Amino Acid-Based Boron Carriers in Boron Neutron Capture Therapy (BNCT)
Abstract
:1. Introduction
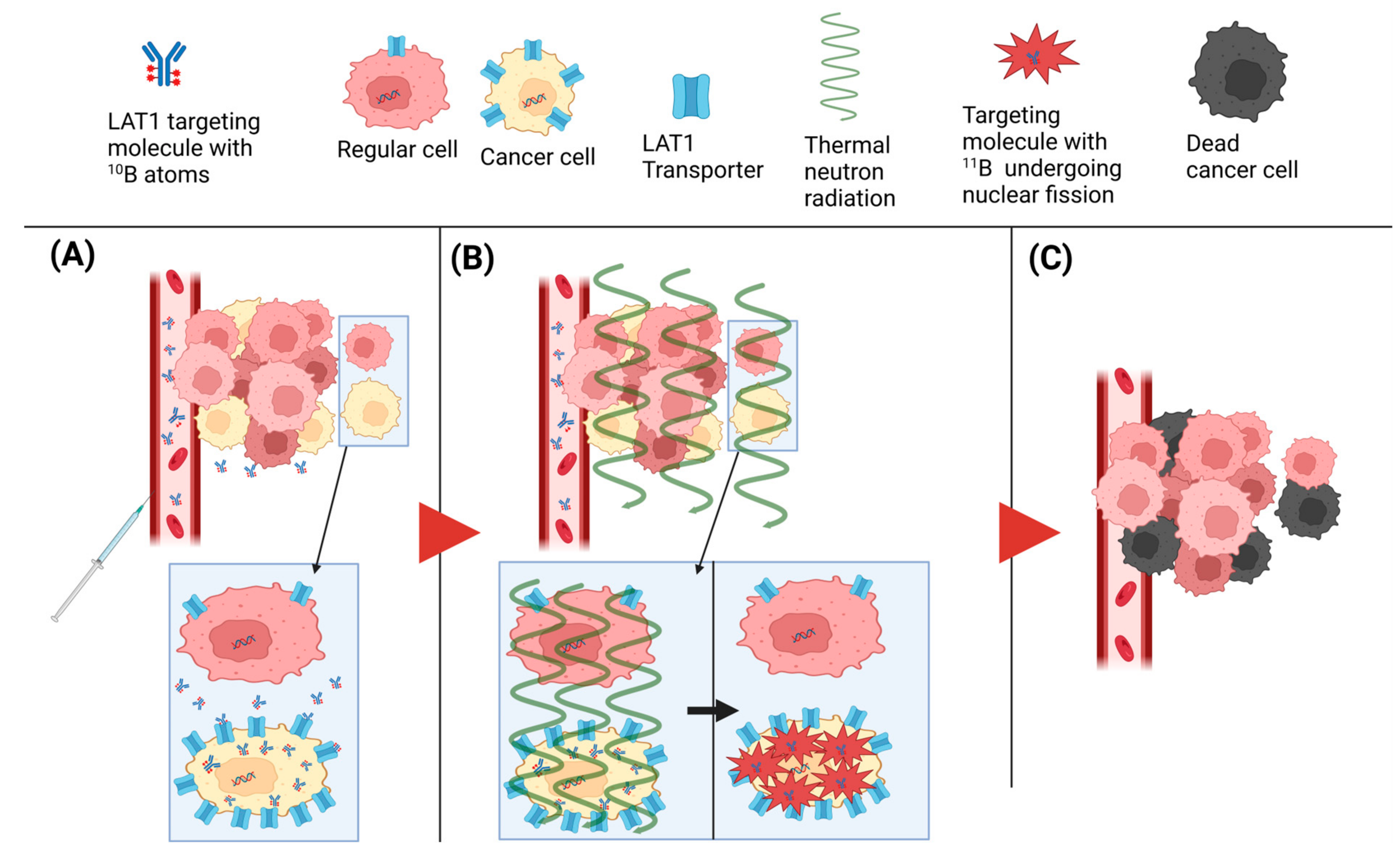
2. BNCT
3. Amino Acid-Based Boron Carriers for BNCT: A Journey through Development and Challenges
- First generation: In the 1950s and early 1960s, inorganic boron compounds, e.g., boric acid, borax, and pentaborates, were synthesized and tested in clinical trials. However, poor tumor accumulation was observed, resulting in much lower concentrations of tumors compared to the brain [49].
- Second generation: The two most efficient and prominent boron compounds, BPA and BSH, emerged in the 1960s. These boron carriers had significantly less toxicity and persisted longer in animal tumors than other tested compounds. In addition, T/B and T/N ratios were shown to be higher than 1. Promising results in vitro and in vivo resulted in these boron compounds being transferred to clinical trials and eventually to clinical use [51].
- Third generation: Recently, boron clusters with high hydrolytic stability have emerged as tools for BNCT; however, their toxicity and lack of tumor selectivity limit their direct benefits in BNCT. There are a wide variety of intellectual developments in this regard, such as polyamines, unnatural amino acids, peptides, proteins, nucleosides, sugars, porphyrins, antibodies, liposomes, and nanoparticles conjugated with either BPA, BSH, or other boronated compounds to develop a better delivery system [52].
3.1. Prehistory—Treatment of Melanoma with BPA and Its Isomers
3.2. The Challenge of Non-Invasive Detection—Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI)
3.3. The Challenge of Boron Accumulation—Polyhedral Boron Cages for Higher Load
3.4. The Challenge of Water Solubility—Complexes with Saccharides and Incorporation of Hydroxyl Groups
3.5. The Challenge of Targeting—Pure Enantiomers and Novel Amino Acid Scaffolds
4. Discussion
4.1. The Role of LAT1 in Cancer Metabolism and Its Potential as a Therapeutic Target
4.2. Current State of Amino Acid-Based Boron Carrier Development
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Savolainen, S.; Kortesniemi, M.; Timonen, M.; Reijonen, V.; Kuusela, L.; Uusi-Simola, J.; Salli, E.; Koivunoro, H.; Seppälä, T.; Lönnroth, N.; et al. Boron neutron capture therapy (BNCT) in Finland: Technological and physical prospects after 20 years of experiences. Phys. Med. 2013, 29, 233–248. [Google Scholar] [CrossRef]
- Barth, R.F.; Vicente, M.G.H.; Harling, O.K.; Kiger, W.S.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 1. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Barth, R.F.; Joensuu, H. Boron neutron capture therapy for the treatment of glioblastomas and extracranial tumours: As effective, more effective or less effective than photon irradiation? Radiother. Oncol. 2007, 82, 119–122. [Google Scholar] [CrossRef]
- Miyatake, S.I.; Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Suzuki, M.; Ono, K. Boron Neutron Capture Therapy of Malignant Gliomas. Prog. Neurol. Surg. 2018, 32, 48–56. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Mäkitie, A.; et al. Boron Neutron Capture Therapy in the Treatment of Locally Recurred Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 475–482. [Google Scholar] [CrossRef]
- Wang, L.W.; Liu, Y.W.H.; Chou, F.I.; Jiang, S.H. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing-Hua Open Pool Reactor. Cancer Commun. 2018, 38, 37. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, O.; Sakurai, Y.; Tanaka, H.; Kondo, N.; Kinashi, Y.; Masunaga, S.; Maruhashi, A.; Ono, K. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT). Int. Cancer Conf. J. 2012, 1, 235–238. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Välimäki, P.; Beule, A.; Collan, J.; Kortesniemi, M.; Uusi-Simola, J.; Kotiluoto, P.; Auterinen, I.; et al. L-boronophenylalanine-mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: A phase I study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Cai, J.; Asao, N.; Iwamoto, S.; Yamamoto, Y. Synthesis and Biological Properties of Water-Soluble p-Boronophenylalanine Derivatives. Relationship between Water Solubility, Cytotoxicity, and Cellular Uptake. J. Med. Chem. 1995, 38, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Wittig, A.; Collette, L.; Appelman, K.; Bührmann, S.; Jäckel, M.C.; Jöckel, K.H.; Schmid, K.W.; Ortmann, U.; Moss, R.; Sauerwein, W.A.G. EORTC trial 11001: Distribution of two 10B-compounds in patients with squamous cell carcinoma of head and neck, a translational research/phase 1 trial. J. Cell. Mol. Med. 2009, 13, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.; Li, J.; Hosmane, N.S.; Zhu, Y. Next generation of boron neutron capture therapy (BNCT) agents for cancer treatment. Med. Res. Rev. 2023, 43, 1809–1830. [Google Scholar] [CrossRef] [PubMed]
- Leśnikowski, Z.J.; Paradowska, E.; Olejniczak, A.B.; Studzińska, M.; Seekamp, P.; Schüßler, U.; Gabel, D.; Schinazi, R.F.; Plešek, J. Towards new boron carriers for boron neutron capture therapy: Metallacarboranes and their nucleoside conjugates. Bioorganic Med. Chem. 2005, 13, 4168–4175. [Google Scholar] [CrossRef] [PubMed]
- Imperio, D.; Panza, L. Sweet Boron: Boron-Containing Sugar Derivatives as Potential Agents for Boron Neutron Capture Therapy. Symmetry 2022, 14, 182. [Google Scholar] [CrossRef]
- Zharkov, D.O.; Yudkina, A.V.; Riesebeck, T.; Loshchenova, P.S.; Mostovich, E.A.; Dianov, G.L. Boron-containing nucleosides as tools for boron-neutron capture therapy. Am. J. Cancer Res. 2021, 11, 4668–4682. [Google Scholar]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro. Oncol. 2014, 16, iv1–iv63. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. Neurotherapeutics 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Pan, J. The role of L-type amino acid transporter 1 in human tumors. Intractable Rare Dis. Res. 2015, 4, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Uchino, H.; Kanai, Y.; Kim, D.K.; Wempe, M.F.; Chairoungdua, A.; Morimoto, E.; Anders, M.W.; Endou, H. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): Insights into the mechanisms of substrate recognition. Mol. Pharmacol. 2002, 61, 729–737. [Google Scholar] [CrossRef]
- Rautio, J.; Gynther, M.; Laine, K. LAT1-mediated prodrug uptake: A way to breach the blood-brain barrier? Ther. Deliv. 2013, 4, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Carpenter, D.E. Rodent Brain Tumor Models for Studies Focusing on Boron Neutron Capture Therapy. Cancer Biother. Radiopharm. 2023, 38, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Monti Hughes, A.; Schwint, A.E. Animal Tumor Models for Boron Neutron Capture Therapy Studies (Excluding Central Nervous System Solid Tumors). Cancer Biother. Radiopharm. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Timonen, J.M. Amino Acids in Boron Neutron Capture Therapy—Prospects for Precise Treatment of Malignant Brain Tumors. Gen. Chem. 2020, 6, 190024. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, Y.Y.; Lin, C.F.; Pan, P.S.; Chen, J.K.; Wang, C.W.; Hsu, S.M.; Kuo, Y.C.; Lan, T.L.; Hsu, S.P.C.; et al. Salvage boron neutron capture therapy for malignant brain tumor patients in compliance with emergency and compassionate use: Evaluation of 34 cases in Taiwan. Biology 2021, 10, 334. [Google Scholar] [CrossRef]
- Cheng, X.; Li, F.; Liang, L. Boron Neutron Capture Therapy: Clinical Application and Research Progress. Curr. Oncol. 2022, 29, 7868–7886. [Google Scholar] [CrossRef]
- Wang, L.W.; Chen, Y.W.; Ho, C.Y.; Hsueh Liu, Y.W.; Chou, F.I.; Liu, Y.H.; Liu, H.M.; Peir, J.J.; Jiang, S.H.; Chang, C.W.; et al. Fractionated Boron Neutron Capture Therapy in Locally Recurrent Head and Neck Cancer: A Prospective Phase I/II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 396–403. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front. Oncol. 2021, 11, 351. [Google Scholar] [CrossRef]
- Maitz, C.A.; Brockman, J.D.; Yang, M.; Zhang, S.; Stannard, J.; Volgas, D.; Gahl, J.M. Demonstration of the bactericidal effects of the boron neutron capture reaction. Appl. Radiat. Isot. 2018, 137, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Halfon, S.; Paul, M.; Steinberg, D.; Nagler, A.; Arenshtam, A.; Kijel, D.; Polacheck, I.; Srebnik, M. High power accelerator-based boron neutron capture with a liquid lithium target and new applications to treatment of infectious diseases. Appl. Radiat. Isot. 2009, 67, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Azzi, E.; Alberti, D.; Parisotto, S.; Oppedisano, A.; Protti, N.; Altieri, S.; Geninatti-Crich, S.; Deagostino, A. Design, synthesis and preliminary in-vitro studies of novel boronated monocarbonyl analogues of Curcumin (BMAC) for antitumor and β-amiloyd disaggregation activity. Bioorg. Chem. 2019, 93, 103324. [Google Scholar] [CrossRef]
- Miyatake, S.I.; Wanibuchi, M.; Hu, N.; Ono, K. Boron neutron capture therapy for malignant brain tumors. J. Neurooncol. 2020, 149, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kiyanagi, Y.; Sakurai, Y.; Kumada, H.; Tanaka, H. Status of accelerator-based BNCT projects worldwide. AIP Conf. Proc. 2019, 2160, 050012. [Google Scholar] [CrossRef]
- Kawabata, S.; Suzuki, M.; Hirose, K.; Tanaka, H.; Kato, T.; Goto, H.; Narita, Y.; Miyatake, S.-I. Accelerator-based BNCT for patients with recurrent glioblastoma: A multicenter phase II study. Neuro-Oncol. Adv. 2021, 3, vdab067. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Konno, A.; Hiratsuka, J.; Yoshimoto, S.; Kato, T.; Ono, K.; Otsuki, N.; Hatazawa, J.; Tanaka, H.; Takayama, K.; et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother. Oncol. 2021, 155, 182–187. [Google Scholar] [CrossRef]
- Coderre, J.A.; Morris, G.M. The radiation biology of boron neutron capture therapy. Radiat. Res. 1999, 151, 1–18. [Google Scholar] [CrossRef]
- Koivunoro, H.; Kankaanranta, L.; Seppälä, T.; Haapaniemi, A.; Mäkitie, A.; Joensuu, H. Boron neutron capture therapy for locally recurrent head and neck squamous cell carcinoma: An analysis of dose response and survival. Radiother. Oncol. 2019, 137, 153–158. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Z.; Li, B.; Chen, G.; Xie, X.; Wei, Y.; Wu, J.; Zhou, Y.; Du, Z. Boron neutron capture therapy induces cell cycle arrest and cell apoptosis of glioma stem/progenitor cells in vitro. Radiat. Oncol. 2013, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Faião-Flores, F.; Coelho, P.R.P.; Arruda-Neto, J.D.T.; Maria-Engler, S.S.; Maria, D.A. Cell cycle arrest, extracellular matrix changes and intrinsic apoptosis in human melanoma cells are induced by Boron Neutron Capture Therapy. Toxicol. Vitr. 2013, 27, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kanda, K. Analytical calculation of boron-10 dosage in cell nucleus of neutron capture therapy. Radiat. Res. 1982, 91, 77–94. [Google Scholar] [CrossRef]
- Soloway, A.H.; Barth, R.F.; Gahbauer, R.A.; Blue, T.E.; Goodman, J.H. The rationale and requirements for the development of boron neutron capture therapy of brain tumors. J. Neurooncol. 1997, 33, 09–18. [Google Scholar] [CrossRef] [PubMed]
- Pazirandeh, A.; Torkamani, A.; Taheri, A. Design and simulation of a neutron source based on an electron linear accelerator for BNCT of skin melanoma. Appl. Radiat. Isot. 2011, 69, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Coderre, J.A.; Glass, J.D.; Fairchild, R.G.; Roy, U.; Cohen, S.; Fand, I. Selective Targeting of Boronophenylalanine to Melanoma in BALB/c Mice for Neutron Capture Therapy. Cancer Res. 1987, 47, 6377–6383. [Google Scholar] [PubMed]
- Coderre, J.A.; Fairchild, R.G.; Micca, P.L.; Joel, D.D.; Glass, J.D.; Fand, I. Selective Delivery of Boron by the Melanin Precursor Analogue p-Boronophenylalanine to Tumors Other Than Melanoma. Cancer Res. 1990, 50, 138–141. [Google Scholar]
- Wongthai, P.; Hagiwara, K.; Miyoshi, Y.; Wiriyasermkul, P.; Wei, L.; Ohgaki, R.; Kato, I.; Hamase, K.; Nagamori, S.; Kanai, Y. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015, 106, 279–286. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Lamba, M.; Goswami, A.; Bandyopadhyay, A. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem. Commun. 2021, 57, 827–839. [Google Scholar] [CrossRef]
- Barth, R.F.; Soloway, A.H.; Fairchild, R.G. Boron Neutron Capture Therapy for Cancer without damaging normal tissues in the process. Sci. Am. 1990, 263, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Snyder, H.R.; Reedy, A.J.; Lennarz, W.J. Synthesis of Aromatic Boronic Acids. Aldehydo Boronic Acids and a Boronic Acid Analog of Tyrosine. J. Am. Chem. Soc. 1958, 80, 835–838. [Google Scholar] [CrossRef]
- Soloway, A.H.; Whitman, B.; Messer, J.R. Penetration of Brain and Brain Tumor by Aromatic Compounds as a Function of Molecular Substituents. III. J. Med. Pharm. Chem. 1962, 5, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Soloway, A.H.; Wright, R.L.; Messer, J.R. Evaluation of boron compounds for use in neutron capture therapy of brain tumors. I. Animal investigations. J. Pharmacol. Exp. Ther. 1961, 134, 117–122. [Google Scholar] [PubMed]
- Packer, S.; Coderre, J.; Saraf, S.; Fairchild, R.; Hansrote, J.; Perry, H. Boron neutron capture therapy of anterior chamber melanoma with p-boronophenylalanine. Investig. Ophthalmol. Vis. Sci. 1992, 33, 395–403. [Google Scholar]
- Belkhou, R.; Abbé, J.C.; Pham, P.; Jasner, N.; Sahel, J.; Dreyfus, H.; Moutaouakkil, M.; Massarelli, R. Uptake and metabolism of boronophenylalanine in human uveal melanoma cells in culture Relevance to boron neutron capture therapy of cancer cells. Amino Acids 1995, 8, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Kondoh, H.; Hiratsuka, J.; Mishima, Y. Enhanced Melanogenesis Induced by Tyrosinase Gene-Transfer Increases Boron-Uptake and Killing Effect of Boron Neutron Capture Therapy for Amelanotic Melanoma. Pigment Cell Res. 1998, 11, 275–282. [Google Scholar] [CrossRef]
- Hiratsuka, J.; Yoshino, K.; Kondoh, H.; Imajo, Y.; Mishima, Y. Biodistribution of boron concentration on melanoma-bearing hamsters after administration of p-, m-, o-boronophenylalanine. Jpn. J. Cancer Res. 2000, 91, 446–450. [Google Scholar] [CrossRef]
- Chandra, S.; Lorey, D.R. SIMS ion microscopy imaging of boronophenylalanine (BPA) and 13C15N-labeled phenylalanine in human glioblastoma cells: Relevance of subcellular scale observations to BPA-mediated boron neutron capture therapy of cancer. Int. J. Mass Spectrom. 2007, 260, 90–101. [Google Scholar] [CrossRef]
- Barth, R.F.; Yang, W.; Rotaru, J.H.; Moeschberger, M.L.; Joel, D.D.; Nawrocky, M.M.; Goodman, J.H.; Soloway, A.H. Boron neutron capture therapy of brain tumors: Enhanced survival following intracarotid injection of either sodium borocaptate or boronophenylalanine with or without blood-brain barrier disruption. Cancer Res. 1997, 57, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Rossini, A.E.; Dagrosa, M.A.; Portu, A.; Saint Martin, G.; Thorp, S.; Casal, M.; Navarro, A.; Juvenal, G.J.; Pisarev, M.A. Assessment of biological effectiveness of boron neutron capture therapy in primary and metastatic melanoma cell lines. Int. J. Radiat. Biol. 2015, 91, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Imahori, Y.; Ueda, S.; Ohmori, Y.; Kusuki, T.; Ono, K.; Fujii, R.; Ido, T. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J. Nucl. Med. 1998, 39, 325–333. [Google Scholar] [PubMed]
- Hattori, Y.; Yamamoto, H.; Ando, H.; Kondoh, H.; Asano, T.; Kirihata, M.; Yamaguchi, Y.; Wakamiya, T. Synthesis and evaluation as MRI probe of the trifluoromethylated p-boronophenylalanine and its alcohol derivative. Bioorganic Med. Chem. 2007, 15, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Asano, T.; Niki, Y.; Kondoh, H.; Kirihata, M.; Yamaguchi, Y.; Wakamiya, T. Study on the compounds containing 19F and 10B atoms in a single molecule for the application to MRI and BNCT. Bioorganic Med. Chem. 2006, 14, 3258–3262. [Google Scholar] [CrossRef]
- Ishiwata, K.; Ido, T.; Mejia, A.A.; Ichihashi, M.; Mishima, Y. Synthesis and radiation dosimetry of 4-borono-2-[18F]fluoro-d,l-phenylalanine: A target compound for PET and boron neutron capture therapy. Int. J. Radiat. Appl. Instrum. Part 1991, 42, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Yamada, S.; Ishiwata, K.; Tada, M.; Ido, T.; Kubota, K. Cellular accumulation of 18F-labelled boronophenylalanine depending on DNA synthesis and melanin incorporation: A double-tracer microautoradiographic study of B16 melanomas in vivo. Br. J. Cancer 1993, 67, 701–705. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Honda, N.; Kurihara, H.; Hiroi, K.; Nakamura, S.; Ito, M.; Shikano, N.; Itami, J.; Fujii, H. Non-invasive estimation of 10B-4-borono-L-phenylalanine-derived boron concentration in tumors by PET using 4-borono-2-18F-fluoro-phenylalanine. Cancer Sci. 2018, 109, 1617–1626. [Google Scholar] [CrossRef]
- Deng, J.P.; Yu, C.S. Recent Development of Radiofluorination of Boron Agents for Boron Neutron Capture Therapy of Tumor: Creation of 18F-Labeled C-F and B-F Linkages. Pharmaceuticals 2023, 16, 93. [Google Scholar] [CrossRef]
- Wingelhofer, B.; Kreis, K.; Mairinger, S.; Muchitsch, V.; Stanek, J.; Wanek, T.; Langer, O.; Kuntner, C. Preloading with L-BPA, L-tyrosine and L-DOPA enhances the uptake of [18F]FBPA in human and mouse tumour cell lines. Appl. Radiat. Isot. 2016, 118, 67–72. [Google Scholar] [CrossRef]
- Grunewald, C.; Sauberer, M.; Filip, T.; Wanek, T.; Stanek, J.; Mairinger, S.; Rollet, S.; Kudejova, P.; Langer, O.; Schütz, C.; et al. On the applicability of [18F]FBPA to predict L-BPA concentration after amino acid preloading in HuH-7 liver tumor model and the implication for liver boron neutron capture therapy. Nucl. Med. Biol. 2017, 44, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, Y.; Zhang, Z.; Liu, H.; Lang, L.; Liu, T.; Chen, X.; Liu, Z. A Metabolically Stable Boron-Derived Tyrosine Serves as a Theranostic Agent for Positron Emission Tomography Guided Boron Neutron Capture Therapy. Bioconjug. Chem. 2019, 30, 2870–2878. [Google Scholar] [CrossRef] [PubMed]
- Leukart, O.; Caviezel, M.; Eberle, A.; Escher, E.; Tun-Kyi, A.; Schwyzer, R. L-o-Carboranylalanine, a Boron Analogue of Phenylalanine. Helv. Chim. Acta 1976, 59, 2184–2187. [Google Scholar] [CrossRef]
- Brattsev, V.; Stanko, V. β-(O-barenyl) alanine. Zh Obs. Khim 1969, 39, 1175–1176. [Google Scholar]
- Yong, J.-H.; Barth, R.F.; Wyzlic, I.M.; Soloway, A.H.; Rotaru, J.H. In vitro and in vivo evaluation of o-Carboranylalanine as a potential boron delivery agent for neutron capture therapy. Anticancer Res. 1995, 16, 113–120. [Google Scholar]
- Wyzlic, I.M.; Soloway, A.I. A General, Convenient Way to Carborane-Containing Amino Acids for Boron Neutron Capture Therapy. Tetrahedron 1992, 33, 7489–7490. [Google Scholar] [CrossRef]
- Yong, J.H.; Barth, R.F.; Rotaru, J.H.; Wyzlic, I.M.; Soloway, A.H. Evaluation of in vitro cytotoxicity of carboranyl amino acids, their chemical precursors and nido carboranyl amino acids for Boron Neutron Capture Therapy. Anticancer Res. 1995, 15, 2039–2043. [Google Scholar]
- Olsson, P.; Black, M.; Capala, J.; Coderre, J.; Hartman, T.; Makar, M.; Malmquist, J.; Pettersson, J.; Tilly, N.; Sjoberg, S.; et al. Uptake, toxicity and radiation effects of the boron compounds DAAC-1 and DAC-1 in cultured human glioma cells. Int. J. Radiat. Oncol. Biol. 1998, 73, 103–112. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusaka, S.; Mukumoto, M.; Uehara, K.; Asano, T.; Suzuki, M.; Masunaga, S.; Ono, K.; Tanimori, S.; Kirihata, M. Biological Evaluation of Dodecaborate-Containing L-Amino Acids for Boron Neutron Capture Therapy. J. Med. Chem. 2012, 10, 6980–6984. [Google Scholar] [CrossRef]
- He, T.; Musah, R.A. Evaluation of the Potential of 2-Amino-3-(1,7-dicarba- closo-dodecaboranyl-1-thio)propanoic acid as a boron neutron capture therapy agent. ACS Omega 2019, 4, 3820–3826. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Kabalka, G.W. Syntheses of 1-Amino-3-[2-(7-(2-hydroxyethyl)-1,7-dicarba-closo-dodecaboran(12)-1-yl)ethyl] cyclobutanecarboxylic Acid and Its nido-Analogue: Potential BNCT Agents. J. Org. Chem. 1997, 62, 8730–8734. [Google Scholar] [CrossRef]
- Yoshino, K.; Suzuki, A.; Mori, Y.; Kakihana, H.; Honda, C.; Mishima, Y.; Kobayashi, T.; Kanda, K. Improvement of solubility of p-boronophenylalanine by complex formation with monosaccharides. Strahlenther. Onkol. 1989, 165, 127–129. [Google Scholar] [PubMed]
- Heikkinen, S.; Savolainen, S.; Melkko, P. In vitro studies on stability of L-p-boronophenylalanine-fructose complex (BPA-F). J. Radiat. Res. 2011, 52, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Shull, B.K.; Spielvogel, D.E.; Head, G.; Gopalaswamy, R.; Sankar, S.; Devito, K. Studies on the Structure of the Complex of the Boron Neutron Capture Therapy Drug, L-p-Boronophenylalanine, with Fructose and Related Carbohydrates: Chemical and 13C NMR evidence for the β-D-Fructofuranose 2,3,6-(p-Phenylalanylorthoboronate) Structure. J. Pharm. Sci. 2000, 89, 215–222. [Google Scholar] [CrossRef]
- Kirihata, M.; Morimoto, T.; Mizuta, T.; Ichimoto, I. Tumor-targetted boron-containing amino acid. Part II. Synthesis of p-boronophenylserine, a new boron-containing amino acid for boron neutron capture therapy. Biosci. Biotechnol. Biochem. 1995, 59, 2317–2318. [Google Scholar] [CrossRef]
- Das, B.C.; Kabalka, G.W.; Srivastava, R.R.; Bao, W.; Das, S.; Li, G. Synthesis of a water soluble boron neutron capture therapy agent: 1-amino-3-[2-(7-(3-[2-(2-hydroxymethyl-ethoxy)-1-(2-hydroxy-1-hydroxymethyl-ethoxymethyl)ethoxy]propyl)-1,7-di-carba-closo-dodecaboran-1-yl)ethyl] cyclobutanecarboxylic acid. J. Organomet. Chem. 2000, 614, 255–261. [Google Scholar] [CrossRef]
- Sjoberg, S.; Hawthorne, M.F.; Wilmouth, S.; Lindstrom, P. Asymmetric Synthesis of Carboranyl Amino Acids with Potential Use in BNCT. Chem. A Eur. J. 1995, 1, 430–435. [Google Scholar] [CrossRef]
- Malmquist, J.; Sjoberg, S. Asymmetric Synthesis of p-Carboranylalanine (p-Car) and 2-Methyl-o-Carboranylalanine (Me-o-Car). Tetrahedron 1996, 52, 9207–9218. [Google Scholar] [CrossRef]
- Karnborg, W.; Musiol, H.-J.; Moroder, L. Enantioselective Synthesis of S-o-Carboranylalanine via Methylated Bislactim Ethers of 2,5-Diketopiperazines. Tetrahedron 1995, 51, 1187–1196. [Google Scholar]
- Kessels, M.M.; Qualmann, B.; Baumgartner, M. Facile Enantioselective Synthesis of (S)-5-(2-Methyl-1,2-dicarba_closo-dodecaborane(l2)-1-yl)-2-aminopentanoic Acid (L-MeCBA) Using the Bislactim Ether Method. J. Fur Prakt. Chemie Chem. 1996, 338, 89–91. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. L-4-Boronophenylalanine (all around the one molecule). Arkivoc 2008, 2008, 47–61. [Google Scholar] [CrossRef]
- Zaidlewicz, M.; Cytarska, J.; Dzielendziak, A.; Ziegler-Borowska, M. Synthesis of boronated phenylalanine analogues with a quaternary center for boron neutron capture therapy. Arkivoc 2004, 2004, 11–21. [Google Scholar] [CrossRef]
- Harada, S.; Kajihara, R.; Muramoto, R.; Jutabha, P.; Anzai, N.; Nemoto, T. Catalytic asymmetric synthesis of α-methyl-p-boronophenylalanine. Bioorganic Med. Chem. Lett. 2018, 28, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Hübner, K.F.; Thie, J.A.; Smith, G.T.; Kabalka, G.W.; Keller, I.B.; Kliefoth, A.B.; Campbell, S.K.; Buonocore, E. Positron Emission Tomography (PET) with 1-Aminocyclobutane-1-[11C]carboxylic Acid (1-[11C]-ACBC) for Detecting Recurrent Brain Tumors. Clin. Positron Imaging 1998, 1, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Wu, Z.Z.; Yao, M.; Natarajan, N. The syntheses and in vivo biodistribution of novel boronated unnatural amino acids. Appl. Radiat. Isot. 2004, 61, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Yao, M.L. Synthesis of 1-amino-3-[(dihydroxyboryl)methyl]-cyclobutanecarboxylic acid as a potential therapy agent. J. Org. Chem. 2004, 69, 8280–8286. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Shaikh, A.L.; Barth, R.F.; Huo, T.; Yang, W.; Gordnier, P.M.; Chandra, S. Boronated unnatural cyclic amino acids as potential delivery agents for neutron capture therapy. Appl. Radiat. Isot. 2011, 69, 1778–1781. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Yao, M.L.; Marepally, S.R.; Chandra, S. Biological evaluation of boronated unnatural amino acids as new boron carriers. Appl. Radiat. Isot. 2009, 67, S374–S379. [Google Scholar] [CrossRef]
- Chandra, S.; Barth, R.F.; Haider, S.A.; Yang, W.; Huo, T.; Shaikh, A.L.; Kabalka, G.W. Biodistribution and Subcellular Localization of an Unnatural Boron-Containing Amino Acid (Cis-ABCPC) by Imaging Secondary Ion Mass Spectrometry for Neutron Capture Therapy of Melanomas and Gliomas. PLoS ONE 2013, 8, e75377. [Google Scholar] [CrossRef]
- Barth, R.F.; Kabalka, G.W.; Yang, W.; Huo, T.; Nakkula, R.J.; Shaikh, A.L.; Haider, S.A.; Chandra, S. Evaluation of unnatural cyclic amino acids as boron delivery agents for treatment of melanomas and gliomas. Appl. Radiat. Isot. 2014, 88, 38–42. [Google Scholar] [CrossRef]
- Chandra, S.; Ahmad, T.; Barth, R.F.; Kabalka, G.W. Quantitative evaluation of boron neutron capture therapy (BNCT) drugs for boron delivery and retention at subcellular-scale resolution in human glioblastoma cells with imaging secondary ion mass spectrometry (SIMS). J. Microsc. 2014, 254, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Wu, Z.; Yao, M.L. Synthesis of a series of boronated unnatural cyclic amino acids as potential boron neutron capture therapy agents. Appl. Organomet. Chem. 2008, 22, 516–522. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Singhaus, R.R.; Kabalka, G.W. 4-Dihydroxyborylphenyl Analogues of 1-Aminocyclobutanecarboxylic Acids: Potential Boron Neutron Capture Therapy Agents. J. Org. Chem. 1999, 64, 8495–8500. [Google Scholar] [CrossRef]
- Futamura, G.; Kawabata, S.; Nonoguchi, N.; Hiramatsu, R.; Toho, T.; Tanaka, H.; Masunaga, S.I.; Hattori, Y.; Kirihata, M.; Ono, K.; et al. Evaluation of a novel sodium borocaptate-containing unnatural amino acid as a boron delivery agent for neutron capture therapy of the F98 rat glioma. Radiat. Oncol. 2017, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kusaka, S.; Mukumoto, M.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Uehara, K.; Asano, T.; Suzuki, M.; Masunaga, S.I.; et al. Synthesis and in vitro evaluation of thiododecaborated α, α-Cycloalkylamino acids for the treatment of malignant brain tumors by boron neutron capture therapy. Amino Acids 2014, 46, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Miao, L.; Li, Y. Boron Neutron Capture Therapy: Current Status and Challenges. Front. Oncol. 2022, 12, 788770. [Google Scholar] [CrossRef] [PubMed]
- Youland, R.S.; Kitange, G.J.; Peterson, T.E.; Pafundi, D.H.; Ramiscal, J.A.; Pokorny, J.L.; Giannini, C.; Laack, N.N.; Parney, I.F.; Lowe, V.J.; et al. The role of LAT1 in 18F-DOPA uptake in malignant gliomas. J. Neurooncol. 2013, 111, 11–18. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, I.J.; Hwang, S.; Kook, J.H.; Lee, M.C.; Shin, B.A.; Bae, C.S.; Yoon, J.H.; Ahn, S.G.; Kim, S.A.; et al. System L-amino acid transporters are differently expressed in rat astrocyte and C6 glioma cells. Neurosci. Res. 2004, 50, 437–446. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ishii, Y.; Takayama, T. Expression of L-Type Amino Acid Transporter 1 (LAT1) in Esophageal Carcinoma. J. Surg. Oncol. 2005, 1, 233–238. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron Rev. 2008, 2, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Smith, Q.R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 2000, 130, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.R.; Fuller, R.W.; Ahmad, S.; Vistica, D.T.; Marquez, V.E. Selective cytotoxicity of a system L specific amino acid nitrogen mustard. J. Med. Chem. 2002, 30, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Ylikangas, H.; Peura, L.; Malmioja, K.; Leppänen, J.; Laine, K.; Poso, A.; Lahtela-Kakkonen, M.; Rautio, J. Structure-activity relationship study of compounds binding to large amino acid transporter 1 (LAT1) based on pharmacophore modeling and in situ rat brain perfusion. Eur. J. Pharm. Sci. 2013, 48, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Kankaanranta, L.; Saarilahti, K.; Mäkitie, A.; Välimäki, P.; Tenhunen, M.; Joensuu, H. Boron neutron capture therapy (BNCT) followed by intensity modulated chemoradiotherapy as primary treatment of large head and neck cancer with intracranial involvement. Radiother. Oncol. 2011, 99, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Miyatake, S.I.; Kuroiwa, T.; Yokoyama, K.; Doi, A.; Iida, K.; Miyata, S.; Nonoguchi, N.; Michiue, H.; Takahashi, M.; et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J. Radiat. Res. 2009, 50, 51–60. [Google Scholar] [CrossRef]
- Miyatake, S.I.; Kawabata, S.; Hiramatsu, R.; Kuroiwa, T.; Suzuki, M.; Kondo, N.; Ono, K. Boron neutron capture therapy for malignant brain tumors. Neurol. Med. Chir. 2016, 56, 361–371. [Google Scholar] [CrossRef]
- Nawashiro, H.; Otani, N.; Uozumi, Y.; Ooigawa, H.; Toyooka, T.; Suzuki, T.; Katoh, H.; Tsuzuki, N.; Ohnuki, A.; Shima, K.; et al. High expression of L-type amino acid transporter 1 in infiltrating glioma cells. Brain Tumor Pathol. 2005, 22, 89–91. [Google Scholar] [CrossRef]
- Haining, Z.; Kawai, N.; Okada, M.; Okubo, S.; Zhang, X.; Fei, Z.; Tamiya, T. Relation of 4F2hc expression to pathological grade proliferation and angiogenesis in human brain gliomas. Chin. J. Clin. Oncol. 2012, 39, 1161–1164. [Google Scholar] [CrossRef]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.I.; Fukasawa, Y.; et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta Biomembr. 2001, 1514, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Yunger, L.M.; Cramer, R.D. Measurement and correlation of partition coefficients of polar amino acids. Mol. Pharmacol. 1981, 20, 602–608. [Google Scholar] [PubMed]
- Chollet, J.F.; Delétage, C.; Faucher, M.; Miginiac, L.; Bonnemain, J.L. Synthesis and structure-activity relationships of some pesticides with an α-amino acid function. Biochim. Biophys. Acta Gen. Subj. 1997, 1336, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Cormerais, Y.; Giuliano, S.; LeFloch, R.; Front, B.; Durivault, J.; Tambutté, E.; Massard, P.A.; De La Ballina, L.R.; Endou, H.; Wempe, M.F.; et al. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 2016, 76, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F. INEL BNCT Research Program Publications: The Role of Chemistry in the Development of Boron Neutron Capture Therapy of Cancer. Angew. Chem. Int. Ed. Engl. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Wada, Y.; Hirose, K.; Harada, T.; Sato, M.; Watanabe, T.; Anbai, A.; Hashimoto, M.; Takai, Y. Impact of oxygen status on 10B-BPA uptake into human glioblastoma cells, referring to significance in boron neutron capture therapy. J. Radiat. Res. 2018, 59, 122–128. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef]
- Bartolucci, S.; Bartoccini, F.; Righi, M.; Piersanti, G. Direct, regioselective, and chemoselective preparation of novel boronated tryptophans by friedel-crafts alkylation. Org. Lett. 2012, 14, 600–603. [Google Scholar] [CrossRef]
- Chio, C.M.; Huang, Y.C.; Chou, Y.C.; Hsu, F.C.; Lai, Y.B.; Yu, C.S. Boron Accumulation in Brain Tumor Cells through Boc-Protected Tryptophan as a Carrier for Boron Neutron Capture Therapy. ACS Med. Chem. Lett. 2020, 11, 589–596. [Google Scholar] [CrossRef]
- Bonjoch, J.; Drew, M.G.B.; González, A.; Greco, F.; Jawaid, S.; Osborn, H.M.I.; Williams, N.A.O.; Yaqoob, P. Synthesis and evaluation of novel boron-containing complexes of potential use for the selective treatment of malignant melanoma. J. Med. Chem. 2008, 51, 6604–6608. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Hirano, F.; Temma, T. Evaluation of 3-Borono-L-Phenylalanine as a Water-Soluble Boron Neutron Capture Therapy Agent. Pharmaceutics 2022, 14, 1106. [Google Scholar] [CrossRef] [PubMed]
- Raitano, A.; Martin, T.; Zhang, C.; Malinao, M.C.; Capo, L.; Ikeura, M.; Carroll, R.; Quintana, J.C.; Dlamini, S.; Kulenovic, L.; et al. Boronotyrosine, a Borylated Amino Acid Mimetic with Enhanced Solubility, Tumor Boron Delivery, and Retention for the Re-emerging Boron Neutron Capture Therapy Field. J. Med. Chem. 2023, 66, 13809–13820. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Morizane, Y.; Tokumaru, Y.; Tamaki, S.; Maemunah, I.R.; Akiyama, Y.; Sato, F.; Murata, I. Cerebrospinal fluid-based boron delivery system may help increase the uptake boron for boron neutron capture therapy in veterinary medicine: A preliminary study with normal rat brain cells. Res. Vet. Sci. 2022, 148, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Morizane, Y.; Tokumaru, Y.; Tamaki, S.; Maemunah, I.R.; Akiyama, Y.; Sato, F.; Murata, I. Boron Delivery to Brain Cells via Cerebrospinal Fluid (CSF) Circulation for BNCT in a Rat Melanoma Model. Biology 2022, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Watanabe, T.; Tanaka, H.; Ono, K.; Kirihata, M. Detection of boronic acid derivatives in cells using a fluorescent sensor. Org. Biomol. Chem. 2015, 13, 6927–6930. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Kondo, N.; Hagimori, M.; Temma, T. Development of a switching-type fluorescence sensor for the detection of boronic acid-containing agents. Anal. Sci. 2022, 38, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Takada, S.; Hagimori, M. Development of a 2-(2-Hydroxyphenyl)-1H-benzimidazole-Based Fluorescence Sensor Targeting Boronic Acids for Versatile Application in Boron Neutron Capture Therapy. Cancers 2023, 15, 1862. [Google Scholar] [CrossRef]
- Yu, L.Y.; Hsu, C.H.; Li, C.Y.; Hong, S.Y.; Chen, C.R.; Chen, C.S. Evaluating the biological effectiveness of boron neutron capture therapy by using microfluidics-based pancreatic tumor spheroids. Analyst 2023, 148, 3045–3056. [Google Scholar] [CrossRef]
- Delgrosso, E.; Scocozza, F.; Cansolino, L.; Riva, F.; Conti, M.; Loi, G.; Auricchio, F.; Postuma, I.; Bortolussi, S.; Cobianchi, L.; et al. 3D bioprinted osteosarcoma model for experimental boron neutron capture therapy (BNCT) applications: Preliminary assessment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 1571–1580. [Google Scholar] [CrossRef]
- Silarski, M.; Dziedzic-Kocurek, K.; Szczepanek, M. Combined BNCT and PET for theranostics. Bio-Algorithms Med-Syst. 2021, 17, 293–300. [Google Scholar] [CrossRef]
- Langen, K.J.; Galldiks, N. Update on amino acid pet of brain tumours. Curr. Opin. Neurol. 2018, 31, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Hall, L.T. Molecular Imaging of Brain Metastases with PET. In Metastasis, 1st ed.; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- Nakaichi, T.; Nakamura, S.; Ito, K.; Takahashi, K.; Takemori, M.; Kashihara, T.; Kunito, K.; Murakami, N.; Iijima, K.; Chiba, T.; et al. Analyzing spatial distribution between 18F-fluorodeoxyglucose and 18F-boronophenylalanine positron emission tomography to investigate selection indicators for boron neutron capture therapy. EJNMMI Phys. 2022, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Isohashi, K.; Kanai, Y.; Aihara, T.; Hu, N.; Fukushima, K.; Baba, I.; Hirokawa, F.; Kakino, R.; Komori, T.; Nihei, K.; et al. Exploration of the threshold SUV for diagnosis of malignancy using 18F-FBPA PET/CT. Eur. J. Hybrid Imaging 2022, 6, 35. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Järvinen, J.; Pulkkinen, H.; Rautio, J.; Timonen, J.M. Amino Acid-Based Boron Carriers in Boron Neutron Capture Therapy (BNCT). Pharmaceutics 2023, 15, 2663. https://doi.org/10.3390/pharmaceutics15122663
Järvinen J, Pulkkinen H, Rautio J, Timonen JM. Amino Acid-Based Boron Carriers in Boron Neutron Capture Therapy (BNCT). Pharmaceutics. 2023; 15(12):2663. https://doi.org/10.3390/pharmaceutics15122663
Chicago/Turabian StyleJärvinen, Juulia, Herkko Pulkkinen, Jarkko Rautio, and Juri M. Timonen. 2023. "Amino Acid-Based Boron Carriers in Boron Neutron Capture Therapy (BNCT)" Pharmaceutics 15, no. 12: 2663. https://doi.org/10.3390/pharmaceutics15122663
APA StyleJärvinen, J., Pulkkinen, H., Rautio, J., & Timonen, J. M. (2023). Amino Acid-Based Boron Carriers in Boron Neutron Capture Therapy (BNCT). Pharmaceutics, 15(12), 2663. https://doi.org/10.3390/pharmaceutics15122663







