, 193, 257–269. [Google Scholar] [CrossRef]
- Roustit, M.; Blaise, S.; Cracowski, J.-L. Trials and tribulations of skin iontophoresis in therapeutics. Br. J. Clin. Pharmacol. 2015, 77, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Sieg, A.; Guy, R.; Delgado-Charro, M.B. Electroosmosis in transdermal iontophoresis: Implications for noninvasive and calibration-free glucose monitoring. Biophys. J. 2004, 87, 3344–3350. [Google Scholar] [CrossRef]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001, 46, 281–305. [Google Scholar] [CrossRef]
- Curdy, C.; Kalia, Y.N.; Guy, R.H. Post-iontophoresis recovery of human skin impedance in vivo. Eur. J. Pharm. Biopharm. 2002, 53, 15–21. [Google Scholar] [CrossRef]
- Hama, S.; Kimura, Y.; Mikami, A.; Shiota, K.; Toyoda, M.; Tamura, A.; Nagasaki, Y.; Kanamura, K.; Kajimoto, K.; Kogure, K. Electric stimulus opens intracellular space in skin. J. Biol. Chem. 2014, 289, 2450–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.; Nishimoto, A.; Ohgita, T.; Hama, S.; Kashida, H.; Asanuma, H.; Kogure, K. Faint electric treatment-induced rapid and efficient delivery of extraneous hydrophilic molecules into the cytoplasm. J. Control. Release 2016, 228, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Tarashima, N.; Fujikawa, K.; Ohgita, T.; Hama, S.; Tanaka, T.; Saito, H.; Minakawa, N.; Kogure, K. The novel functional nucleic acid iRed effectively regulates target genes following cytoplasmic delivery by faint electric treatment. Sci. Technol. Adv. Mater. 2016, 17, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Hama, S.; Kogure, K. Low electric treatment activates rho gtpase via heat shock protein 90 and protein kinase c for intracellular delivery of siRNA. Sci. Rep. 2019, 9, 4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torao, T.; Mimura, M.; Oshima, Y.; Fujikawa, K.; Hasan, M.; Shimokawa, T.; Yamazaki, N.; Ando, H.; Ishida, T.; Fukuta, T.; et al. Characteristics of unique endocytosis induced by weak current for cytoplasmic drug delivery. Int. J. Pharm. 2019, 576, 119010. [Google Scholar] [CrossRef]
- Fabrowski, P.; Necakov, A.S.; Mumbauer, S.; Loeser, E.; Reversi, A.; Streichan, S.; Briggs, J.A.G.; Renzis, S.D. Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila. Nat. Commun. 2013, 4, 2244. [Google Scholar] [CrossRef] [Green Version]
- Banga, A.K.; Bose, S.; Ghosh, T.K. Iontophoresis and electroporation: Comparisons and contrasts. Int. J. Pharm. 1999, 179, 1–19. [Google Scholar] [CrossRef]
- Lauer, A.C.; Lieb, L.M.; Ramachandran, C.; Flynn, G.L.; Weiner, N.D. Transfollicular Drug Delivery. Pharm. Res. 1995, 12, 179–186. [Google Scholar] [CrossRef]
- Hinsberg, W.H.C.; Verhoef, J.C.; Bax, L.J.; Junginger, H.E.; Bodde, H.E. Role of appendages in skin resistance and iontophoretic peptide flux: Human versus snake skin. Pharm. Res. 1995, 12, 1506–1512. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Electrobiochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Kigasawa, K.; Kajimoto, K.; Hama, S.; Saito, A.; Kanamura, K.; Kogure, K. Noninvasive delivery of siRNA into the epidermis by iontophoresis using an atopic dermatitis-like model rat. Int. J. Pharm. 2010, 383, 157–160. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuefermann, P.; Baumgarten, G.; Koch, A.; Schwederski, M.; Velten, M.; Ehrentraut, H.; Mersmann, J.; Meyer, R.; Hoeft, A.; Zacharowski, K.; et al. CpG oligonucleotide activates Toll-like receptor 9 and causes lung inflammation in vivo. Respir. Res. 2007, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashem, S.; Haniffa, M.; Kaplan, D.H. Antigen-Presenting cells in the skin. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Kigasawa, K.; Kajimoto, K.; Nakamura, T.; Hama, S.; Kanamura, K.; Harashima, H.; Kogure, K. Noninvasive and efficient transdermal delivery of CPG-oligodeoxynucleotide for cancer immune therapy. J. Control. Release 2011, 150, 256–265. [Google Scholar] [CrossRef]
- Toyoda, M.; Hama, S.; Ikeda, Y.; Nagasaki, Y.; Kogure, K. Anti-cancer vaccination by transdermal delivery of antigen peptide-loaded nanogels via iontophoresis. Int. J. Pharm. 2015, 483, 110–114. [Google Scholar] [CrossRef]
- Hay, R.; Augustin, M.; Griffiths, C.; Sterry, W. The global challenge for skin health. Br. J. Dermatol. 2015, 172, 1469–1472. [Google Scholar] [CrossRef] [Green Version]
- Shang-Hung, L.; Hung-Yi, C.; Ji-Chen, H.; Chih-Hung, L.; Chang-Chun, H. Treatment with TNF- α inhibitor rectifies M1 macrophages polarization from blood CD14+ monocytes in patents with psoriasis independents of STAT1 and IRF-1 activation. J. Dermatol. Sci. 2018, 91, 276–284. [Google Scholar]
- Rachael, A.C.; Thomas, S.K. Misbehaving macrophages in the pathogeneses of psoriasis. J. Clin. Investig. 2006, 116, 2084–2087. [Google Scholar]
- Mizutani, H.; Ohmoto, Y.; Mzutani, T.; Murata, M.; Shimizu, M. Role of increased production of monocytes TNF-alpha, IL-1beta, IL-6 in psoriasis: Relation to focal infection, disease activity and responses to treatments. J. Dermatol. Sci. 1997, 14, 145–153. [Google Scholar] [CrossRef]
- Whan, B.K.; Dana, J.; Jensen, Y. Diagnosis and management of psoriasis. Can. Pharm. Physician 2017, 63, 278–285. [Google Scholar]
- Thappa, D.M.; Malathi, M. Topical therapy of psoriasis: Where we stand? J. Postgrad. Med. 2017, 63, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.; Horn, E.J.; Balkrishnan, R.; Basra, M.K.; Finlay, A.Y.; McCoy, D.; Menter, A.; Van De Kerkhof, P.C. Psoriasis: Improving adherence to topical therapy. J. Am. Acad. Dermatol. 2008, 59, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Jyotisterna, M.; Bharat, B.M. Intramatricial injections for nail psoriasis: An open label comparative study of triamcinolone, methotrexate, and cyclosporine. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 419–423. [Google Scholar]
- Lebwohl, M.; Drake, L.; Menter, A.; Koo, J.; Gottlieb, A.B.; Zanolli, M.; Young, M.; McClelland, P. Consensus conference: Acitretin in combination with UVB or PUVA in the treatment of psoriasis. J. Am. Acad. Dermatol. 2001, 45, 544–553. [Google Scholar] [CrossRef]
- Leon, H.K.; James, Q.D.R. Anti-TNF agents for the treatment of psoriasis. J. Drugs Dermatol. 2009, 8, 546–549. [Google Scholar]
- Mease, P.J.; Goffe, B.S.; Metz, J.; VanderStoep, A.; Finck, B.; Burge, D.J. Etanercept in the treatment of psoriatic arthritis and psoriasis: A randomized trial. Lancet 2000, 356, 385–390. [Google Scholar] [CrossRef]
- Fukuta, T.; Tanaka, D.; Inoue, S.; Michuue, K.; Kogure, K. Overcoming thickened pathological skin in psoriasis via iontophoresis combined with tight junction-opening peptide AT1002 foe intradermal delivery of NF-kB decoy oligodeoxynucleotide. Int. J. Pharm. 2021, 602, 120601. [Google Scholar] [CrossRef]
- Uchida, T.; Kanazawa, T.; Takashima, Y.; Okada, H. Development of an efficient transdermal delivery system of small inter-fering RNA using functional peptides, Tat and AT-1002. Chem. Pharm. Bull. 2011, 59, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Kallini, J.R.; Hamed, N.; Khachemoune, A. Squamous cell carcinoma of the skin: Epidemiology, classification, management, and novel trends. Int. J. Dermatol. 2015, 54, 130–140. [Google Scholar] [CrossRef]
- Ozanne, B.; Richards, C.S.; Hendler, F.; Burns, D.; Gusterson, B. Overexpression of the EGF receptor is a hallmark of squamous cell carcinomas. J. Pathol. 1986, 149, 9–14. [Google Scholar] [CrossRef]
- Vincenzi, B.; Zoccoli, A.; Pantano, F.; Venditti, O.; Galluzzo, S. CETUXIMAB: From Bench to Bedside. Curr. Cancer Drug Targets 2010, 10, 80–95. [Google Scholar] [CrossRef]
- Lapteva, M.; Sallam, M.A.; Goyon, A.; Guillarme, D.; Veuthey, J.-L.; Kalia, Y.N. Non-invasive targeted iontophoretic delivery of cetuximab to skin. Expert Opin. Drug Deliv. 2020, 17, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Zhu, Y.T.; Ding, J.; Zhou, F.Y.; Xue, J.X.; Jung, J.H.; Li, Z.J.; Gao, W.Y. Distribution of fibroblast growth factors and their roles in skin fibroblast cell migration. Mol. Med. Rep. 2016, 14, 3336–3342. [Google Scholar] [CrossRef]
- Yamanaka, K.-I.; Inaba, T.; Nomura, E.; Hurwitz, D.; Jones, D.A.; Hakamada, A.; Isoda, K.; Kupper, T.S.; Mizutani, H. Basic fibroblast growth factor treatment for skin ulcerations in scleroderma. Cutis 2005, 76, 373–376. [Google Scholar] [PubMed]
- Hayashida, K.; Fujioka, M.; Morooka, S.; Saijo, H.; Akita, S. Effectiveness of basic fibroblast growth factor for pediatric hand burns. J. Tissue Viability 2016, 25, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Perozzo, R.; Scapozza, L.; Kalia, Y.N. Noninvasive transdermal iontophoretic delivery of biologically active hu-man basic fibroblast growth factor. Mol. Pharm. 2011, 4, 1322–1331. [Google Scholar] [CrossRef]
- Hasan, M.; Fukuta, T.; Inoue, S.; Mori, H.; Kagawa, M.; Kogure, K. Iontophoresis-mediated direct delivery of nucleic acid therapeutics, without use of carriers, to internal organs via non-blood circulatory pathways. J. Control. Release 2022, 343, 392–399. [Google Scholar] [CrossRef]
- Sato, Y.; Murase, K.; Kato, J.; Kobune, M.; Sato, T.; Kawano, Y.; Takimoto, R.; Takada, K.; Miyanishi, K.; Matsunaga, T.; et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 2008, 26, 431–442. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tsuchiya, H.; Hama, S.; Kajimoto, K.; Kogure, K. Resistin affects lipid metabolism during adipocyte maturation of 3T3-L1 cells. FEBS J. 2013, 280, 5884–5895. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Shi, Z.; Liu, X.; Tan, Y.; Wei, L.; Zhu, X.; Zhang, H.; Zhu, X.; Meng, X.; Ji, W.; et al. Acute elevated resistin exacerbates mitochondrial damage and aggravates liver steatosis through AMPK/PGC-1a signaling pathway in male NFLD mice. Horm. Metab. Res. 2021, 53, 132–144. [Google Scholar] [PubMed]
- Lam, S.; Cheng, K. Long term survival outcome of laparoscopic liver resection for hepatocellular carcinoma. World J. Gastrointest. Surg. 2021, 13, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.L.; Hogg, M.E. Current state of minimally invasive pancreatic surgery. J. Surg. Oncol. 2021, 123, 1370–1386. [Google Scholar] [CrossRef]
- Aljuffali, A.A.; Lin, Y.; Fang, J. Noninvasive approach for enhancing small interfering RNA delivery percutaneously. Expert Opin. Drug Deliv. 2016, 13, 265–280. [Google Scholar] [CrossRef]
- Dharamdasani, V.; Mandal, A.; Qi, Q.M.; Suzuki, I.; Bentley, M.V.L.B.; Mitragotri, S. Topical delivery of siRNA into skin using ionic liquids. J. Control. Release 2020, 323, 475–482. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Ye, R.; Zheng, Y.; Li, X.; Chen, Y.; Xie, X.; Jiang, L. Smartphone-powered iontophoresis-microneedle array patch for controlled transdermal delivery. Microsyst. Nanoeng. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Noh, G.; Keum, T.; Seo, J.; Bashyal, S.; Eum, N.; Kweon, M.J.; Lee, S.; Sohn, D.H.; Lee, S. Iontophoretic Transdermal Delivery of Human Growth Hormone (hGH) and the Combination Effect of a New Type Microneedle, Tappy Tok Tok®. Pharmaceutics 2018, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Kigasawa, K.; Miyashita, M.; Kajimoto, K.; Kanamura, K.; Harashima, H.; Kogure, K. Efficient transdermal delivery of superoxide dismutase using a combination of liposomes and iontophoresis against UV-induced skin damage. Biol. Pharm. Bull. 2012, 35, 781–785. [Google Scholar] [CrossRef] [Green Version]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective skin cancer treatment by topical co-delivery of curcumin and stat3 sirna using cationic liposomes. AAPS PharmSciTech 2017, 19, 166–175. [Google Scholar] [CrossRef]
- Liu, K.-C.; Green, C.R.; Alany, R.; Rupenthal, I.D. Synergistic effect of chemical penetration enhancer and iontophoresis on transappendageal transport of oligodeoxynucleotides. Int. J. Pharm. 2012, 441, 687–692. [Google Scholar] [CrossRef]
- Venuganti, V.V.K.; Saraswathy, M.; Dwivedi, C.; Kaushik, R.S.; Perumal, O.P. Topical gene silencing by iontophoretic delivery of an antisense oligonucleotide–dendrimer nanocomplex: The proof of concept in a skin cancer mouse model. Nanoscale 2014, 7, 3903–3914. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, N.K.; Dua, A. Iontophoresis Analgesic Medications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wang, Y.; Zeng, L.; Song, W.; Liu, J. Influencing factors and drug application of iontophoresis in transdermal drug delivery: An overview of recent progress. Drug Deliv. Transl. Res. 2021, 12, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chandrappa, N.K.A.; Ravikumar, B.C.; Rangegowda, S.M. Iontophoretic delivery of methotrexate in the treatment of palmar psoriasis: A randomized controlled study. Australas. J. Dermatol. 2020, 61, 140–146. [Google Scholar] [CrossRef]
- Gaillard-Bigot, F.; Roustit, M.; Blaise, S.; Cracowski, C.; Seinturier, C.; Imbert, B.; Carpentier, P.; Cracowski, J. Treprostinil iontophoresis improves digital blood flow during local cooling in systemic sclerosis. Microcirculation 2016, 23, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Korsten, M.A.; Lyons, B.L.; Radulovic, M.; Cummings, T.M.; Sikka, G.; Singh, K.; Hobson, J.C.; Sabiev, A.; Spungen, A.M.; Bauman, W.A. Delivery of neostigmine and glycopyrrolate by iontophoresis: A nonrandomized study in individuals with spinal cord injury. Spinal Cord 2017, 56, 212–217. [Google Scholar] [CrossRef]
- Yamada, M.; Prow, T.W. Physical drug delivery enhancement for aged skin, UV damaged skin and skin cancer: Translational and commercialization. Adv. Drug Deliv. Rev. 2020, 153, 2–17. [Google Scholar] [CrossRef]
| Methods & References | Driving Forces | Advantages | Disadvantages |
|---|---|---|---|
| Iontophoresis [42] | Weak electric current (<0.5 mA/cm2). |
|
|
| Electroporation [43] | High-voltage electric pulses (30–500 Volt) for micro to milli second. |
|
|
| Ultrasound [44] | High frequency ultrasound (0.7–3 MHz), low frequency ultrasound (20–100 kHz). |
|
|
| Microneedles [45] | Mechanically 100–1000 μm needles penetrate through the SC. |
|
|
| Pyro jet injector [46] | High velocity liquid jet (100–200 m/s). |
|
|
| Thermal ablation [47] | Microsecond heat pulse selectively removes SC. |
|
|
| Biological Macromolecular Drugs | Dose of IP | Model | Important Outcome of the Study | Reference |
|---|---|---|---|---|
| Anti-IL-10 siRNA | 0.3 mA/cm2, for 1 h | Ovalbumin-induced atopic dermatitis rat | IP-mediated delivery of siRNA into the epidermis significantly reduced IL-10 mRNA expression. | [72] |
| CpG-ODN | 0.3 mA/cm2, for 1 h | B16F1 melanoma bearing mouse | Transdermal delivery of CpG-ODN by IP induced pro-inflammatory cytokine production and inhibited the tumor growth. | [76] |
| GP 100 | 0.4 mA/cm2, 1 h | B16F1 melanoma bearing mouse | IP-mediated transdermal delivery of GP 100 activated immune responses and inhibited the tumor growth. | [77] |
| NF-κB decoy ODN | 0.34 mA/cm2, 1 h | IMQ-induced psoriasis rat | IP-mediated transdermal delivery of NF-κB decoy ODN significantly reduced proinflammatory cytokine production and reduced epidermal hyperplasia. | [89] |
| TNF-α drug etanercept | 0.34 mA/cm2, 1 h | IMQ-induced psoriasis rat | IP-mediated delivery of TNF-α drug etanercept into the epidermis significantly reduced epidermal hyperplasia. | [48] |
| Cetuximab | 0.5 mA/cm2, 2, 4, 8 h | Porcine skin | IP induced transdermal permeation of cetuximab. | [95] |
| hbFGF | 0.15, 0.3, 0.5 mA/cm2, 8 h | Porcine skin, Human skin | IP induced transdermal delivery of hbRGF. | [99] |
| Anti-HSP47 siRNA | 0.34 mA/cm2, 30 min | CCl4-induced fibrosis mice | IP employed hepatic delivery of siRNA and significantly suppressed HSP47 expression leading to the reduction of collagen deposition in fibrotic liver. | [100] |
| Anti-resistin siRNA | 0.34 mA/cm2, 30 min | KKAy obesity model mice | IP-mediated hepatic delivery of anti-resistin siRNA significantly reduced lipid accumulation in liver. | [100] |
| Anti-Pdx-1 siRNA | 0.34 mA/cm2, 30 min | BALB/c Mice | IP employed pancreatic delivery of siRNA and induced significant RNA interference effect. | [100] |
| Biological Macromolecules | Method Combined with IP | Model/IP Dose | Outcome | References |
|---|---|---|---|---|
| Insulin | Microneedles | Type 1 diabetic rat (In vivo)/Microneedle array/1 mA, 1 h | Induced controlled insulin delivery and significant hypoglycemic effect. | [108] |
| hGH | Microneedles | Rat Skin (in vitro)/ 0.5 mA/cm2, 4 h | Increased transdermal delivery of hGH as of 6-fold compared to single applications. | [109] |
| Insulin | Liposomes DOTAP/EPC/Chol = 2:2:1 (molar ratio) | Diabetic Rats (In vivo)/ 0.45 mA/cm2, 1 h | Gradually reduced blood glucose level up to 24 h. | [29] |
| superoxide dismutase | Liposomes DOTAP/EPC/Chol = 2:2:1 (molar ratio) | UV irradiated Rats (In vivo)/0.45 mA/cm2, 1 h | Suppressed skin damage-associated marker. | [110] |
| STAT3 siRNA with curcumin | Liposomes DOTAP/DOPE/C6 Ceramide/Sodium Cholate = 50:30:10:10 (w/w) | Melanoma bearing mice (In vivo)/0.47 mA/cm2, 2 h | Exhibited greater tumor suppression compared to single applications. | [111] |
| Antisense oligonucleotide | Chemical enhancer (limonene/ethanol (1:1)) | Pig ear skin (In vitro)/ 1.25 mA/cm2, 4 h | Synergistic effect increased transdermal delivery of antisense oligonucleotide. | [112] |
| Antisense oligonucleotide | PAMAM dendrimer | Skin cancer mice (In vivo)/0.5 mA/cm2, 2 h | Combined application suppressed 45% of tumor volume. | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Khatun, A.; Kogure, K. Iontophoresis of Biological Macromolecular Drugs. Pharmaceutics 2022, 14, 525. https://doi.org/10.3390/pharmaceutics14030525
Hasan M, Khatun A, Kogure K. Iontophoresis of Biological Macromolecular Drugs. Pharmaceutics. 2022; 14(3):525. https://doi.org/10.3390/pharmaceutics14030525
Chicago/Turabian StyleHasan, Mahadi, Anowara Khatun, and Kentaro Kogure. 2022. "Iontophoresis of Biological Macromolecular Drugs" Pharmaceutics 14, no. 3: 525. https://doi.org/10.3390/pharmaceutics14030525
APA StyleHasan, M., Khatun, A., & Kogure, K. (2022). Iontophoresis of Biological Macromolecular Drugs. Pharmaceutics, 14(3), 525. https://doi.org/10.3390/pharmaceutics14030525





