Alternative Pharmacokinetic Metrics in Single-Dose Studies to Ensure Bioequivalence of Prolonged-Release Products at Steady State—A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Sampling and Analytical Methods
2.3. Pharmacokinetic and Statistical Analysis
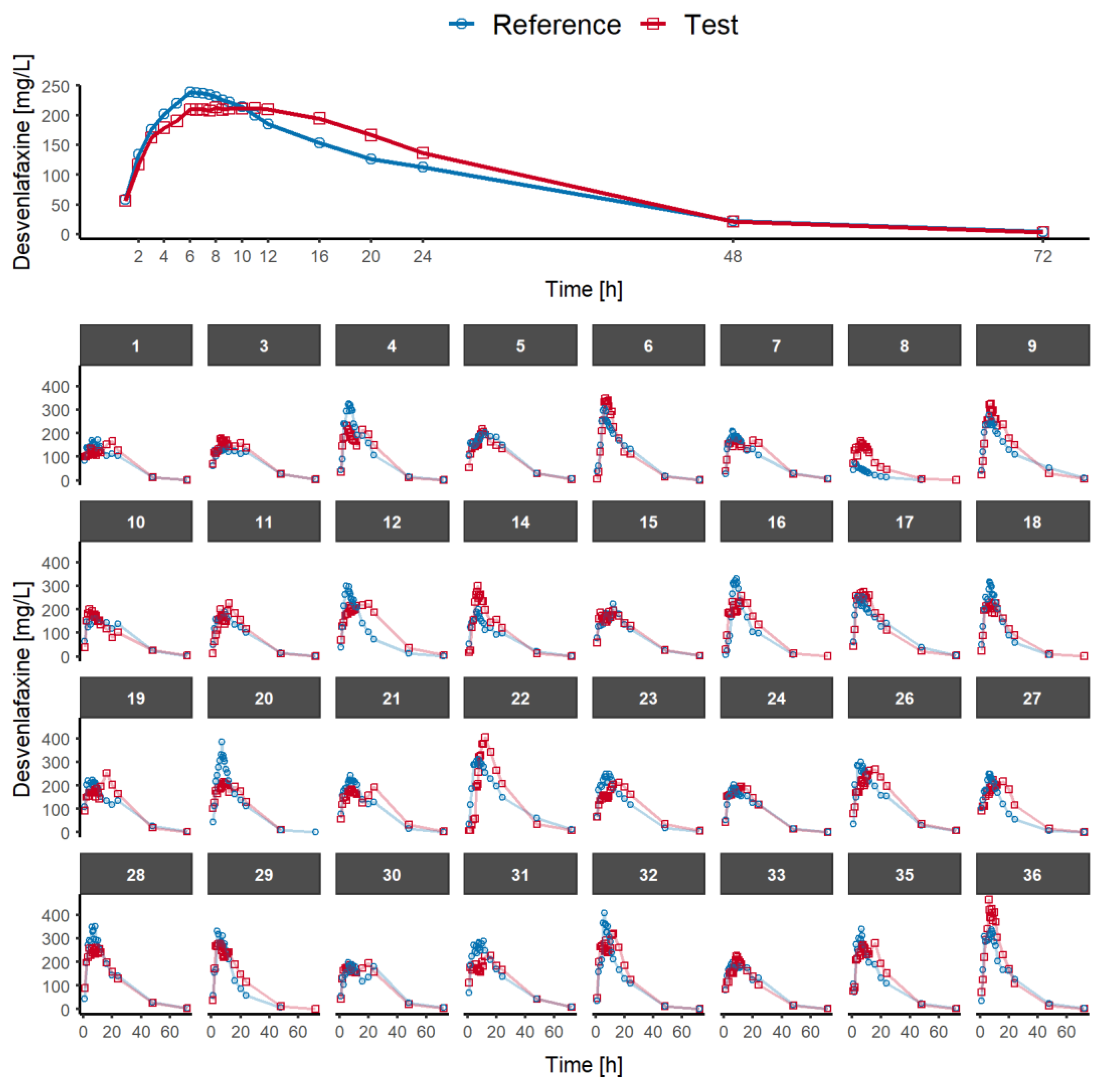
- Cτ: the concentration at the end of the dosing interval in the single-dose study.
- pAUC0–8h and pAUC8h-t: the partial areas under the curve with a cut-off point of 8 h, i.e., from 0 to 8 h (11 data points), and from 8 h to the last measurable concentration (11 data points).
- pAUC0–10h and pAUC10h-t: the partial areas under the curve with a cut-off point of 10 h, i.e., from 0 to 10 h (14 data points), and from 10 h to the last measurable concentration (8 data points).
- pAUC0–12h and pAUC112h-t: the partial areas under the curve with a cut-off point of 12 h, i.e., from 0 to 12 h (16 data points), and from 12 h to the last measurable concentration (6 data points).
- pAUC0–16h and pAUC116h-t: the partial areas under the curve with a cut-off point of 16 h, i.e., from 0 to 12 h (17 data points), and from 12 h to the last measurable concentration (5 data points).
- pAUC0–20h and pAUC120h-t: the partial areas under the curve with a cut-off point of 20 h, i.e., from 0 to 12 h (18 data points), and from 12 h to the last measurable concentration (4 data points).
- pAUC0–24h vs. pAUC24h-t: the partial areas under the curve with a cut-off point of 24 h, i.e., from 0 to 24 h (19 data points), and from 24 h to the last measurable concentration (3 data points).
- Half-value duration (HVD or T50%Cmax): the time span during which the plasma concentrations are equal to or higher than half of the Cmax value [19], calculated according to the linear trapezoidal rule.
2.4. Prediction of the Multiple-Dose Fasted-State Study Using a Model-Based Approach
2.4.1. Data Analysis
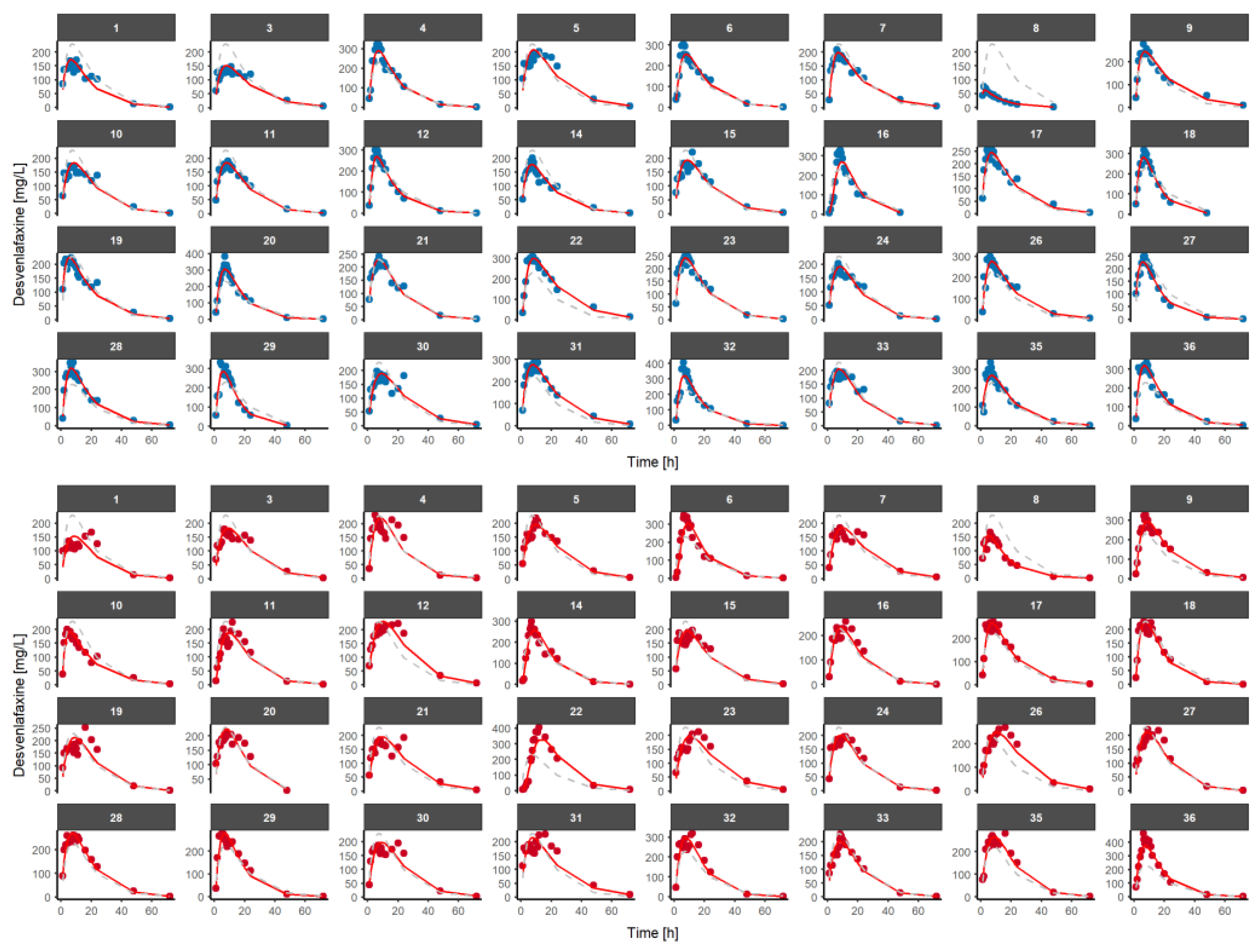
2.4.2. Model Selection
2.4.3. Model Evaluation
3. Results
3.1. Single-Dose Fasted-State Study
3.2. Population Pharmacokinetic Model of Desvenlafaxine
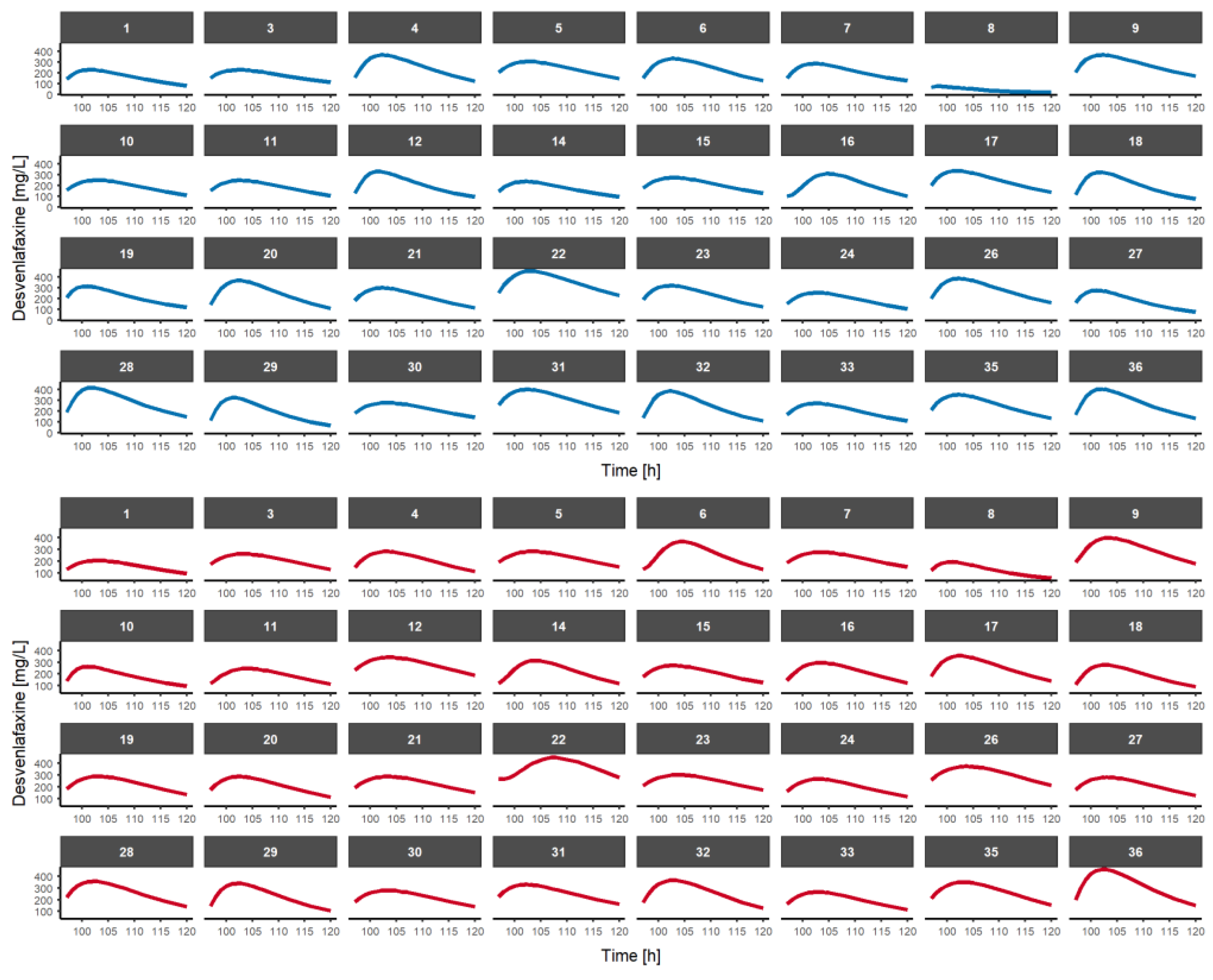
3.3. Multiple-Dose Fasted-State Study Using a Model-Based Approach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roost, M.S.; Potthast, H.; Walther, C.; Garcia-Arieta, A.; Abalos, I.; Agostinho Freitas Fernandes, E.; Mendes Lima Santos, G.; Rodriguez Martinez, Z.; Tam, A.; Rodrigues, C.; et al. Requirements for Additional Strength Biowaivers for Modified Release Solid Oral Dosage Forms in International Pharmaceutical Regulators Programme Participating Regulators and Organisations: Differences and Commonalities. J. Pharm. Pharm. Sci. 2021, 24, 548–562. [Google Scholar] [CrossRef] [PubMed]
- EMA. Guideline on the Pharmacokinetic and Clinical Evaluation of Modified Release Dosage Forms. EMA/CHMP/EWP/280/96 Rev1; Committee for Medicinal Products for Human Use: London, UK, 2014. [Google Scholar]
- FDA. Draft Guidance Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an Abbreviated New Drug Application. Center for Drug Evaluation and Research; U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- el-Tahtawy, A.A.; Jackson, A.J.; Ludden, T.M. Comparison of single and multiple dose pharmacokinetics using clinical bioequivalence data and Monte Carlo simulations. Pharm. Res. 1994, 11, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- el-Tahtawy, A.A.; Jackson, A.J.; Ludden, T.M. Evaluation of bioequivalence of highly variable drugs using Monte Carlo simulations. I. Estimation of rate of absorption for single and multiple dose trials using Cmax. Pharm. Res. 1995, 12, 1634–1641. [Google Scholar] [CrossRef]
- el-Tahtawy, A.A.; Tozer, T.N.; Harrison, F.; Lesko, L.; Williams, R. Evaluation of bioequivalence of highly variable drugs using clinical trial simulations. II: Comparison of single and multiple-dose trials using AUC and Cmax. Pharm. Res. 1998, 15, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Teruel, C.; Gonzalez-Alvarez, I.; Navarro-Fontestad, C.; Garcia-Arieta, A.; Bermejo, M.; Casabo, V.G. Computer simulations of bioequivalence trials: Selection of design and analyte in BCS drugs with first-pass hepatic metabolism: Part II. Non-linear kinetics. Eur. J. Pharm. Sci. 2009, 36, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Teruel, C.; Nalda Molina, R.; Gonzalez-Alvarez, I.; Navarro-Fontestad, C.; Garcia-Arieta, A.; Casabo, V.G.; Bermejo, M. Computer simulations of bioequivalence trials: Selection of design and analyte in BCS drugs with first-pass hepatic metabolism: Linear kinetics (I). Eur. J. Pharm. Sci. 2009, 36, 137–146. [Google Scholar] [CrossRef]
- Jackson, A.J. Prediction of steady-state bioequivalence relationships using single dose data I-linear kinetics. Biopharm Drug Dispos. 1987, 8, 483–496. [Google Scholar] [CrossRef]
- Jackson, A.J. Prediction of steady state bioequivalence relationships using single dose data II-nonlinear kinetics. Biopharm Drug Dispos. 1989, 10, 489–503. [Google Scholar] [CrossRef]
- Mangas-Sanjuan, V.; Navarro-Fontestad, C.; Garcia-Arieta, A.; Troconiz, I.F.; Bermejo, M. Computer simulations for bioequivalence trials: Selection of analyte in BCS class II and IV drugs with first-pass metabolism, two metabolic pathways and intestinal efflux transporter. Eur. J. Pharm. Sci. 2018, 117, 193–203. [Google Scholar] [CrossRef]
- Navarro-Fontestad, C.; Gonzalez-Alvarez, I.; Fernandez-Teruel, C.; Garcia-Arieta, A.; Bermejo, M.; Casabo, V.G. Computer simulations for bioequivalence trials: Selection of analyte in BCS drugs with first-pass metabolism and two metabolic pathways. Eur. J. Pharm. Sci. 2010, 41, 716–728. [Google Scholar] [CrossRef]
- Cuesta-Gragera, A.; Navarro-Fontestad, C.; Mangas-Sanjuan, V.; Gonzalez-Alvarez, I.; Garcia-Arieta, A.; Troconiz, I.F.; Casabo, V.G.; Bermejo, M. Semi-physiologic model validation and bioequivalence trials simulation to select the best analyte for acetylsalicylic acid. Eur. J. Pharm. Sci. 2015, 74, 86–94. [Google Scholar] [CrossRef]
- Cuesta-Gragera, A.; Navarro-Fontestad, C.; Mangas-Sanjuan, V.; Gonzalez-Alvarez, I.; Garcia-Arieta, A.; Troconiz, I.F.; Casabo, V.G.; Bermejo, M. Validation of a semi-physiological model for caffeine in healthy subjects and cirrhotic patients. Eur. J. Pharm. Sci. 2015, 73, 57–63. [Google Scholar] [CrossRef]
- Aldhous, M.; Franey, C.; Wright, J.; Arendt, J. Plasma concentrations of melatonin in man following oral absorption of different preparations. Br. J. Clin. Pharmacol. 1985, 19, 517–521. [Google Scholar] [CrossRef]
- EMA. Octreotide Acetate Depot Powder and Solvent for Suspension for Injection 10 mg, 20 mg and 30 mg Product-Specific Bioequivalence Guidance. EMA/CHMP/291571/2018; Committee for Medicinal Products for Human Use: London, UK, 2019. [Google Scholar]
- Paixao, P.; Gouveia, L.F.; Morais, J.A. An alternative single dose parameter to avoid the need for steady-state studies on oral extended-release drug products. Eur. J. Pharm. Biopharm. 2012, 80, 410–417. [Google Scholar] [CrossRef]
- Garcia-Arieta, A.; Morales-Alcelay, S.; Herranz, M.; de la Torre-Alvarado, J.M.; Blazquez-Perez, A.; Suarez-Gea, M.L.; Alvarez, C. Investigation on the need of multiple dose bioequivalence studies for prolonged-release generic products. Int. J. Pharm. 2012, 423, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Endrenyi, L.; Tothfalusi, L. Metrics for the evaluation of bioequivalence of modified-release formulations. AAPS J. 2012, 14, 813–819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- FDA. Draft Guidance on Desvenlafaxine Succinate. Center for Drug Evaluation and Research; U.S. Department of Health and Human Services: Washington, DC, USA, 2010.
- EMA. Guideline for good clinical practice E6(R2); Committee for Medicinal Products for Human Use: London, UK, 2017. [Google Scholar]
- WMA. Declaration of Helsinki; World Medical Association: London, UK, 2013. [Google Scholar]
- Wade, J.R.; Beal, S.L.; Sambol, N.C. Interaction between structural, statistical, and covariate models in population pharmacokinetic analysis. J. Pharmacokinet Biopharm. 1994, 22, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- Bonate, P.L. The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm. Res. 1999, 16, 709–717. [Google Scholar] [CrossRef]
- Comets, E.; Brendel, K.; Mentre, F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: The npde add-on package for R. Comput. Methods Programs Biomed. 2008, 90, 154–166. [Google Scholar] [CrossRef]
- Lindbom, L.; Pihlgren, P.; Jonsson, N. PsN-Toolkit—A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.C.C.; Chiann, C.; Storpirtis, S. Assessment of the impact of partial area under the curve in a bioavailability/bioequivalence study on generic prolonged-release formulations. Eur. J. Pharm. Sci. 2022, 171, 106127. [Google Scholar] [CrossRef] [PubMed]
- Doki, K.; Darwich, A.S.; Patel, N.; Rostami-Hodjegan, A. Virtual bioequivalence for achlorhydric subjects: The use of PBPK modelling to assess the formulation-dependent effect of achlorhydria. Eur. J. Pharm. Sci. 2017, 109, 111–120. [Google Scholar] [CrossRef] [PubMed]

| PK Parameter | Point Estimate (%) | Lower Limit of 90% CI (%) | Upper Limit of 90% CI (%) | Intra-Subject CV (%) | BE Conclusion |
|---|---|---|---|---|---|
| AUC0-t | 111.67 | 104.39 | 119.45 | 15.97 | Yes |
| AUC0-inf 1 | 111.33 | 103.97 | 119.22 | 15.95 | Yes |
| Cmax | 97.29 | 89.74 | 105.48 | 19.21 | Yes |
| PK Parameter | Point Estimate (%) | Lower Limit of 90% CI (%) | Upper Limit of 90% CI (%) | Intra-Subject CV (%) | BE Conclusion |
|---|---|---|---|---|---|
| Cτ | 125.51 | 112.57 | 139.93 | 26.06 | No |
| pAUC0–8h | 90.18 | 83.36 | 97.56 | 18.69 | Yes |
| pAUC8h-t | 118.68 | 109.37 | 128.70 | 19.35 | No |
| pAUC0–10h | 92.05 | 85.39 | 99.24 | 17.86 | Yes |
| pAUC10h-t | 121.23 | 111.21 | 132.14 | 20.53 | No |
| pAUC0–12h | 94.91 | 88.14 | 102.19 | 17.56 | Yes |
| pAUC12h-t | 122.91 | 112.17 | 134.67 | 21.79 | No |
| pAUC0–16h | 101.08 | 94.30 | 108.33 | 16.45 | Yes |
| pAUC16h-t | 123.70 | 111.78 | 136.89 | 24.22 | No |
| pAUC0–20h | 106.04 | 99.29 | 113.24 | 15.58 | Yes |
| pAUC20-t | 122.11 | 109.36 | 136.35 | 26.44 | No |
| pAUC0–24h | 108.90 | 102.15 | 116.09 | 15.16 | Yes |
| pAUC24h-t | 120.14 | 106.93 | 134.97 | 27.97 | No |
| HVD | 123.79 | 110.19 | 139.07 | 27.96 | No |
| Population PK Model Estimates | Bootstrap Results | |||||
|---|---|---|---|---|---|---|
| Fixed effect | Value | RSE (%) | Shrinkage (%) | Median | RSE (%) | 95%CI |
| ka (h–1) | 7.37 × 10–2 | 21 | 7.35 × 10–2 | 22 | (6.84–7.56) × 10–2 | |
| ktr (h–1) | 18.50 | 31 | 18.78 | 32 | (16.75–24.32) | |
| CL (L/h) | 18.65 | 12 | 18.20 | 14 | (11.42–26.98) | |
| V1 (L) | 83.31 | 19 | 82.92 | 17 | (65.31–101.64) | |
| Inter-individual variability | ||||||
| ka (%) | 15 | 19 | 21 | 15 | 20 | (14–28) |
| ktr (%) | 39 | 31 | 29 | 38 | 33 | (22–51) |
| CL (%) | 17 | 12 | 10 | 17 | 13 | (10–31) |
| V1 (%) | 43 | 22 | 16 | 42 | 21 | (30–58) |
| Inter-occasion variability | ||||||
| ka (%) | 13 | 9 | 16 | 14 | 11 | (7–33) |
| ktr (%) | 27 | 8 | 26 | 26 | 10 | (19–41) |
| CL (%) | 14 | 10 | 12 | 14 | 9 | (8–21) |
| Residual unexplained variability | ||||||
| Plasma (%) | 18 | 7 | 8 | 17 | 8 | (11–23) |
| PK Parameter | Point Estimate (%) | Lower Limit of 90% CI (%) | Upper Limit of 90% CI (%) | Intra-Subject CV (%) | BE Conclusion |
|---|---|---|---|---|---|
| AUC0-τ | 106.37 | 99.30 | 113.94 | 16.31 | Yes |
| Cmax,ss | 102.79 | 95.28 | 110.89 | 18.02 | Yes |
| Cτ,ss | 123.41 | 111.08 | 137.10 | 25.18 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangas-Sanjuán, V.; Simón, M.; González-Rojano, E.; Ochoa, D.; Abad-Santos, F.; Román, M.; Ramos, M.; Govantes, C.; García-Arieta, A. Alternative Pharmacokinetic Metrics in Single-Dose Studies to Ensure Bioequivalence of Prolonged-Release Products at Steady State—A Case Study. Pharmaceutics 2023, 15, 409. https://doi.org/10.3390/pharmaceutics15020409
Mangas-Sanjuán V, Simón M, González-Rojano E, Ochoa D, Abad-Santos F, Román M, Ramos M, Govantes C, García-Arieta A. Alternative Pharmacokinetic Metrics in Single-Dose Studies to Ensure Bioequivalence of Prolonged-Release Products at Steady State—A Case Study. Pharmaceutics. 2023; 15(2):409. https://doi.org/10.3390/pharmaceutics15020409
Chicago/Turabian StyleMangas-Sanjuán, Víctor, Marta Simón, Esperanza González-Rojano, Dolores Ochoa, Francisco Abad-Santos, Manuel Román, Mercedes Ramos, Carlos Govantes, and Alfredo García-Arieta. 2023. "Alternative Pharmacokinetic Metrics in Single-Dose Studies to Ensure Bioequivalence of Prolonged-Release Products at Steady State—A Case Study" Pharmaceutics 15, no. 2: 409. https://doi.org/10.3390/pharmaceutics15020409
APA StyleMangas-Sanjuán, V., Simón, M., González-Rojano, E., Ochoa, D., Abad-Santos, F., Román, M., Ramos, M., Govantes, C., & García-Arieta, A. (2023). Alternative Pharmacokinetic Metrics in Single-Dose Studies to Ensure Bioequivalence of Prolonged-Release Products at Steady State—A Case Study. Pharmaceutics, 15(2), 409. https://doi.org/10.3390/pharmaceutics15020409









