A Comprehensive Review of the Evolution of Insulin Development and Its Delivery Method
Abstract
:1. Introduction
2. The Pathogenic Landscape of Diabetes Mellitus (DM)
3. The Evolution of Insulin Development
4. Improvement in Pharmacokinetic/Pharmacodynamic (PK/PD) Profiles
4.1. Bolus Insulin Analogs
4.2. Basal Insulin Analogs
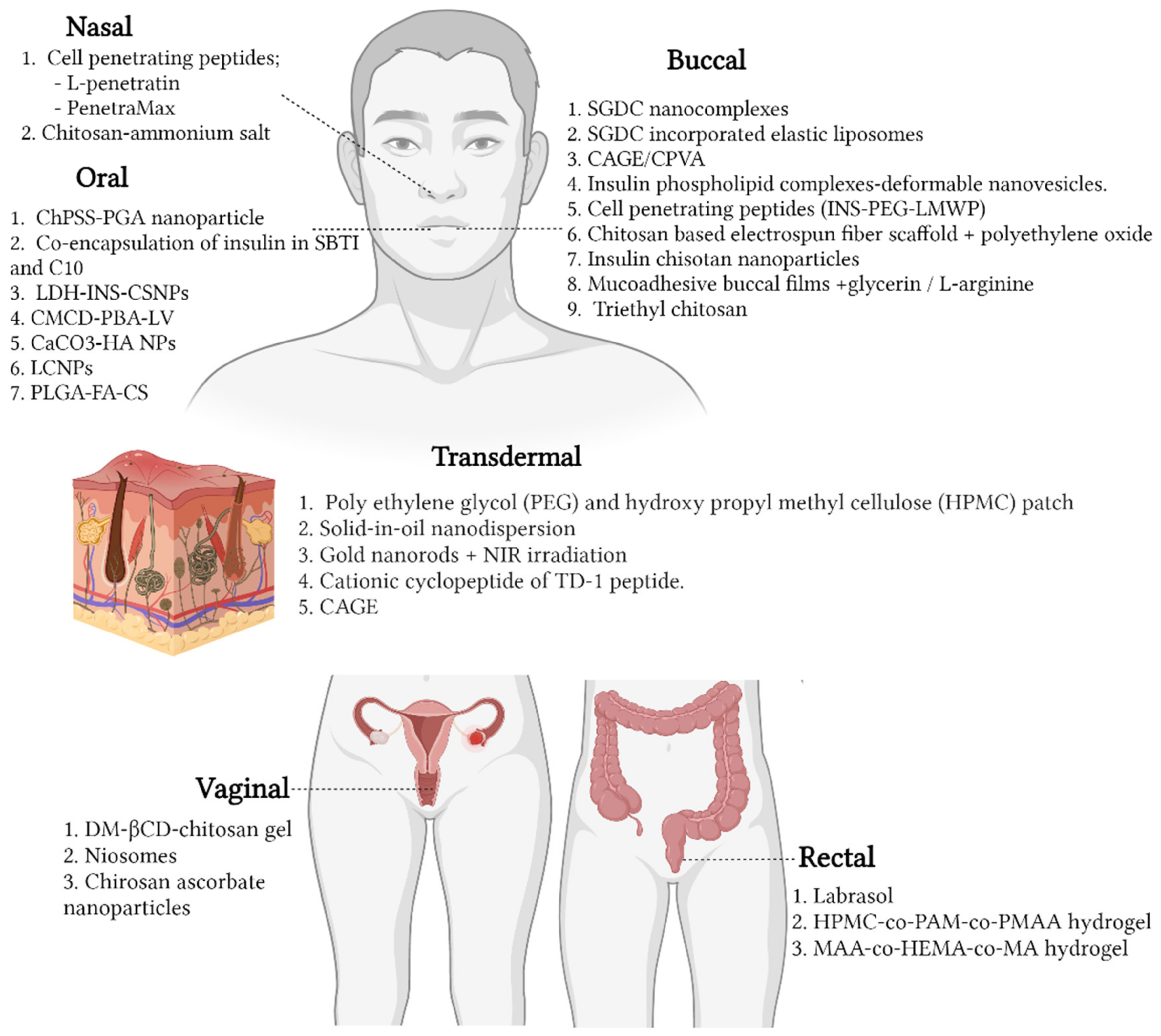
5. Advancements in Insulin Delivery Technology
5.1. Chemical Enhancers
5.1.1. Nasal
- In Vitro
- In Vivo
5.1.2. Buccal
- In Vitro
- In Vivo
5.1.3. Oral
- In Vitro
- In Vivo
5.1.4. Transdermal
- In Vitro
- In Vitro
5.1.5. Vaginal
- In Vitro
- In Vivo
5.1.6. Rectum
- In Vitro
- In Vivo
5.2. Physical Enhancers
5.2.1. Iontophoresis
5.2.2. Microneedling
5.2.3. Inhalation
6. Advancement of Alternative Routes for Insulin Delivery to Clinical Trials
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mathieu, C.; Martens, P.-J.; Vangoitsenhoven, R. One hundred years of insulin therapy. Nat. Rev. Endocrinol. 2021, 17, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Oras, A.; Peet, A.; Giese, T.; Tillmann, V.; Uibo, R. A study of 51 subtypes of peripheral blood immune cells in newly diagnosed young type 1 diabetes patients. Clin. Exp. Immunol. 2019, 198, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Jeker, L.T.; Bour-Jordan, H.; Bluestone, J.A. Breakdown in peripheral tolerance in type 1 diabetes in mice and humans. Cold Spring Harb. Perspect. Med. 2012, 2, a007807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugliese, A. Autoreactive T cells in type 1 diabetes. J. Clin. Investig. 2017, 127, 2881–2891. [Google Scholar] [CrossRef]
- Tanaka, S.; Nishida, Y.; Aida, K.; Maruyama, T.; Shimada, A.; Suzuki, M.; Shimura, H.; Takizawa, S.; Takahashi, M.; Akiyama, D.; et al. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: A mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes 2009, 58, 2285–2291. [Google Scholar] [CrossRef] [Green Version]
- Op de Beeck, A.; Eizirik, D.L. Viral infections in type 1 diabetes mellitus—Why the β cells? Nat. Rev. Endocrinol. 2017, 12, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, Y.; Hanafusa, T.; Imagawa, A. Pathogenesis of fulminant type 1 diabetes: Genes, viruses and the immune mechanism, and usefulness of patient-derived induced pluripotent stem cells for future research. J. Diabetes Investig. 2019, 10, 1158–1164. [Google Scholar] [CrossRef]
- Morel, P. Dendritic Cell Subsets in Type 1 Diabetes: Friend or Foe? Front. Immunol. 2013, 4, 415. [Google Scholar] [CrossRef] [Green Version]
- Hopp, A.-K.; Rupp, A.; Lukacs-Kornek, V. Self-Antigen Presentation by Dendritic Cells in Autoimmunity. Front. Immunol. 2014, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Hotta-Iwamura, C.; Tarbell, K.V. Type 1 diabetes genetic susceptibility and dendritic cell function: Potential targets for treatment. J. Leukoc. Biol. 2016, 100, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Katz, J.D.; Janssen, E.M. Breaking T cell tolerance to beta cell antigens by merocytic dendritic cells. Cell. Mol. Life Sci. 2011, 68, 2873–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audiger, C.; Chabot-Roy, G.; Lesage, S. Characterization of merocytic dendritic cells homeostasis. J. Immunol. 2017, 198, 221. [Google Scholar]
- Audiger, C.; Lesage, S. BIM determines the number of merocytic dendritic cells, a cell type that breaks immune tolerance. Immunol. Cell Biol. 2018, 96, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Audiger, C.; Fois, A.; Thomas, A.L.; Janssen, E.; Pelletier, M.; Lesage, S. Merocytic Dendritic Cells Compose a Conventional Dendritic Cell Subset with Low Metabolic Activity. J. Immunol. 2020, 205, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.; Fraker, C.A. Natural Killer Cells as Key Mediators in Type I Diabetes Immunopathology. Front. Immunol. 2021, 12, 3381. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 23, 15019. [Google Scholar] [CrossRef]
- Biden, T.J.; Boslem, E.; Chu, K.Y.; Sue, N. Lipotoxic endoplasmic reticulum stress, β cell failure, and type 2 diabetes mellitus. Trends Endocrinol. Metab. 2014, 25, 389–398. [Google Scholar] [CrossRef]
- Ebato, C.; Uchida, T.; Arakawa, M.; Komatsu, M.; Ueno, T.; Komiya, K.; Azuma, K.; Hirose, T.; Tanaka, K.; Kominami, E.; et al. Autophagy Is Important in Islet Homeostasis and Compensatory Increase of Beta Cell Mass in Response to High-Fat Diet. Cell Metab. 2008, 8, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef]
- Rocha, M.; Apostolova, N.; Diaz-Rua, R.; Muntane, J.; Victor, V.M. Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome. Trends Endocrinol. Metab. 2020, 31, 725–741. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Ahluwalia, T.S.; Groop, L. Genetics of Type 2 Diabetes. Clin. Chem. 2011, 57, 241–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, D.T. The story of insulin discovery. Diabetes Res. Clin. Pract. 2011, 93, S2–S8. [Google Scholar] [CrossRef]
- Lawrence, M. Landmarks in Insulin Research. Front. Endocrinol. 2011, 2, 76. [Google Scholar]
- De Meyts, P.; Whittaker, J. Structural biology of insulin and IGF1 receptors: Implications for drug design. Nat. Rev. Drug Discov. 2002, 1, 769–783. [Google Scholar] [CrossRef]
- Haeusler, R.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2017, 19, 31–44. [Google Scholar] [CrossRef]
- Bliss, M. The History of Insulin. Diabetes Care 1993, 16, 4–7. [Google Scholar] [CrossRef]
- Hirsch, I.B.; Juneja, R.; Beals, J.M.; Antalis, C.J.; Wright, E.E. The Evolution of Insulin and How it Informs Therapy and Treatment Choices. Endocr. Rev. 2020, 41, 733–755. [Google Scholar] [CrossRef]
- Saleem, F.; Sharma, A. NPH Insulin; BTI-StatPearls Publishing: Tresure Island, FL, USA, 2021. [Google Scholar]
- Bollinger, M.E.; Hamilton, R.G.; Wood, R.A. Protamine allergy as a complication of insulin hypersensitivity: A case report. J. Allergy Clin. Immunol. 1999, 104, 462–465. [Google Scholar] [CrossRef]
- Johnson, I.S. Human Insulin from Recombinant DNA Technology. Science 1983, 219, 632–637. [Google Scholar] [CrossRef]
- Chance, R.E.; Frank, B.H. Research, Development, Production, and Safety of Biosynthetic Human Insulin. Diabetes Care 1993, 16, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Riggs, A.D. Making, Cloning, and the Expression of Human Insulin Genes in Bacteria: The Path to Humulin. Endocr. Rev. 2020, 42, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, M.A.; Dhayalan, B.; Chen, Y.-S.; Chatterjee, D.; Varas, N.; Weiss, M.A. Structural principles of insulin formulation and analog design: A century of innovation. Mol. Metab. 2021, 52, 101325. [Google Scholar] [CrossRef] [PubMed]
- Kurtzhals, P.; Nishimura, E.; Haahr, H.; Høeg-Jensen, T.; Johansson, E.; Madsen, P.; Sturis, J.; Kjeldsen, T. Commemorating insulin’s centennial: Engineering insulin pharmacology towards physiology. Trends Pharmacol. Sci. 2021, 42, 620–639. [Google Scholar] [CrossRef] [PubMed]
- Kucera, M.L.; Graham, J.P. Insulin lispro, a new insulin analog. Pharmacotherapy 1998, 18, 526–538. [Google Scholar]
- Koivisto, V.A. The human insulin analogue insulin lispro. Ann. Med. 1998, 30, 260–266. [Google Scholar] [CrossRef]
- Lindholm, A.; Jacobsen, L.V. Clinical Pharmacokinetics and Pharmacodynamics of Insulin Aspart. Clin. Pharmacokinet. 2001, 40, 641–659. [Google Scholar] [CrossRef]
- Homko, C.; Deluzio, A.; Jimenez, C.; Kolaczynski, J.W.; Boden, G. Comparison of insulin aspart and lispro: Pharmacokinetic and metabolic effects. Diabetes Care 2003, 26, 2027–2031. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.K.; Ellis, S.L.; Ulrich, H. Insulin glulisine: A new rapid-acting insulin analogue for the treatment of diabetes. Expert Opin. Pharmacother. 2005, 6, 643–651. [Google Scholar] [CrossRef]
- Van Bon, A.C.; Bode, B.W.; Sert-Langeron, C.; DeVries, J.H.; Charpentier, G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: A randomized controlled trial. Diabetes Technol. Ther. 2011, 13, 607–614. [Google Scholar] [CrossRef]
- Melo, K.F.S.; Bahia, L.R.; Pasinato, B.; Porfirio, G.J.M.; Martimbianco, A.L.; Riera, R.; Calliari, L.E.P.; Minicucci, W.J.; Turatti, L.A.A.; Pedrosa, H.C.; et al. Short-acting insulin analogues versus regular human insulin on postprandial glucose and hypoglycemia in type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.; Ceriello, A.; Di Bartolo, P.; Corcos, A.; Federici, M.O. Rapid-Acting Insulin Analogues Versus Regular Human Insulin: A Meta-Analysis of Effects on Glycemic Control in Patients with Diabetes. Diabetes Ther. 2019, 11, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fullerton, B.; Siebenhofer, A.; Jeitler, K.; Horvath, K.; Semlitsch, T.; Berghold, A.; Gerlach, F.M. Short-acting insulin analogues versus regular human insulin for adult, non-pregnant persons with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2018, 12, CD013228. [Google Scholar] [CrossRef]
- Monami, M.; Marchionni, N.; Mannucci, E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes. Metab. 2009, 11, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.; Jeitler, K.; Berghold, A.; Ebrahim, S.H.; Gratzer, T.W.; Plank, J.; Kaiser, T.; Pieber, T.R.; Siebenhofer, A. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2007, 18, CD005613. [Google Scholar] [CrossRef]
- Semlitsch, T.; Engler, J.; Siebenhofer, A.; Jeitler, K.; Berghold, A.; Horvath, K. (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD005613. [Google Scholar] [CrossRef]
- Bolli, G.B.; Owens, D.R. Insulin glargine. Lancet 2000, 356, 443–445. [Google Scholar] [CrossRef]
- Goykhman, S.; Drincic, A.; Desmangles, J.C.; Rendell, M. Insulin Glargine: A review 8 years after its introduction. Expert Opin. Pharmacother. 2009, 10, 705–718. [Google Scholar] [CrossRef]
- Scheen, A.; Radermecker, R.; Philips, J.-C.; Paquot, N. Le medicament du mois. L’insuline detemir (Levemir). Revue Médicale de Liège 2005, 60, 814. [Google Scholar]
- Dorchy, H.; Sternon, J. Insulin analogues: Place of detemir (levemir). Rev. Medicale de Brux. 2006, 27, 89–94. [Google Scholar]
- Swinnen, S.G.; Simon, A.C.; Holleman, F.; Hoekstra, J.B.; Devries, J.H. Insulin detemir versus insulin glargine for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2011, 2011, CD006383. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.; Koenen, C.; Bode, B. Comparison of insulin detemir and insulin glargine in a basal-bolus regimen, with insulin aspart as the mealtime insulin, in patients with type 1 diabetes: A 52-week, multinational, randomized, open-label, parallel-group, treat-to-target noninferiority trial. Clin. Ther. 2009, 31, 2086–2097. [Google Scholar] [PubMed] [Green Version]
- Renard, E.; Dubois-Laforgue, D.; Guerci, B. Non-inferiority of insulin glargine versus insulin detemir on blood glucose variability in type 1 diabetes patients: A multicenter, randomized, crossover study. Diabetes Technol. Ther. 2011, 13, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, I.; Havelund, S.; Hoeg-Jensen, T.; Steensgaard, D.B.; Wahlund, P.-O.; Ribel, U. Design of the Novel Protraction Mechanism of Insulin Degludec, an Ultra-long-Acting Basal Insulin. Pharm. Res. 2012, 29, 2104–2114. [Google Scholar] [CrossRef] [Green Version]
- Vora, J.; Cariou, B.; Evans, M.; Gross, J.L.; Harris, S.; Landstedt-Hallin, L.; Mithal, A.; Rodriguez, M.R.; Meneghini, L. Clinical use of insulin degludec. Diabetes Res. Clin. Pract. 2015, 109, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Holmes, R.S.; Crabtree, E.; McDonagh, M.S. Comparative effectiveness and harms of long-acting insulins for type 1 and type 2 diabetes: A systematic review and meta-analysis. Diabetes, Obes. Metab. 2018, 21, 984–992. [Google Scholar] [CrossRef]
- Kjeldsen, T.B.; Hubálek, F.; Hjørringgaard, C.U.; Tagmose, T.M.; Nishimura, E.; Stidsen, C.E.; Porsgaard, T.; Fledelius, C.; Refsgaard, H.H.F.; Gram-Nielsen, S.; et al. Molecular Engineering of Insulin Icodec, the First Acylated Insulin Analog for Once-Weekly Administration in Humans. J. Med. Chem. 2021, 64, 8942–8950. [Google Scholar] [CrossRef]
- Nishimura, E.; Pridal, L.; Glendorf, T.; Hansen, B.F.; Hubálek, F.; Kjeldsen, T.; Kristensen, N.R.; Lützen, A.; Lyby, K.; Madsen, P.; et al. Molecular and pharmacological characterization of insulin icodec: A new basal insulin analog designed for once-weekly dosing. BMJ Open Diabetes Res. Care 2021, 9, e002301. [Google Scholar] [CrossRef]
- Holleman, F.; Gale, E.A.M. Nice insulins, pity about the evidence. Diabetologia 2007, 50, 1783–1790. [Google Scholar] [CrossRef] [Green Version]
- Wilde, M.I.; McTavish, D. Insulin lispro: A review of its pharmacological properties and therapeutic use in the management of diabetes mellitus. Drugs 1997, 54, 597–614. [Google Scholar] [CrossRef]
- Stubbs, D.J.; Levy, N.; Dhatariya, K. Diabetes medication pharmacology. BJA Educ. 2017, 17, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Noble, S.L.; Johnston, E.; Walton, B. Insulin lispro: A fast-acting insulin analog. Am. Fam. Physician 1998, 57, 279–286. [Google Scholar] [PubMed]
- Hermansen, K.; Bohl, M.; Schioldan, A.G. Insulin Aspart in the Management of Diabetes Mellitus: 15 Years of Clinical Experience. Drugs 2015, 76, 41–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolli, G.B.; Luzio, S.; Marzotti, S.; Porcellati, F.; Sert-Langeron, C.; Charbonnel, B.; Zair, Y.; Owens, D.R. Comparative pharmacodynamic and pharmacokinetic characteristics of subcutaneous insulin glulisine and insulin aspart prior to a standard meal in obese subjects with type 2 diabetes. Diabetes Obes. Metab. 2011, 13, 251–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helms, K.L.; Kelley, K.W. Insulin Glulisine: An Evaluation of Its Pharmacodynamic Properties and Clinical Application. Ann. Pharmacother. 2009, 43, 658–668. [Google Scholar] [CrossRef]
- Becker, R.H.A.; Frick, A.D. Clinical Pharmacokinetics and Pharmacodynamics of Insulin Glulisine. Clin. Pharmacokinet. 2008, 47, 7–20. [Google Scholar] [CrossRef]
- Mavrogiannaki, A.N.; Migdalis, I.N. Long-acting basal insulin analogs: Latest developments and clinical usefulness. Ther. Adv. Chronic Dis. 2012, 3, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Goldman-Levine, J.D.; Lee, K.W. Insulin Detemir—A New Basal Insulin Analog. Ann. Pharmacother. 2005, 39, 502–507. [Google Scholar] [CrossRef]
- Kurtzhals, P. Pharmacology of Insulin Detemir. Endocrinol. Metab. Clin. N. Am. 2007, 36, 14–20. [Google Scholar] [CrossRef]
- Plank, J.; Bodenlenz, M.; Sinner, F.; Magnes, C.; Görzer, E.; Regittnig; Endahl, L.A.; Draeger, E.; Zdravkovic, M.; Pieber, T.R. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care 2005, 28, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Haahr, H.; Heise, T. A Review of the Pharmacological Properties of Insulin Degludec and Their Clinical Relevance. Clin. Pharmacokinet. 2014, 53, 787–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambascia, M.A.; Eliaschewitz, F.G. Degludec: The new ultra-long insulin analogue. Diabetol. Metab. Syndr. 2015, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, R.C.L. Chapter 42—Insulin, other hypoglycemic drugs, and glucagon. In Side Effects of Drugs Annual; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 35, pp. 763–787. [Google Scholar]
- Rosenstock, J.; Bajaj, H.S.; Janež, A.; Silver, R.; Begtrup, K.; Hansen, M.V.; Jia, T.; Goldenberg, R. Once-Weekly Insulin for Type 2 Diabetes without Previous Insulin Treatment. N. Engl. J. Med. 2020, 383, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Hövelmann, U.; Brøndsted, L.; Kristensen, N.R.; Ribel-Madsen, R.; Devries, J.H.; Heise, T.; Haahr, H. 237-OR: Insulin Icodec: An Insulin Analog Suited for Once-Weekly Dosing in Type 2 Diabetes. Diabetes 2020, 69, 237-OR. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Lillard, J.W. Past, Present, and Future Technologies for Oral Delivery of Therapeutic Proteins. J. Pharm. Sci. 2008, 97, 2497–2523. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.B.; Shah, V.N.; Patel, M.; Maahs, D.M. Insulin delivery methods: Past, present and future. Int. J. Pharm. Investig. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Al-Tabakha, M.; Arida, A. Recent challenges in insulin delivery systems: A review. Indian J. Pharm. Sci. 2008, 70, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Gradel, A.K.J.; Porsgaard, T.; Lykkesfeldt, J.; Seested, T.; Gram-Nielsen, S.; Kristensen, N.R.; Refsgaard, H.H.F. Factors Affecting the Absorption of Subcutaneously Administered Insulin: Effect on Variability. J. Diabetes Res. 2018, 2018, 1205121. [Google Scholar] [CrossRef]
- Blanco, M.; Hernández, M.; Strauss, K.; Amaya, M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013, 39, 445–453. [Google Scholar] [CrossRef]
- Richardson, T.; Kerr, D. Skin-related complications of insulin therapy: Epidemiology and emerging management strategies. Am. J. Clin. Dermatol. 2003, 4, 661–667. [Google Scholar] [CrossRef]
- Duan, X.; Mao, S. New strategies to improve the intranasal absorption of insulin. Drug Discov. Today 2010, 15, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliffe, M.; Illum, L. Intranasal insulin delivery and therapy. Adv. Drug Deliv. Rev. 1999, 35, 199–234. [Google Scholar] [CrossRef]
- Bahmanpour, A.; Ghaffari, M.; Milan, P.B.; Moztarzadeh, F.; Mozafari, M. Synthesis and characterization of thermosensitive hydrogel based on quaternized chitosan for intranasal delivery of insulin. Biotechnol. Appl. Biochem. 2020, 68, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.K.; Al-Attraqchi, O.; Chandrasekaran, B.; Paradkar, A.; Tekade, R.K. Chapter 16—Protein/Peptide Drug Delivery Systems: Practical Considerations in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 651–684. [Google Scholar]
- Khafagy, E.-S.; Morishita, M.; Isowa, K.; Imai, J.; Takayama, K. Effect of cell-penetrating peptides on the nasal absorption of insulin. J. Control. Release 2009, 133, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.S.; Morishita, M.; Takayama, K. The role of intermolecular interactions with penetratin and its analogue on the enhancement of absorption of nasal therapeutic peptides. Int. J. Pharm. 2010, 388, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.S.; Kamei, N.; Nielsen, E.J.; Nishio, R.; Takeda-Morishita, M. One-month subchronic toxicity study of cell-penetrating peptides for insulin nasal delivery in rats. Eur. J. Pharm. Biopharm 2013, 85, 736–743. [Google Scholar] [CrossRef]
- Hua, S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front. Pharmacol. 2019, 10, 1328. [Google Scholar] [CrossRef] [Green Version]
- Kumria, R.; Goomber, G. Emerging trends in insulin delivery: Buccal route. J. Diabetol. 2011, 2, 1. [Google Scholar]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Lamichhane, S.; Kim, J.H.; Kim, C.H.; Choi, Y.W.; Lee, S. Facilitated Buccal Insulin Delivery via Hydrophobic Ion-Pairing Approach: In vitro and ex vivo Evaluation. Int. J. Nanomed. 2021, 16, 4677–4691. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Choi, Y.W.; Lee, S. Facilitated permeation of insulin across TR146 cells by cholic acid derivatives-modified elastic bilosomes. Int. J. Nanomed. 2018, 13, 5173–5186. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, X.; Zhang, Y.; Ye, J.; Wang, H.L.; Xia, X.; Liu, Y. Mechanisms of deformable nanovesicles based on insulin-phospholipid complex for enhancing buccal delivery of insulin. Int. J. Nanomed. 2018, 13, 7319–7331. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, X.; Wang, N.; Pei, X.; Guo, Y.; Wang, J.; Barth, S.; Yu, F.; Lee, S.J.; He, H.; et al. Cell-penetrating peptide enhanced insulin buccal absorption. Int. J. Pharm. 2020, 584, 119469. [Google Scholar] [CrossRef] [PubMed]
- Lancina, M.G., III; Shankar, R.K.; Yang, H. Chitosan nanofibers for transbuccal insulin delivery. J. Biomed. Mater. Res. A 2017, 105, 1252–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nemrawi, N.K.; Alsharif, S.S.M.; Alzoubi, K.H.; Alkhatib, R.Q. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm. Dev. Technol. 2019, 24, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Rahbarian, M.; Mortazavian, E.; Dorkoosh, F.A.; Tehrani, M.R. Preparation, evaluation and optimization of nanoparticles composed of thiolated triethyl chitosan: A potential approach for buccal delivery of insulin. J. Drug Deliv. Sci. Technol. 2017, 44, 254–263. [Google Scholar] [CrossRef]
- Diab, M.; Sallam, A.-S.; Hamdan, I.; Mansour, R.; Hussain, R.; Siligardi, G.; Qinna, N.; Khalil, E. Characterization of Insulin Mucoadhesive Buccal Films: Spectroscopic Analysis and In Vivo Evaluation. Symmetry 2021, 13, 88. [Google Scholar] [CrossRef]
- Iyire, A.; Alaayedi, M.; Mohammed, A.R. Pre-formulation and systematic evaluation of amino acid assisted permeability of insulin across in vitro buccal cell layers. Sci. Rep. 2016, 1, 32498. [Google Scholar] [CrossRef]
- Bashyal, S.; Seo, J.-E.; Keum, T.; Noh, G.; Lamichhane, S.; Lee, S. Development, Characterization, and Ex Vivo Assessment of Elastic Liposomes for Enhancing the Buccal Delivery of Insulin. Pharmaceutics 2021, 13, 565. [Google Scholar] [CrossRef]
- Vaidya, A.; Mitragotri, S. Ionic liquid-mediated delivery of insulin to buccal mucosa. J. Control. Release 2020, 327, 26–34. [Google Scholar] [CrossRef]
- Fonte, P.; Araújo, F.; Reis, S.; Sarmento, B. Oral Insulin Delivery: How Far are We? J. Diabetes Sci. Technol. 2013, 7, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Urimi, D.; Agrawal, A.K.; Kushwah, V.; Jain, S. Polyglutamic Acid Functionalization of Chitosan Nanoparticles Enhances the Therapeutic Efficacy of Insulin Following Oral Administration. AAPS PharmSciTech 2019, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Franzyk, H.; Klausen, M.T.; Iversen, A.; Bahnsen, J.S.; Skyggebjerg, R.B.; Foderà, V.; Nielsen, H.M. Penetratin-Mediated Transepithelial Insulin Permeation: Importance of Cationic Residues and pH for Complexation and Permeation. AAPS J. 2015, 17, 1200–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McManus, S.A.; Zhang, Y.; Kim, B.; Lee, B.K.; ElSayed, M.E.; Prud’Homme, R.K. Co-encapsulation by Flash NanoPrecipitation of Insulin, Trypsin Inhibitor and Caprate Permeabilization Enhancer for Oral Administration. Precis. Nanomed. 2020, 3, 710–723. [Google Scholar] [CrossRef]
- El-Shahawy, A.; Abdel-Moneim, A.; Eldin, Z.; Youssef, A.; Gouda, Z.; Ramadan, H.; Amar, E.; Taha, E.; Saied, A.; Zanaty, M. Characterization and Efficacy of a Novel Formula of Insulin Chitosan Layered Duple Hydroxide-Nanohybrid for Oral Administration. Nano Biomed. Eng. 2020, 12, 297–305. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Tong, Z.; Kong, X.; Yao, J. Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater. Sci. Eng. C 2017, 70, 278–286. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumar, K.; Swarnakar, N.K.; Kushwah, V.; Jain, S. “Liquid Crystalline Nanoparticles”: Rationally Designed Vehicle to Improve Stability and Therapeutic Efficacy of Insulin Following Oral Administration. Mol. Pharm. 2017, 14, 1874–1882. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, G.; Yu, W.; Li, L.; Tong, Z.; Kong, X.; Yao, J. Oral delivery of insulin using CaCO3-based composite nanocarriers with hyaluronic acid coatings. Mater. Lett. 2016, 188, 263–266. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Liu, Y.; Kong, X.; Yao, J. Preparation of poly(lactic- co -glycolic acid) and chitosan composite nanocarriers via electrostatic self assembly for oral delivery of insulin. Mater. Sci. Eng. C 2017, 78, 420–428. [Google Scholar] [CrossRef]
- Sadhasivam, L.; Dey, N.; Francis, A.P.; Devasena, T. Trandermal patches of chitosan nanoparticles for insulin delivery. Int. J. Pharm. Pharm. Sci. 2015, 7, 84–88. [Google Scholar]
- Tahara, Y.; Honda, S.; Kamiya, N.; Goto, M. Transdermal delivery of insulin using a solid-in-oil nanodispersion enhanced by arginine-rich peptides. MedChemComm 2012, 3, 1496–1499. [Google Scholar] [CrossRef]
- Tahara, Y.; Honda, S.; Kamiya, N.; Piao, H.; Hirata, A.; Hayakawa, E.; Fujii, T.; Goto, M. A solid-in-oil nanodispersion for transcutaneous protein delivery. J. Control. Release 2008, 131, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Li, X.; Sun, Y.; Cheng, F.; Wang, Q.; Xie, X.; Zhao, W.; Tian, X. Effect of Cationic Cyclopeptides on Transdermal and Transmembrane Delivery of Insulin. Mol. Pharm. 2013, 10, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Yerramsetty, K.; Rachakonda, V.; Neely, B.; Madihally, S.; Gasem, K. Effect of different enhancers on the transdermal permeation of insulin analog. Int. J. Pharm. 2010, 398, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, E.E.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nose, K.; Pissuwan, D.; Goto, M.; Katayama, Y.; Niidome, T. Gold nanorods in an oil-base formulation for transdermal treatment of type 1 diabetes in mice. Nanoscale 2012, 4, 3776–3780. [Google Scholar] [CrossRef]
- King, M.J.; Badea, I.; Solomon, J.; Kumar, P.; Gaspar, K.J.; Foldvari, M. Transdermal Delivery of Insulin from a Novel Biphasic Lipid System in Diabetic Rats. Diabetes Technol. Ther. 2002, 4, 479–488. [Google Scholar] [CrossRef]
- King, M.J.; Michel, D.; Foldvari, M. Evidence for lymphatic transport of insulin by topically applied biphasic vesicles. J. Pharm. Pharmacol. 2003, 55, 1339–1344. [Google Scholar] [CrossRef]
- Sintov, A.C.; Wormser, U. Topical iodine facilitates transdermal delivery of insulin. J. Control. Release 2007, 118, 185–188. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Quan, Y.-S.; Zang, L.; Jin, M.-N.; Kamiyama, F.; Katsumi, H.; Yamamoto, A.; Tsutsumi, S. Transdermal delivery of insulin using trypsin as a biochemical enhancer. Biol. Pharm. Bull. 2008, 31, 1574–1579. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.P.J. Transdermal Delivery of Insulin in Mice by Using Lecithin Vesicles as a Carrier. Drug Deliv. 2000, 7, 113–116. [Google Scholar] [CrossRef]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [PubMed] [Green Version]
- Marciello, M.; Rossi, S.; Caramella, C.; Remuñán-López, C. Freeze-dried cylinders carrying chitosan nanoparticles for vaginal peptide delivery. Carbohydr. Polym. 2017, 170, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Deĝim, Z.; DeĞim, T.; Acarturk, F.; Erdogan, D.; Ozogul, C.; Koksal, M. Rectal and vaginal administration of insulin–chitosan formulations: An experimental study in rabbits. J. Drug Target. 2005, 13, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Guo, Y.; Pan, H.; Yu, H.; Gu, Z. Niosomes with Sorbitan Monoester as a Carrier for Vaginal Delivery of Insulin: Studies in Rats. Drug Deliv. 2005, 12, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.J.; Hanning, S.M.; Wu, Z. Advances in rectal drug delivery systems. Pharm. Dev. Technol. 2018, 23, 942–952. [Google Scholar] [CrossRef]
- Matsumoto, A.; Murakami, K.; Watanabe, C.; Murakami, M. Improved systemic delivery of insulin by condensed drug loading in a dimpled suppository. Drug Discov. Ther. 2017, 11, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Xue, J.; Sang, Y.; Xu, X.; Shang, Q. Insulin-loaded hydroxypropyl methyl cellulose-co-polyacrylamide-co-methacrylic acid hydrogels used as rectal suppositories to regulate the blood glucose of diabetic rats. Int. J. Biol. Macromol. 2018, 121, 1346–1353. [Google Scholar] [CrossRef]
- Xue, J.; Shi, Y.; Li, C.; Xu, X.; Xu, S.; Cao, M. Methylcellulose and polyacrylate binary hydrogels used as rectal suppository to prevent type I diabetes. Colloids Surf. B Biointerfaces 2018, 172, 37–42. [Google Scholar] [CrossRef]
- Vadlapatla, R.; Wong, E.Y.; Gayakwad, S.G. Electronic drug delivery systems: An overview. J. Drug Deliv. Sci. Technol. 2017, 41, 359–366. [Google Scholar] [CrossRef]
- Elkhatib, M.M.; Ali, A.I.; Al-Badrawy, A.S. In Vitro and in Vivo Comparative Study of Oral Nanoparticles and Gut Iontophoresis as Oral Delivery Systems for Insulin. Biol. Pharm. Bull. 2021, 44, 251–258. [Google Scholar] [CrossRef]
- Banerjee, A.; Chen, R.; Arafin, S.; Mitragotri, S. Intestinal iontophoresis from mucoadhesive patches: A strategy for oral delivery. J. Control. Release 2019, 297, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Davis, S.P.; Holiday, N.R.; Wang, J.; Gill, H.S.; Prausnitz, M.R. Transdermal Delivery of Insulin Using Microneedles in Vivo. Pharm. Res. 2004, 21, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-P.; Liu, Y.-L.; Wang, H.-L.; Zhang, P.-X.; Zhang, J.-L. Transdermal delivery of insulin using microneedle rollers in vivo. Int. J. Pharm. 2010, 392, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zhang, J.N.; Chen, B.Z.; Wang, Q.L.; Guo, X.D. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017, 7, 15408–15415. [Google Scholar] [CrossRef] [Green Version]
- Ling, M.-H.; Chen, M.-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef]
- Chen, M.-C.; Ling, M.-H.; Kusuma, S.J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015, 24, 106–116. [Google Scholar] [CrossRef]
- Lau, S.; Fei, J.; Liu, H.; Chen, W.; Liu, R. Multilayered pyramidal dissolving microneedle patches with flexible pedestals for improving effective drug delivery. J. Control. Release 2017, 265, 113–119. [Google Scholar] [CrossRef]
- Lee, I.-C.; Lin, W.-M.; Shu, J.-C.; Tsai, S.-W.; Chen, C.-H.; Tsai, M.-T. Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice. J. Biomed. Mater. Res. Part A 2016, 105, 84–93. [Google Scholar] [CrossRef]
- Lee, I.-C.; Wu, Y.-C.; Tsai, S.-W.; Chen, C.-H.; Wu, M.-H. Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery. RSC Adv. 2017, 7, 5067–5075. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Jiang, G.; Liu, D.; Li, L.; Chen, H.; Liu, Y.; Huang, Q.; Tong, Z.; Yao, J.; Kong, X. Fabrication of biodegradable composite microneedles based on calcium sulfate and gelatin for transdermal delivery of insulin. Mater. Sci. Eng. C 2017, 71, 725–734. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.; Chen, Z.; Khan, S.A.; et al. Core–Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Y.; Jiang, F.; Cao, J.; Lu, S. Combined Silk Fibroin Microneedles for Insulin Delivery. ACS Biomater. Sci. Eng. 2020, 6, 3422–3429. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Shyu, V.B.-H.; Chen, C.-T. Dissolving Microneedle Patches for Transdermal Insulin Delivery in Diabetic Mice: Potential for Clinical Applications. Materials 2018, 11, 1625. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.-Q.; Chen, G.; Xu, W.; Zhou, D.; Li, J.-X.; Huang, Y.-C.; Lin, R.; Gu, Z.; Du, J.-Z. Microneedle-array patch with pH-sensitive formulation for glucose-responsive insulin delivery. Nano Res. 2021, 14, 2689–2696. [Google Scholar] [CrossRef]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2011, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Cavaiola, T.S.; Edelman, S. Inhaled Insulin: A Breath of Fresh Air? A Review of Inhaled Insulin. Clin. Ther. 2014, 36, 1275–1289. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M. Future prospect of insulin inhalation for diabetic patients: The case of Afrezza versus Exubera. J. Control. Release 2015, 215, 25–38. [Google Scholar] [CrossRef]
- Wolpert, H.; Shih, J. New Technologies for Insulin Administration and Glucose Monitoring. In Textbook of Diabetes; Holt, R., Goldstein, C.C., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 440–451. [Google Scholar]
- Bailey, C.J.; Barnett, A.H. Inhaled insulin: New formulation, new trial. Lancet 2010, 375, 2199–2201. [Google Scholar] [CrossRef]
- Mitri, J.; Pittas, A.G. Inhaled insulin—what went wrong. Nat. Clin. Pract. Endocrinol. Metab. 2008, 5, 24–25. [Google Scholar] [CrossRef] [Green Version]
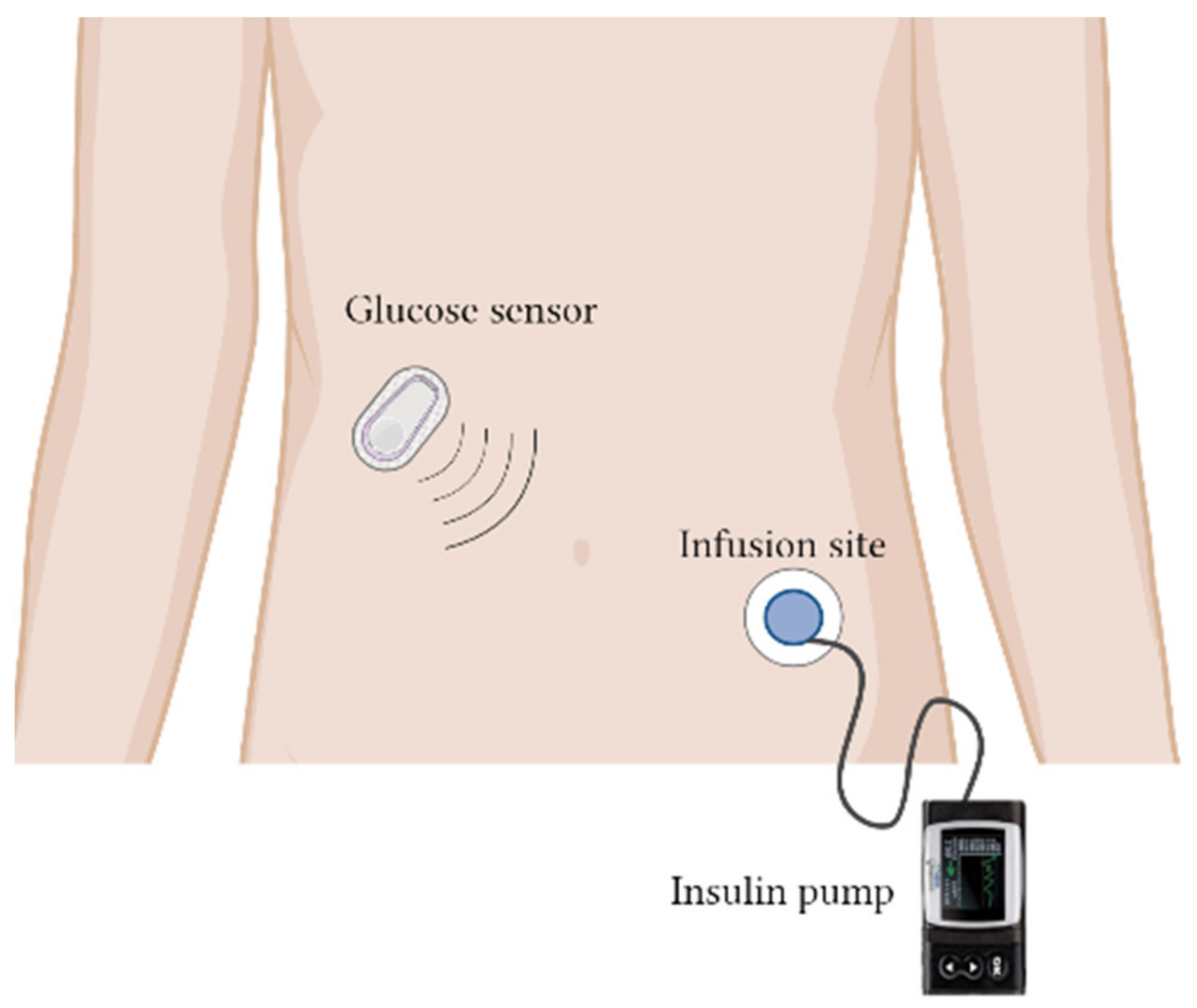
- Aiello, E.M.; Deshpande, S.; Özaslan, B.; Wolkowicz, K.L.; Dassau, E.; Pinsker, J.E.; Doyle, F.J. Review of automated insulin delivery systems for individuals with type 1 diabetes: Tailored solutions for subpopulations. Curr. Opin. Biomed. Eng. 2021, 19, 100312. [Google Scholar] [CrossRef]
- Christiansen, M.; Bartee, A.; Lalonde, A.; Jones, R.E.; Katz, M.; Wolpert, H.; Brazg, R. Performance of an Automated Insulin Delivery System: Results of Early Phase Feasibility Studies. Diabetes Technol. Ther. 2021, 23, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Pinsker, J.E.; Bartee, A.; Katz, M.; Lalonde, A.; Jones, R.; Dassau, E.; Wolpert, H. Predictive Low-Glucose Suspend Necessitates Less Carbohydrate Supplementation to Rescue Hypoglycemia: Need to Revisit Current Hypoglycemia Treatment Guidelines. Diabetes Technol. Ther. 2021, 23, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Brown, M.R.; Raviele, N.A.; Prausnitz, M.R.; Felner, E.I. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2013, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.K.; Lilly, L.C.; Aberer, F.; Korsatko, S.; Strock, E.; Mazze, R.S.; Damsbo, P.; Pieber, T.R. A Feasibility Study of a 3-Day Basal-Bolus Insulin Delivery Device in Individuals with Type 2 Diabetes. Diabetes Care 2014, 37, 1476–1479. [Google Scholar] [CrossRef] [Green Version]
- Mader, J.K.; Lilly, L.C.L.; Aberer, F.; Poettler, T.; Johns, D.; Trautmann, M.; Warner, J.L.; Pieber, T.R. Improved glycaemic control and treatment satisfaction with a simple wearable 3-day insulin delivery device among people with Type 2 diabetes. Diabet. Med. 2018, 35, 1448–1456. [Google Scholar] [CrossRef]
- Russell, S.J.; Balliro, C.; Ekelund, M.; El-Khatib, F.; Graungaard, T.; Greaux, E.; Hillard, M.; Jafri, R.Z.; Rathor, N.; Selagamsetty, R.; et al. Improvements in Glycemic Control Achieved by Altering the t(max) Setting in the iLet(®) Bionic Pancreas When Using Fast-Acting Insulin Aspart: A Randomized Trial. Diabetes Ther. 2021, 12, 2019–2033. [Google Scholar] [CrossRef]
- Lal, R.A.; Basina, M.; Maahs, D.M.; Hood, K.; Buckingham, B.; Wilson, D.M. One Year Clinical Experience of the First Commercial Hybrid Closed-Loop System. Diabetes Care 2019, 42, 2190–2196. [Google Scholar] [CrossRef]
- Dauber, A.; Corcia, L.; Safer, J.; Agus, M.S.D.; Einis, S.; Steil, G.M. Closed-loop insulin therapy improves glycemic control in children aged <7 years: A randomized controlled trial. Diabetes Care 2013, 36, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.; Herrero, P.; El Sharkawy, M.; Pesl, P.; Jugnee, N.; Thomson, H.; Pavitt, D.; Toumazou, C.; Johnston, D.; Georgiou, P.; et al. Feasibility Study of a Bio-inspired Artificial Pancreas in Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2014, 16, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Mora, P.F.; Sutton, D.R.; Gore, A.; Baliga, B.; Goldfaden, R.F.; Nikkel, C.; Ii, J.S.; Adams-Huet, B. Efficacy, safety and cost-effectiveness comparison between U-100 human regular insulin and rapid acting insulin when delivered by V-Go wearable insulin delivery device in type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001832. [Google Scholar] [CrossRef]

| Insulin and Analogs | Time of Onset | Time to Peak Serum Concentration (Cmax) | Duration of Drug Action |
|---|---|---|---|
| Neutral protamine Hagedorn | 1–3 h | 6–10 h | 14–24 h |
| Regular human insulin | 30 min | 1 h | 5–8 h |
| Lispro | 5 to 15 min | 30–60 min | 3–4 h |
| Aspart | 10–20 min | 40–50 min | 3–5 h |
| Glulisine | 20 min | 1 h | 4 h |
| Glargine | 1–2 h | Peakless profile | 24 h |
| Detemir | 1.6 h | Peakless profile | Dosage-dependent; standard dose up to 24 h |
| Degludec | 1 h | Peakless profile | 42 h to 4 days |
| Icodec | N/A | 16 h; albumin-bound | 1 week |
| Analog | Type of Insulin Analog | Modification | Impacts on the Absorption of Insulin |
|---|---|---|---|
| Lispro | Bolus | Reversal of the insulin’s B28 (proline) and B29 (lysine) | The designed modifications prevent formation of a dimer/hexamer or self-association, resulting in faster absorption of insulin monomers when injected subcutaneously. |
| Aspart | Bolus | Substitution of B28 proline with aspartic acid | Reduce monomer–monomer interaction. Enhance repulsion between charged aspartic acid and nearby glutamic acid B21, causing rapid insulin hexamer dissociation into monomers. |
| Glulisine | Bolus | Two modifications:
| These modifications change the isoelectric point from 5.5 (native insulin) to 5.1, improving the solubility of insulin after subcutaneous injection. This enhances stable dimers and monomers at pharmaceutical concentrations in zinc-free buffer. |
| Glargine | Basal |
| The isoelectric point increases to 6.7 to enhance the solubility of insulin. The glycine substitution prevents the deamidation effect of asparagine, causing a more stable insulin aggregation for long-term release. |
| Detemir | Basal | Acylation of myristic acid to lysine at B-chain position 29 | Detemir binds to albumin and forms a reversible bond, resulting in slow release and prolonged action. |
| Degludec | Basal |
| Degludec establishes an insulin depot via insulin multi-hexamer formation in the subcutaneous layer with highly predictable gradual dissociation, resulting in long-term release and action. |
| Icodec | Basal |
| The C20 fatty diacid-containing side chain enforces strong, reversible albumin binding and the gradual release of icodec from albumin. The substituted amino acids result in a slower receptor-mediated clearance, prolonging its half-life. |
| Intervention | Target | Clinical Trial Phase | Purpose of Study | Clinical Trial Number |
|---|---|---|---|---|
| Automated insulin delivery (AID) system (Insulin lispro) | Type 1 diabetes (adult) | - | To evaluate whether the AID system is able to function as designed | NCT03743285 [155] NCT03367390 NCT03848767 |
| Type 1 diabetes (adult) | - | To evaluate whether the AID system is able to function as intended with personalized basal insulin rates when basal insulin rates increase | NCT03849612 [155] | |
| Type 1 diabetes (adult) | - | To determine predictive low glucose suspension (PLGS) feature safety and functionality | NCT03890003 [156] | |
| Microneedle | Type 1 diabetes (children and adolescents) | 2, 3 | To determine the difference in glycemic control between subcutaneous insulin catheters and microneedles for bolus delivery | NCT00837512 [157] |
| PaQ Insulin infusion device (Insulin aspart) | Type 2 diabetes (adult) | - | To evaluate the ability of patients to use PaQ (patch on insulin delivery device) for the control of blood glucose | NCT01535612 [158] |
| PaQ Insulin delivery device (Insulin aspart) | Type 2 diabetes (Adult) | - | To evaluate the efficacy and safety of basal bolus insulin delivery with PaQ in insulin | NCT02419859 [159] |
| iLet bionic pancreas (insulin aspart) | Type 1 diabetes (adult) | 2 | To evaluate the safety of insulin aspart in a different insulin delivery setting in the iLet | NCT03816761 [160] |
| iLet bionic pancreas (BP) (Insulin lispro or aspart) | Type 1 diabetes (children, adult) | - | To compare the insulin-only configuration of the iLet BP system in maintaining normal glycemia compared to usual care in a home-use setting | NCT04200313 |
| Closed-loop control system using JDRF artificial pancreas | Type 1 diabetes (adult) | Early phase 1 | To determine real-time continuous glucose sensing with automated insulin delivery in a closed-loop system | NCT01484457 |
| Closed-loop insulin delivery system 670 G | Type 1 diabetes (children, adult) | - | To track the initiation of the FDA-approved 670G closed-loop insulin delivery system | NCT03017482 [161] |
| Closed-loop insulin device | Type 1 diabetes (children 6 months to 7 years) | - | To evaluate the efficacy of closed-loop insulin pump therapy in delivering insulin in children less than 7 years of age | NCT01421225 [162] |
| Imperial College closed-loop insulin device (bio-inspired artificial pancreas) | Type 1 diabetes (adult) | - | To evaluate the safety and efficacy of closed-loop insulin pump therapy in delivering insulin in people with type 1 diabetes | NCT01534013 [163] |
| V-Go device (Humulin and insulin lispro or aspart) | Type 2 diabetes (adult) | - | To determine the efficacy of regular human insulin on the V-Go device as compared to rapid-acting insulin in the V-Go device | NCT03495908 [164] |
| FMPD (Insulin aspart and glucagon) | Type 1 diabetes (adult) | 2 | To compare the glycemic control in persons with type 1 diabetes using a fading memory proportional derivate (FMPD) algorithm with insulin plus glucagon vs. the FMPD insulin-alone algorithm | NCT00797823 |
| PassPort (R) transdermal insulin delivery system | Type 1 diabetes (adult) | 1, 2 | To evaluate the pharmacodynamics and pharmacokinetics of the PassPort(R) transdermal insulin patch | NCT00519623 |
| Medtronic miniMed implantable pump (human recombinant insulin) | Type 1 diabetes (adult) | 3 | To evaluate the effectiveness of an implantable insulin delivery pump to reduce severe hypoglycemia compared to subcutaneous insulin (MIP310) | NCT00211536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugumar, V.; Ang, K.P.; Alshanon, A.F.; Sethi, G.; Yong, P.V.C.; Looi, C.Y.; Wong, W.F. A Comprehensive Review of the Evolution of Insulin Development and Its Delivery Method. Pharmaceutics 2022, 14, 1406. https://doi.org/10.3390/pharmaceutics14071406
Sugumar V, Ang KP, Alshanon AF, Sethi G, Yong PVC, Looi CY, Wong WF. A Comprehensive Review of the Evolution of Insulin Development and Its Delivery Method. Pharmaceutics. 2022; 14(7):1406. https://doi.org/10.3390/pharmaceutics14071406
Chicago/Turabian StyleSugumar, Vaisnevee, Kuan Ping Ang, Ahmed F. Alshanon, Gautam Sethi, Phelim Voon Chen Yong, Chung Yeng Looi, and Won Fen Wong. 2022. "A Comprehensive Review of the Evolution of Insulin Development and Its Delivery Method" Pharmaceutics 14, no. 7: 1406. https://doi.org/10.3390/pharmaceutics14071406






