Influence of Dose, Particle Size and Concentration on Dermal Penetration Efficacy of Curcumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Production of Curcumin Bulk and Nanosuspensions
2.2.2. Physicochemical Characterization of Curcumin Formulations
Size Characterization
- Photon Correlation Spectroscopy (PCS)
- Laser Diffractometry (LD)
- Light Microscopy (LM)
Zeta Potential (ZP) Analysis
2.2.3. Determination of Stratum Corneum Thickness and Dermal Penetration Efficacy
Digital Image Analysis
2.2.4. Statistical Analysis
3. Results and Discussion
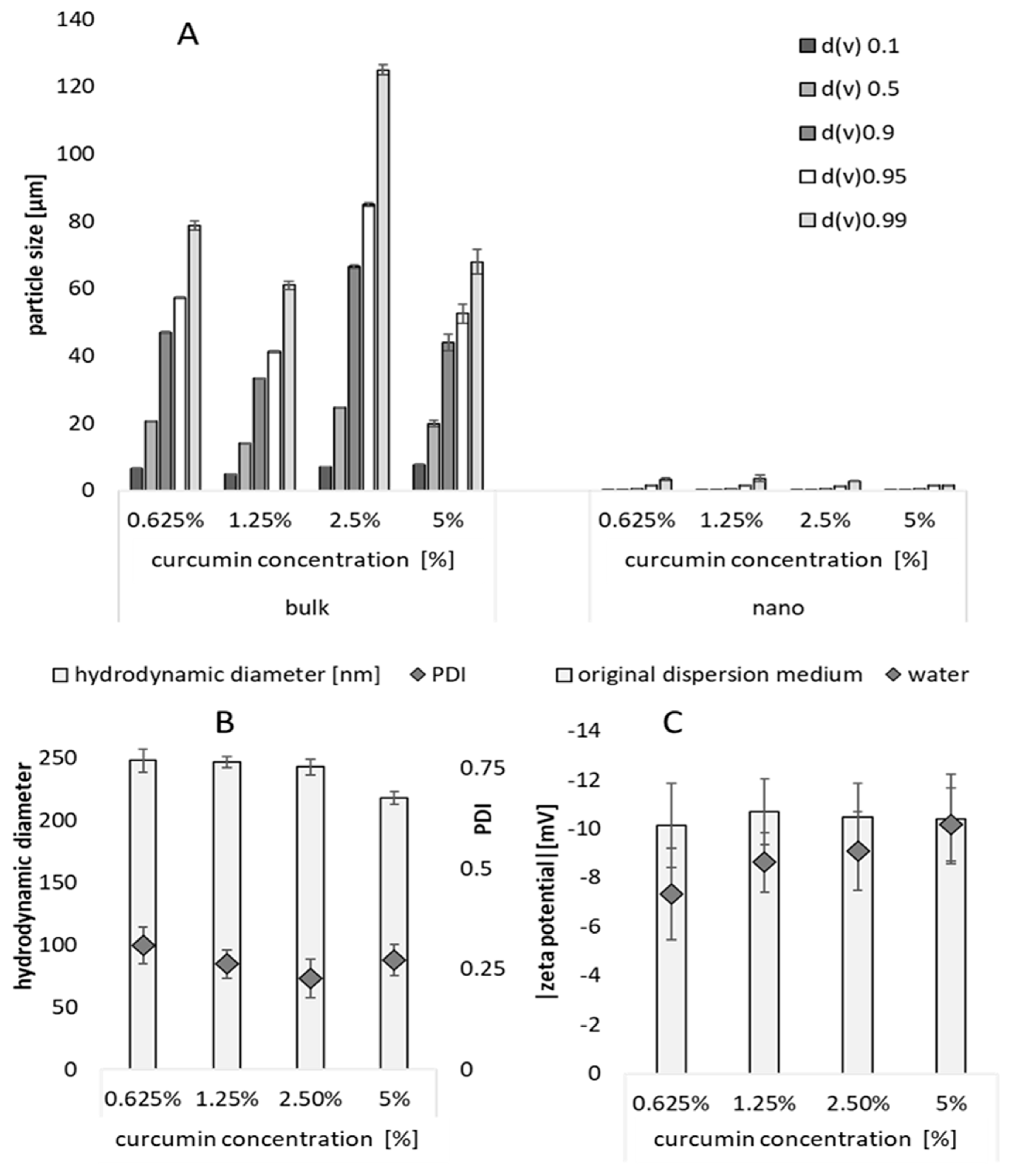
3.1. Production and Characterization of Curcumin Bulk and Nanosuspensions
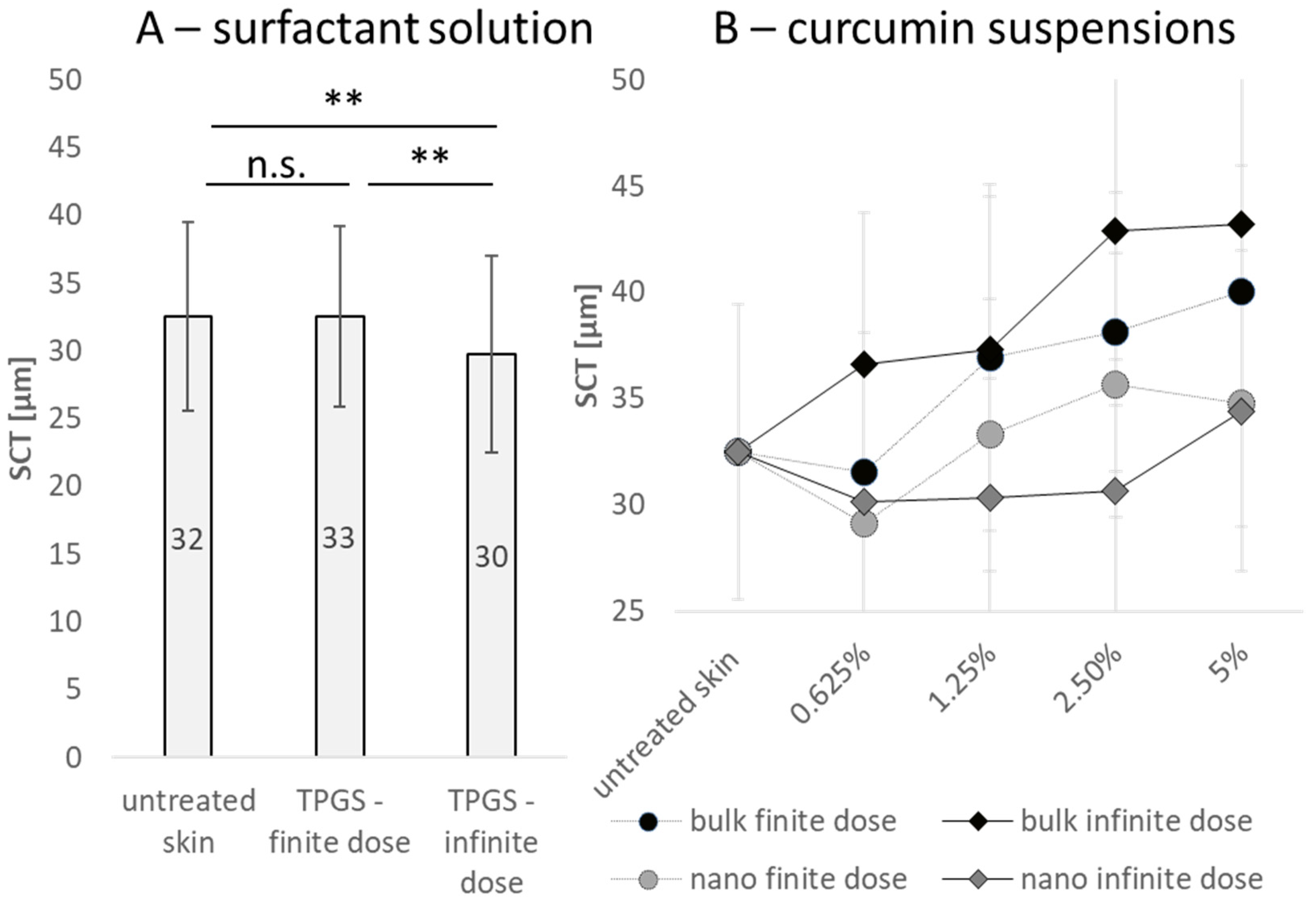
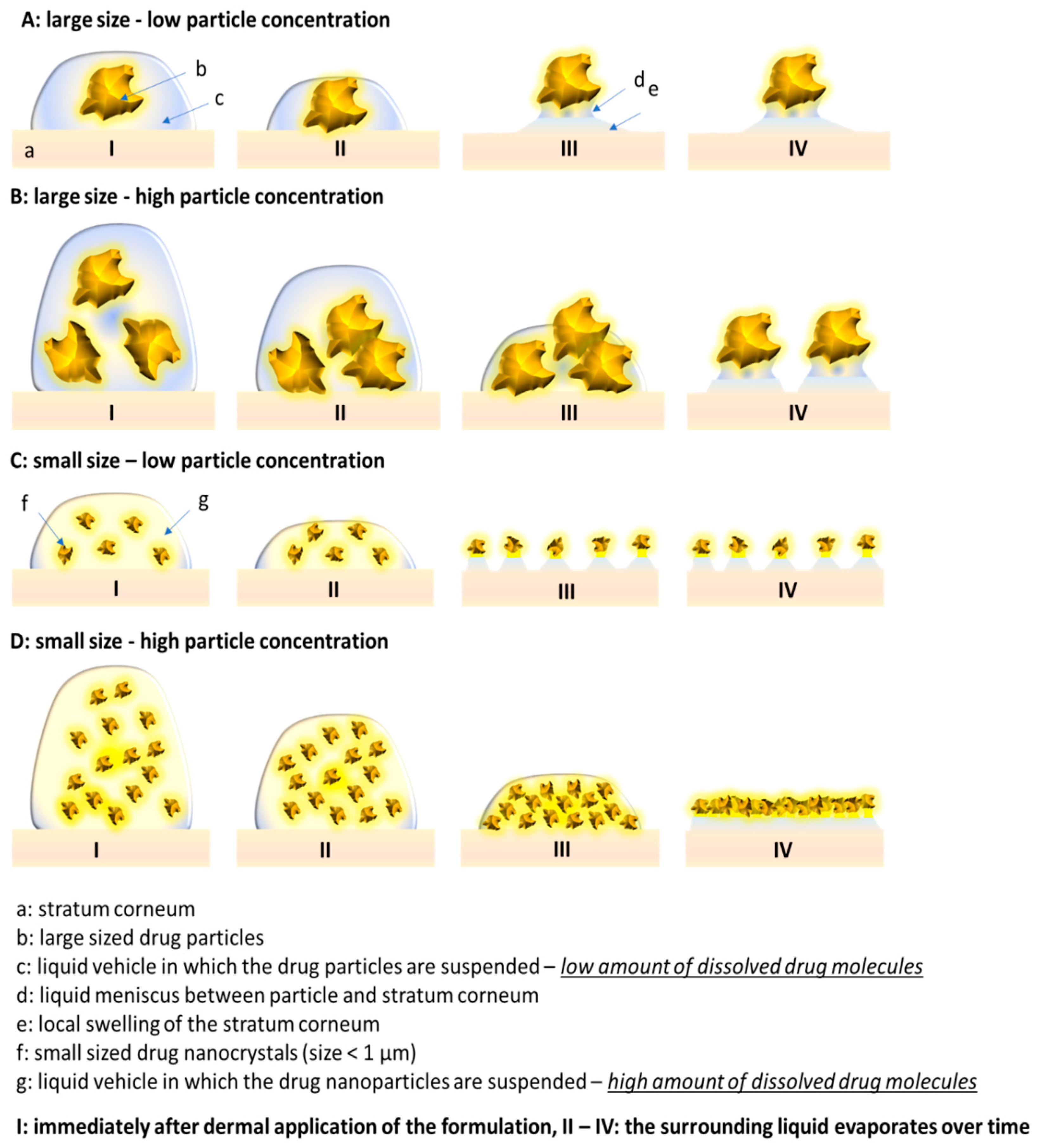
3.2. Influence of Particle Concentration, Dose and Particle Size on SC Thickness
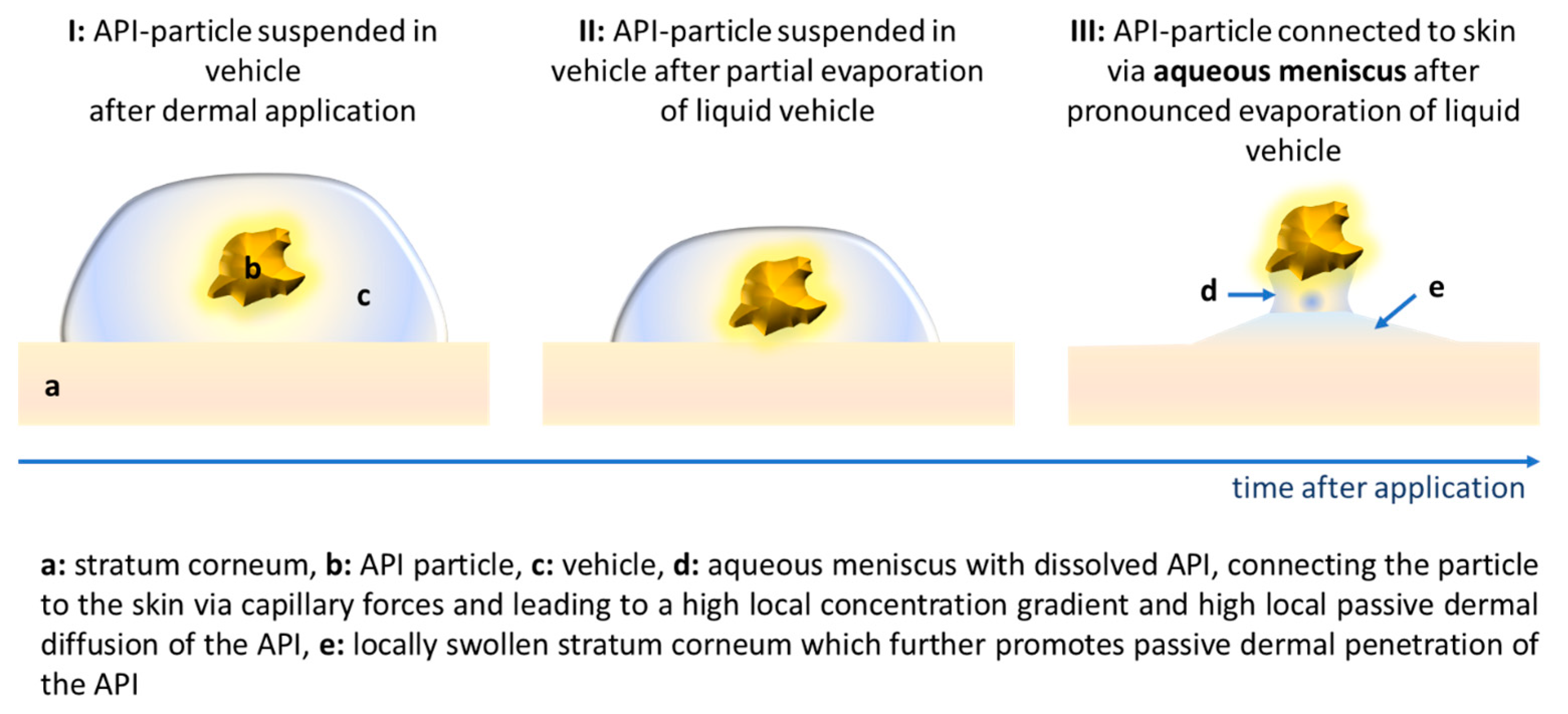
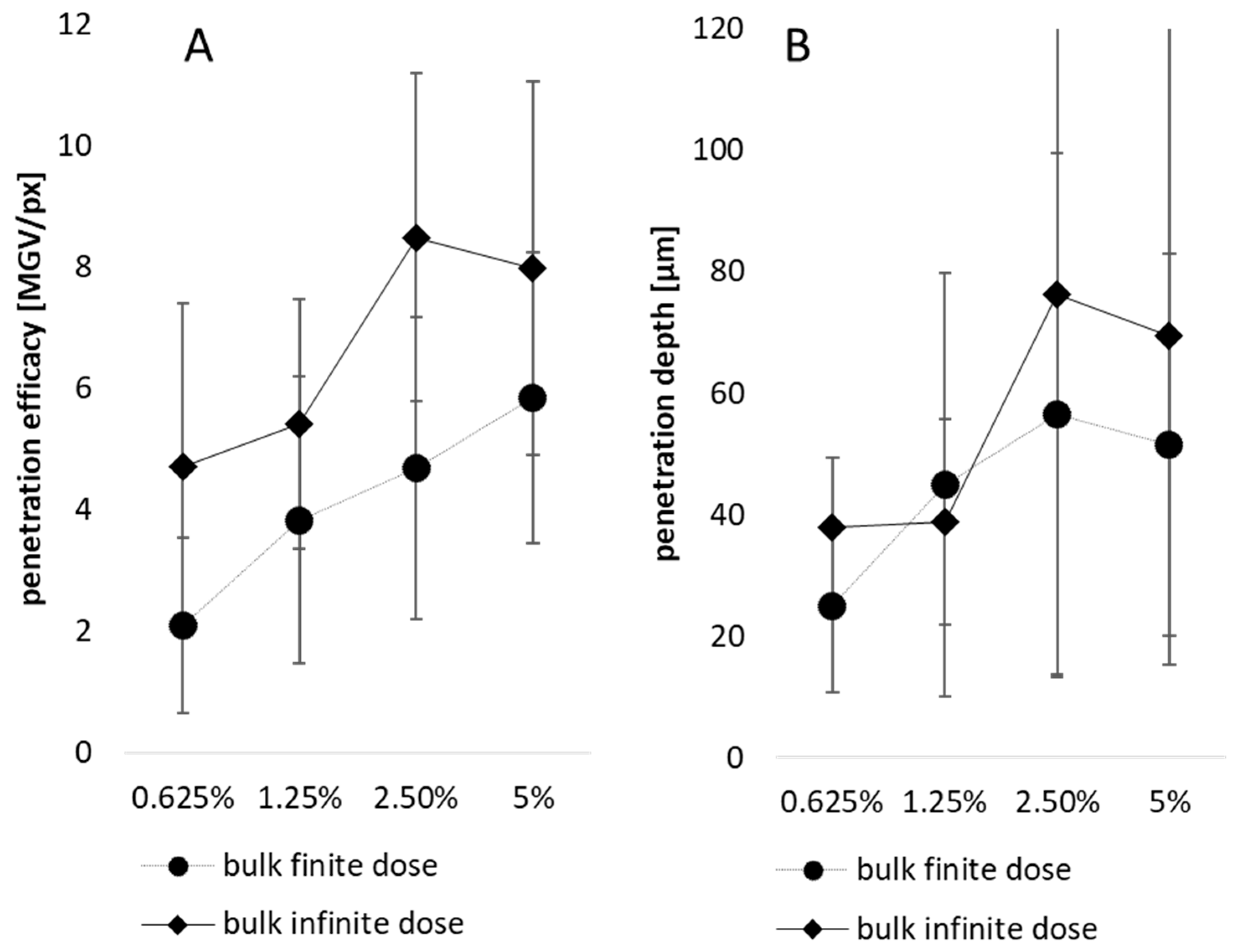
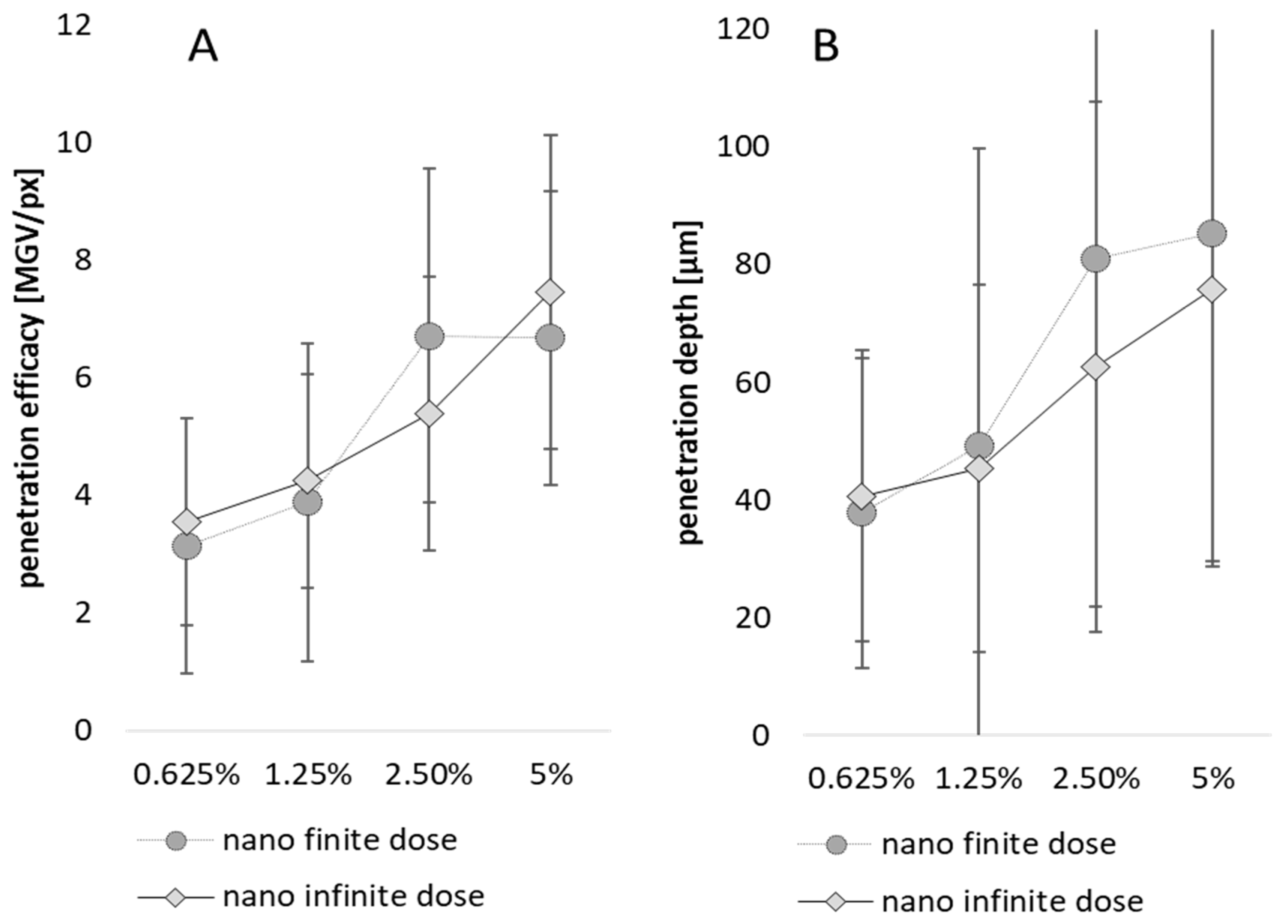
3.3. Influence of Particle Concentration, Dose and Particle Size on Dermal Penetration Efficacy
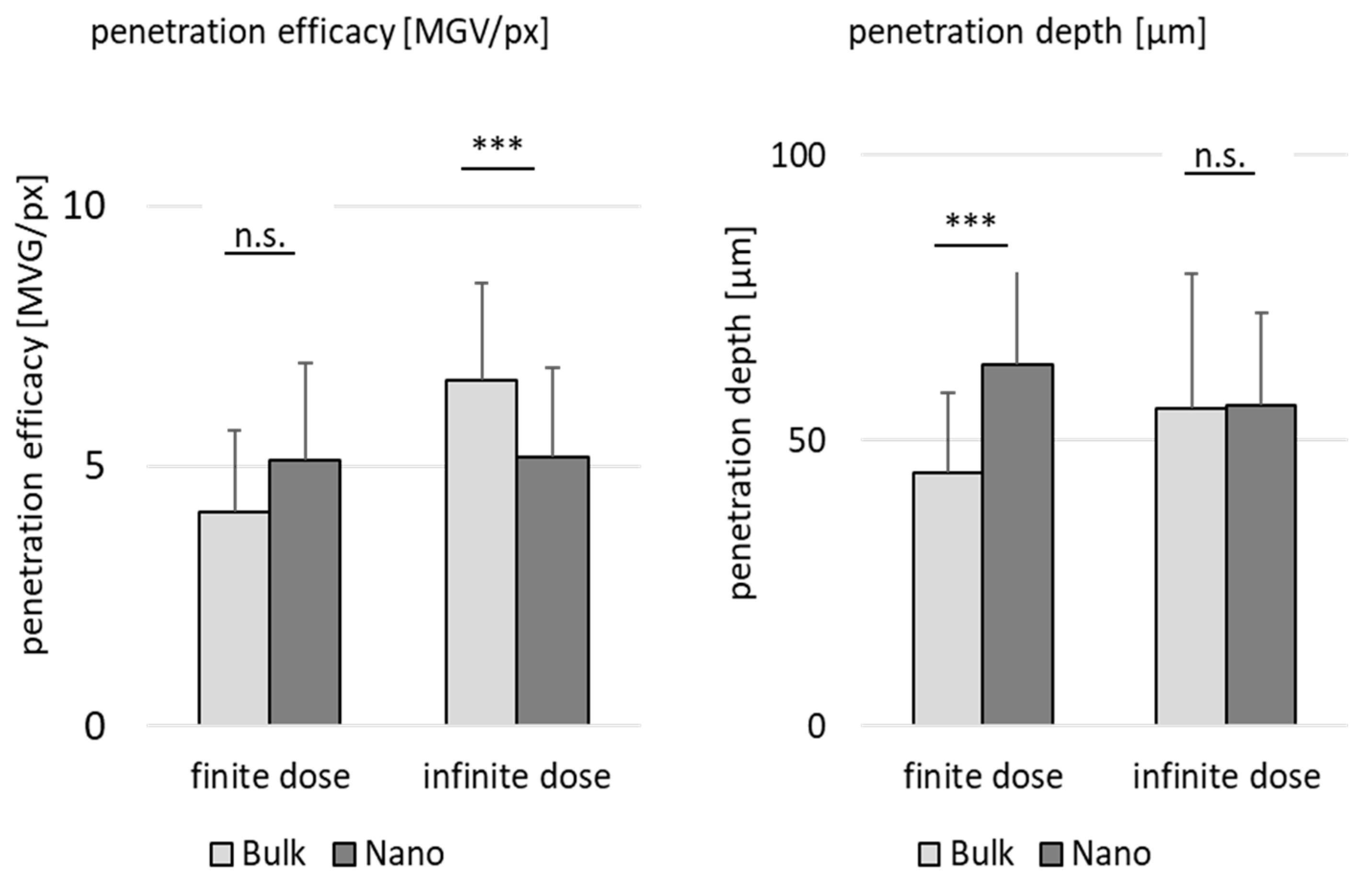
3.4. Comparison of Penetration Efficacy from Bulk Material and Nanocrystals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Gennari, C.G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Medicated foams and film forming dosage forms as tools to improve the thermodynamic activity of drugs to be administered through the skin. Curr. Drug Deliv. 2019, 16, 461–471. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in cosmetics: Recent updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Schroeter, A.; Engelbrecht, T.; Neubert, R.H.H.; Goebel, A.S.B. New nanosized technologies for dermal and transdermal drug delivery. A review. J. Biomed. Nanotechnol. 2010, 6, 511–528. [Google Scholar] [CrossRef]
- Lengert, E.V.; Talnikova, E.E.; Tuchin, V.V.; Svenskaya, Y.I. Prospective Nanotechnology-Based Strategies for Enhanced Intra- and Transdermal Delivery of Antifungal Drugs. Skin Pharmacol. Physiol. 2020, 33, 261–269. [Google Scholar] [CrossRef]
- Borchard, G. Drug Nanocrystals. In Non-Biological Complex Drugs: The Science and the Regulations; Crommelin, D.J., de Vlieger, J., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: Cham, Switzerland, 2015; Volume 20, pp. 171–189. [Google Scholar]
- Bushrab, F.N.; Müller, R.H. Nanocrystals of Poorly Soluble Drugs for Oral Administration. New Drugs 2003, 5, 20–22. [Google Scholar]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef]
- Jarvis, M.; Krishnan, V.; Mitragotri, S. Nanocrystals: A perspective on translational research and clinical studies. Bioeng. Transl. Med. 2019, 4, 5–16. [Google Scholar] [CrossRef]
- Lohan, S.B.; Saeidpour, S.; Colombo, M.; Staufenbiel, S.; Unbehauen, M.; Wolde-Kidan, A.; Netz, R.R.; Bodmeier, R.; Haag, R.; Teutloff, C.; et al. Nanocrystals for Improved Drug Delivery of Dexamethasone in Skin Investigated by EPR Spectroscopy. Pharmaceutics 2020, 12, 400. [Google Scholar] [CrossRef]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.; Benson, H.A.E.; et al. Advances and future perspectives in epithelial drug delivery. Adv. Drug Deliv. Rev. 2022, 186, 114293. [Google Scholar] [CrossRef]
- Yousef, S.A.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Benson, H.A.E.; Sakran, W.; Roberts, M.S. Mechanistic Evaluation of Enhanced Curcumin Delivery through Human Skin In Vitro from Optimised Nanoemulsion Formulations Fabricated with Different Penetration Enhancers. Pharmaceutics 2019, 11, 639. [Google Scholar] [CrossRef]
- Chen, M.L.; John, M.; Lee, S.L.; Tyner, K.M. Development Considerations for Nanocrystal Drug Products. AAPS J. 2017, 19, 642–651. [Google Scholar] [CrossRef]
- Gujar, K.; Wairkar, S. Nanocrystal technology for improving therapeutic efficacy of flavonoids. Phytomedicine 2020, 71, 153240. [Google Scholar] [CrossRef]
- Petersen, R.D. Nanocrystals for Use in Topical Cosmetic Formulations and Method of Production Thereof. US Patent US9114077B2, 25 August 2015. [Google Scholar]
- Pawar, V.K.; Singh, Y.; Meher, J.G.; Gupta, S.; Chourasia, M.K. Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery. J. Control. Release 2014, 183, 51–66. [Google Scholar] [CrossRef]
- Ochi, M.; Kawachi, T.; Toita, E.; Hashimoto, I.; Yuminoki, K.; Onoue, S.; Hashimoto, N. Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors. Int. J. Pharm. 2014, 474, 151–156. [Google Scholar] [CrossRef]
- Dhibar, M.; Chakraborty, S.; Kundu, A.; Laha, P. Chemistry Characterization and Application of Nanocrystals-based Drug Delivery System: Present to Future Perspective. Pharm. Nanotechnol. 2023, 11, 265–275. [Google Scholar] [CrossRef]
- Vidlářová, L.; Romero, G.B.; Hanuš, J.; Štěpánek, F.; Müller, R.H. Nanocrystals for dermal penetration enhancement—Effect of concentration and underlying mechanisms using curcumin as model. Eur. J. Pharm. Biopharm. 2016, 104, 216–225. [Google Scholar] [CrossRef]
- Idson, B. Percutaneous absorption. J. Pharm. Sci. 1975, 64, 901–924. [Google Scholar] [CrossRef]
- Idson, B. Hydration and percutaneous absorption. Curr. Probl. Dermatol. 1978, 7, 132–141. [Google Scholar]
- Idson, B. Vehicle effects in percutaneous absorption. Drug Metab. Rev. 1983, 14, 207–222. [Google Scholar] [CrossRef]
- Schaefer, H.; Redelmeier, T.E. Skin Penetration. In Contact Dermatitis; Frosch, P.J., Menné, T., Lepoittevin, J.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 167–178. [Google Scholar]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation properties on the penetration efficacy. J. Control. Release 2020, 10, 598–613. [Google Scholar] [CrossRef]
- Wiemann, S.; Keck, C.M. Particle-Assisted Dermal Penetration-A Simple Formulation Strategy to Foster the Dermal Penetration Efficacy. Pharmaceutics 2022, 14, 1039. [Google Scholar] [CrossRef]
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals. Molecules 2021, 26, 1633. [Google Scholar] [CrossRef]
- Keck, C.M.; Müller, R.H. Size analysis of submicron particles by laser diffractometry—90% of the published measurements are false. Int. J. Pharm. 2008, 355, 150–163. [Google Scholar] [CrossRef]
- Müller, R.H. Zetapotential und Partikeladung in der Laborpraxis; Band 37; Wissenschaftliche Verlagsgesellschaft: Suttgart, Germany, 1996. [Google Scholar]
- Lau, W.M.; Ng, K.W. Finite and Infinite Dosing. In Percutaneous Penetration Enhancers Drug Penetration into/through the Skin: Methodology and General Considerations; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–44. [Google Scholar]
- Pelikh, O.; Pinnapireddy, S.R.; Keck, C.M. Dermal penetration analysis of curcumin in an ex-vivo porcine ear model using epifluorescence microscopy and digital image processing. Skin Pharmacol. Physiol. 2021, 34, 281–299. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ: Image Processing and Analysis in Java, Version 1.8.0; ASCL: Houghton, MI, USA, 2012. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Keck, C.M. Particle size analysis of nanocrystals: Improved analysis method. Int. J. Pharm. 2010, 390, 3–12. [Google Scholar] [CrossRef]
- Stahr, P.-L.; Grewal, R.; Eckert, G.P.; Keck, C.M. Investigating hesperetin nanocrystals with tailor-made sizes for the prevention and treatment of Alzheimer’s disease. Drug Deliv. Transl. Res. 2021, 11, 659–674. [Google Scholar] [CrossRef]
- Keck, C.M.; Abdelkader, A.; Pelikh, O.; Wiemann, S.; Kaushik, V.; Specht, D.; Eckert, R.W.; Alnemari, R.M.; Dietrich, H.; Brüßler, J. Assessing the Dermal Penetration Efficacy of Chemical Compounds with the Ex-Vivo Porcine Ear Model. Pharmaceutics 2022, 14, 678. [Google Scholar] [CrossRef]
- Wiemann, S.; Keck, C.M. Are lipid nanoparticles really superior? A holistic proof of concept study. Drug Deliv. Transl. Res. 2022, 12, 1433–1444. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, S.; Zhang, W.; Li, Y.; Zhou, Y.; Miao, X. Skin permeation of curcumin nanocrystals: Effect of particle size, delivery vehicles, and permeation enhancer. Colloids Surf. B Biointerfaces 2023, 224, 113203. [Google Scholar] [CrossRef]
- OECD Expert Group on Dermal Absorption. Guidance Notes on Dermal Absorption: OECD Environment, Health and Safety Publications—Series on Testing and Assessment No. 156; OECD: Paris, France, 2019. [Google Scholar]


| Formulation | Curcumin Concentration | Applied Dose = Volume Applied on Skin | |
|---|---|---|---|
| surfactant (1% TPGS) | 0% (w/w) | finite dose = 2.5 µL/cm2 corresponds to a volume of 10 µL that was applied on the skin area of 2 × 2 cm (4 cm2) | infinite dose = 12.5 µL/cm2 corresponds to a volume of 50 µL that was applied on the skin area of 2 × 2 cm (4 cm2) |
| curcumin bulk suspension | 0.625% (w/w) | ||
| 1.25% (w/w) | |||
| 2.5% (w/w) | |||
| 5.0% (w/w) | |||
| curcumin nano suspension | 0.625% (w/w) | ||
| 1.25% (w/w) | |||
| 2.5% (w/w) | |||
| 5.0% (w/w) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiprateep, E.-o.; Wiemann, S.; Eckert, R.W.; Raab, C.; Sengupta, S.; Keck, C.M. Influence of Dose, Particle Size and Concentration on Dermal Penetration Efficacy of Curcumin. Pharmaceutics 2023, 15, 2645. https://doi.org/10.3390/pharmaceutics15112645
Chaiprateep E-o, Wiemann S, Eckert RW, Raab C, Sengupta S, Keck CM. Influence of Dose, Particle Size and Concentration on Dermal Penetration Efficacy of Curcumin. Pharmaceutics. 2023; 15(11):2645. https://doi.org/10.3390/pharmaceutics15112645
Chicago/Turabian StyleChaiprateep, Em-on, Sabrina Wiemann, Ralph W. Eckert, Christian Raab, Soma Sengupta, and Cornelia M. Keck. 2023. "Influence of Dose, Particle Size and Concentration on Dermal Penetration Efficacy of Curcumin" Pharmaceutics 15, no. 11: 2645. https://doi.org/10.3390/pharmaceutics15112645
APA StyleChaiprateep, E.-o., Wiemann, S., Eckert, R. W., Raab, C., Sengupta, S., & Keck, C. M. (2023). Influence of Dose, Particle Size and Concentration on Dermal Penetration Efficacy of Curcumin. Pharmaceutics, 15(11), 2645. https://doi.org/10.3390/pharmaceutics15112645







