3D-Printed Fast-Dissolving Oral Dosage Forms via Fused Deposition Modeling Based on Sugar Alcohol and Poly(Vinyl Alcohol)—Preparation, Drug Release Studies and In Vivo Oral Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Filament and Printing Oral Dosage form by FDM
2.3. Rheological Measurement
2.4. In Vitro Drug Release Test
2.5. In Vivo Oral Absorption Study in Dogs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preparation of a 3D-printed Oral Dosage Form Containing Maltitol
3.1.1. Preparation of Filament Contains Maltitol and Printing Oral Dosage Form
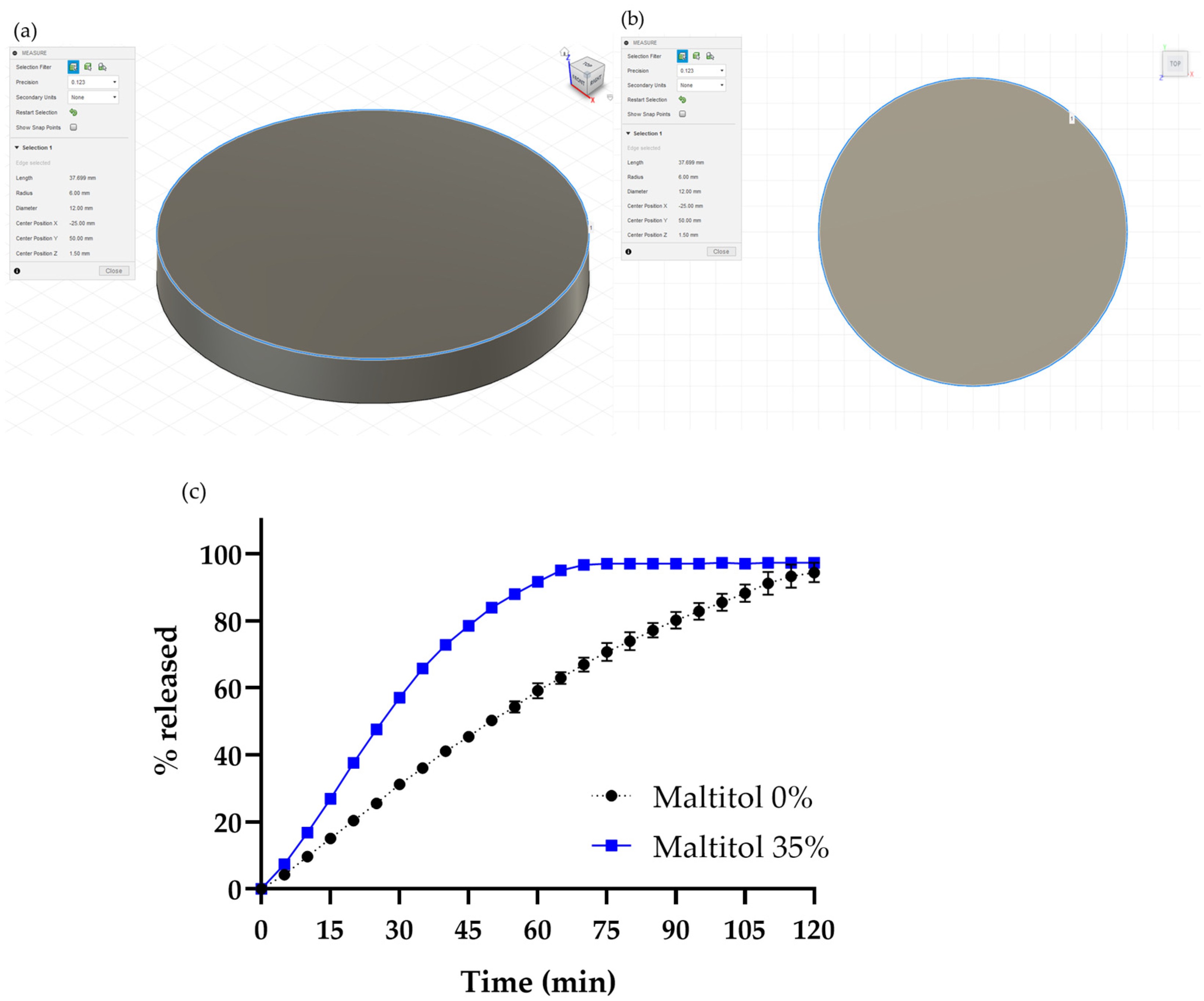
3.1.2. Drug Release Study of the Maltitol-PVA Oral Dosage Form
3.1.3. Changing Shape of the Oral Dosage Form
3.2. Screening of Sugar Alcohol
3.3. Rheological Measurement of the Sugar Alcohol and PVA Mixture
3.4. Effect of the Addition Ratio of Maltitol on Filament Formulation
3.5. In Vivo Oral Absorption Study in Dogs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, R.J.; Hollister, S.J.; Niedner, M.F.; Mahani, M.G.; Park, A.H.; Mehta, D.K.; Ohye, R.G.; Green, G.E. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Sci. Transl. Med. 2015, 7, 285ra264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, H.; Kanno, Y.; Mori, Y.; Suzuki, S.; Ohkubo, K.; Chikazu, D.; Yonehara, Y.; Chung, U.I.; Takato, T. A novel method for designing and fabricating custom-made artificial bones. Int. J. Oral Maxillofac. Surg. 2011, 40, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Aimar, A.; Palermo, B.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 40, 5340616. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, C.; Sun, C.; Yan, X.; He, J.; Shi, C.; Liu, C.; Li, D.; Jiang, T.; Huang, L. Fused Deposition Modeling PEEK Implants for Personalized Surgical Application: From Clinical Need to Biofabrication. Int. J. Bioprinting 2022, 8, 15. [Google Scholar] [CrossRef]
- First 3D-printed pill. Nat. Biotechnol. 2015, 33, 1014. [CrossRef] [PubMed]
- Press Release from Merck Group, “Merck and AMCM/EOS Cooperate in 3D Printing of Tablets”. Available online: https://www.merckgroup.com/press-releases/2020/feb/en/Merck-EOS-AMT-EN.pdf (accessed on 7 December 2022).
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef]
- Okafor-Muo, O.L.; Hassanin, H.; Kayyali, R.; ElShaer, A. 3D Printing of Solid Oral Dosage Forms: Numerous Challenges With Unique Opportunities. J. Pharm. Sci. 2020, 109, 3535–3550. [Google Scholar] [CrossRef]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Manchanda, A.; Mehta, T.; Ma, A.W.K.; Chaudhuri, B. Formulation design for inkjet-based 3D printed tablets. Int. J. Pharm. 2020, 584, 119430. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Yang, X.L.; Huang, W.D.; Liu, J.; Wang, Y.G.; Xu, H. Tablets with material gradients fabricated by three-dimensional printing. J. Pharm. Sci. 2007, 96, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2017, 23, 433–439. [Google Scholar] [CrossRef]
- Chung, M.; Radacsi, N. On the optimization of low-cost FDM 3D printers for accurate replication of patient-specific abdominal aortic aneurysm geometry. 3d Print. Med. 2018, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Macedo, J.; Samaro, A.; Vanhoorne, V.; Vervaet, C.; Pinto, J.F. Processability of poly(vinyl alcohol) Based Filaments With Paracetamol Prepared by Hot-Melt Extrusion for Additive Manufacturing. J. Pharm. Sci. 2020, 109, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Gorkem Buyukgoz, G.; Soffer, D.; Defendre, J.; Pizzano, G.M.; Davé, R.N. Exploring tablet design options for tailoring drug release and dose via fused deposition modeling (FDM) 3D printing. Int. J. Pharm. 2020, 591, 119987. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M. Sugar alcohols-their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mori, C.; Kondo, H. Effect of gastric acidity regulation on the gastrointestinal transit time and secretion of gastric fluids in beagle dogs. J. Drug Deliv. Sci. Technol. 2006, 16, 467–472. [Google Scholar] [CrossRef]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS PharmSciTech 2018, 19, 3403–3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelber, M.K.; Hurst, G.; Comi, T.J.; Bhargava, R. Model-guided design and characterization of a high-precision 3D printing process for carbohydrate glass. Addit. Manuf. 2018, 22, 38–50. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M.S. Suitability of sugar alcohols as antidiabetic supplements: A review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Thommes, M. Investigation into mixing capability and solid dispersion preparation using the DSM Xplore Pharma Micro Extruder. J. Pharm. Pharmacol. 2014, 66, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: A comprehensive review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef]
- Nashed, N.; Lam, M.; Nokhodchi, A. A comprehensive overview of extended release oral dosage forms manufactured through hot melt extrusion and its combination with 3D printing. Int. J. Pharm. 2021, 596, 120237. [Google Scholar] [CrossRef]
- Kolter, K.; Karl, M.; Gryczke, A. Hot-Melt Extrusion with BASF Pharma Polymers—Extrusion Compendium, 2nd ed.; BASF SE Pharma Ingredients & Services: Ludwigshafen, Germany, 2012. [Google Scholar]







| Formulation | Maltitol 0% | Maltitol 35% |
|---|---|---|
| API | 20 | 20 |
| PVA | 79 | 40 |
| Maltitol | - | 35 |
| TEC | 1 | 5 |
| Thickness (mm) | 1.59 ± 0.01 | 1.56 ± 0.11 |
| Weight (mg) | 208.0 ± 0.5 | 239.2 ± 12.9 |
| Formulation | Maltitol 20% | Maltitol 35% | Maltitol 55% |
|---|---|---|---|
| Thickness (mm) | 2.36 ± 0.09 | 2.62 ± 0.18 | 2.59 ± 0.13 |
| Weight (mg) | 229.5 ± 2.4 | 245.6 ± 6.8 | 263.2 ± 30.3 |
| Samples | Cmax (µg/mL) | Tmax (h) | AUC0–24 h (µg·h/mL) |
|---|---|---|---|
| Conventional tablet | 4.2 ± 2.2 | 1.4 ± 0.6 | 34.0 ± 11.1 |
| 3D-printed oral dosage form | 5.6 ± 1.8 | 1.2 ± 0.8 | 35.1 ± 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, S.; Kobayashi, M.; Aoki, S.; Terukina, T.; Kanazawa, T.; Kojima, H.; Kondo, H. 3D-Printed Fast-Dissolving Oral Dosage Forms via Fused Deposition Modeling Based on Sugar Alcohol and Poly(Vinyl Alcohol)—Preparation, Drug Release Studies and In Vivo Oral Absorption. Pharmaceutics 2023, 15, 395. https://doi.org/10.3390/pharmaceutics15020395
Ikeda S, Kobayashi M, Aoki S, Terukina T, Kanazawa T, Kojima H, Kondo H. 3D-Printed Fast-Dissolving Oral Dosage Forms via Fused Deposition Modeling Based on Sugar Alcohol and Poly(Vinyl Alcohol)—Preparation, Drug Release Studies and In Vivo Oral Absorption. Pharmaceutics. 2023; 15(2):395. https://doi.org/10.3390/pharmaceutics15020395
Chicago/Turabian StyleIkeda, Sorato, Masanori Kobayashi, Soken Aoki, Takayuki Terukina, Takanori Kanazawa, Hiroyuki Kojima, and Hiromu Kondo. 2023. "3D-Printed Fast-Dissolving Oral Dosage Forms via Fused Deposition Modeling Based on Sugar Alcohol and Poly(Vinyl Alcohol)—Preparation, Drug Release Studies and In Vivo Oral Absorption" Pharmaceutics 15, no. 2: 395. https://doi.org/10.3390/pharmaceutics15020395







