Layer-by-Layer Hollow Mesoporous Silica Nanoparticles with Tunable Degradation Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
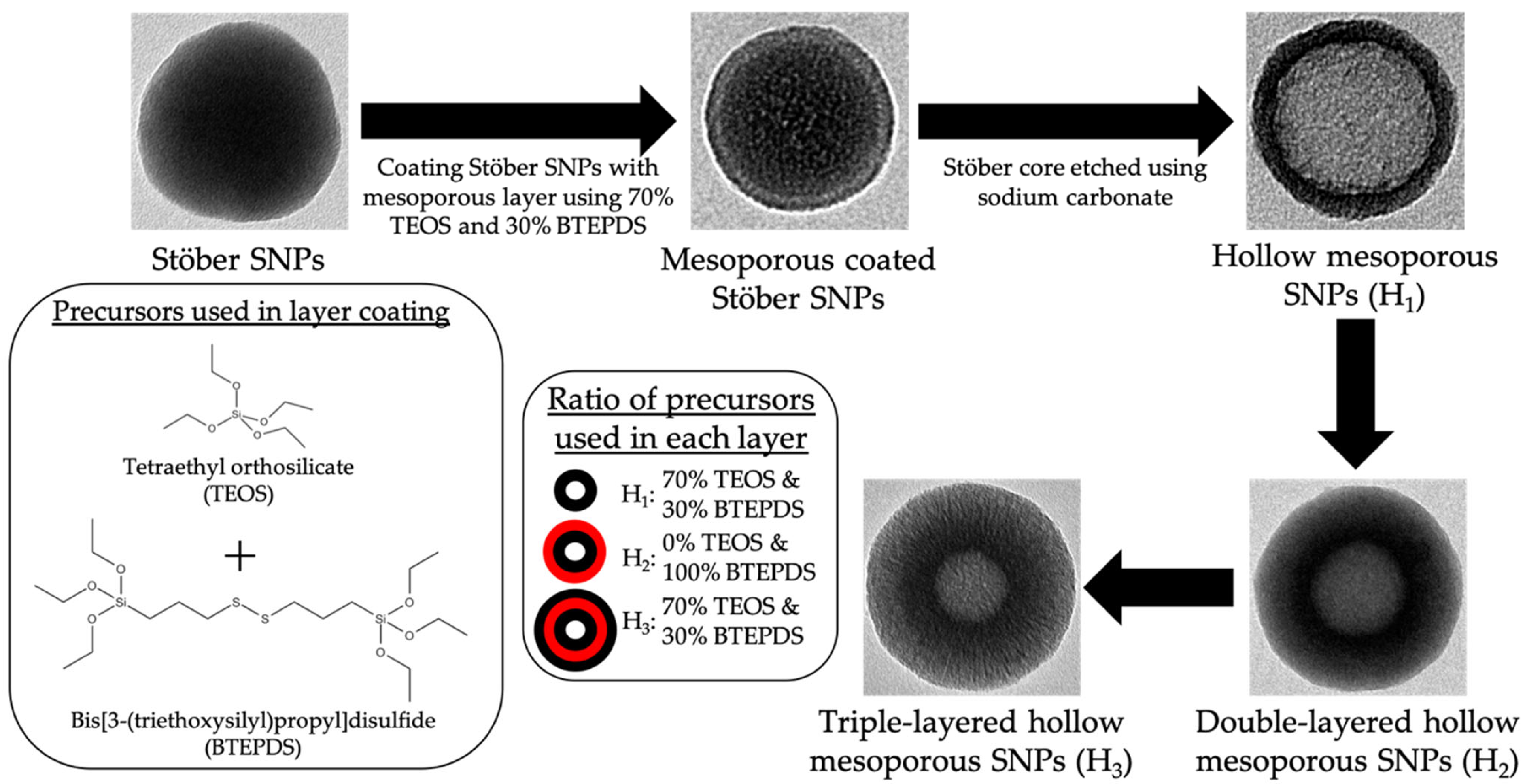
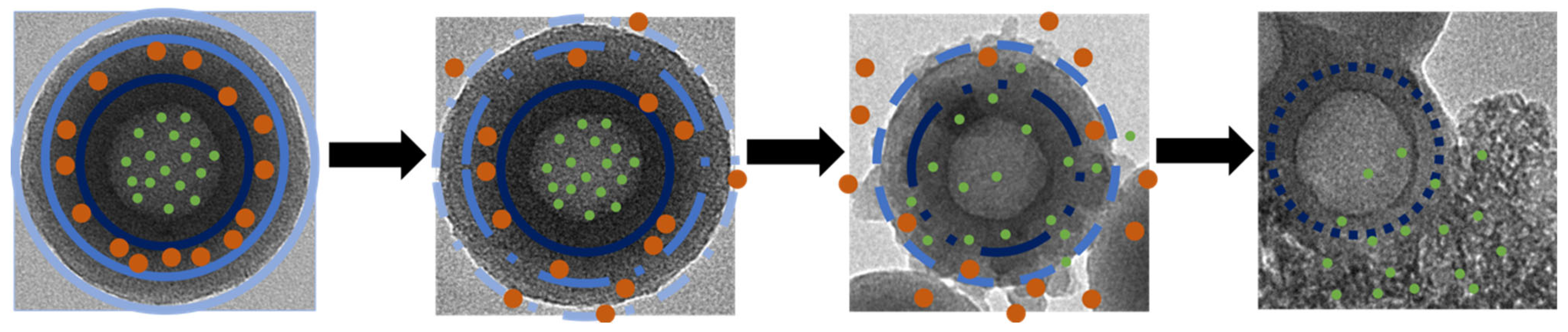
2.2.1. Synthesis of Layer-by-Layer HMSNPs
- Stöber nanospheres were prepared as follows: 3.6 mL ammonium hydroxide, 2.8 mL deionized water, and 100 mL absolute ethanol were mixed in a 250 mL Erlenmeyer flask for 10 min at a stirring rate of 400 rpm. Next, 3.5 mL TEOS was added dropwise and the reaction was left stirring for 24 h at room temperature. The synthesized particles were collected by centrifugation using an Avanti J-15R (Beckman Coulter Inc., Indianapolis, IN, USA) at 11,420 RCF for 10 min, and washed thrice with deionized water and 95% ethanol. Final particles were suspended in 50 mL deionized water.
- Mesoporous coated Stöber SNPs were prepared as follows: 2.73 mL absolute ethanol, 20 mL deionized water, 27 μL TEA, and 70 mg CTAB were mixed in a 100 mL round bottom flask under a stirring rate of 600 rpm in an oil bath with reaction temperature at 80 °C, using a water condenser, for 30 min. 10 mL of previously synthesized Stöber SNPs were added to the solution and left stirring for 15 min. Stirring rate was increased to 1400 rpm and 87.5 μL TEOS and 37.5 μL BTEPDS were added simultaneously to the solution. The reaction was left stirring for 3 h. The synthesized particles were collected by centrifugation, washed once with 95% ethanol, and suspended in 10mL deionized water.
- HMSNPs (H1) were prepared as follows: 1540 mg sodium carbonate was dissolved in 10 mL deionized water in a 100 mL round bottom flask under a stirring rate of 1200 rpm with reaction temperature at 50 °C. After 1 h, the previously fabricated mesoporous coated Stöber SNPs were added and left under stirring for 10 h. The particles were collected by centrifugation and washed thrice with deionized water and 95% ethanol. To remove the CTAB, particles were suspended in acidic ethanol (1 mL concentrated HCl in 30 mL absolute ethanol) and refluxed at 80 °C for 6 h. This CTAB removal process was performed twice. Particles were washed thrice with deionized water and 95% ethanol. Final particles were suspended in 10 mL deionized water.
- Double-layered HMSNPs (H2) were fabricated as follows: 2.73 mL absolute ethanol, 20 mL deionized water, 27.5 μL TEA, and 70 mg CTAB were mixed in a 100 mL round bottom flask under a stirring rate of 600 rpm in oil bath with reaction temperature at 80 °C, using a water condenser, for 30 min. The previously synthesized H1 was added to the solution and left stirring for 15 min. Stirring rate was increased to 1400 rpm and 142 μL BTEPDS was added to the solution. The reaction was left stirring for 3 h. The synthesized particles were collected by centrifugation, washed thrice with water and 95% ethanol, and CTAB was removed via acidic ethanol wash twice. Particles were washed thrice with deionized water and 95% ethanol. Final particles were suspended in 10 mL deionized water.
- Triple-layered HMSNPs (H3) were fabricated as follows: 2.73 mL absolute ethanol, 20 mL deionized water, 27.5 μL TEA, and 70 mg CTAB were mixed in a 100 mL round bottom flask under a stirring rate of 600 rpm in oil bath with reaction temperature at 80 °C, using a water condenser, for 30 min. The previously synthesized H2 was added to the solution and left stirring for 15 min. Stirring rate was increased to 1400 rpm and 140 μL TEOS and 60 μL BTEPDS were added simultaneously to the solution. The reaction was left stirring for 3 h. The synthesized particles were collected by centrifugation, washed thrice with water and 95% ethanol, and CTAB was removed via acidic ethanol twice. Particles were washed thrice with deionized water and 95% ethanol. Final particles were suspended in 10 mL deionized water.
2.2.2. Physicochemical Characterization of Nanoparticles
2.2.3. Cytotoxicity Assay
2.2.4. Evaluation of In Vitro Degradation Profiles
2.2.5. Statistical Analysis
3. Results and Discussion
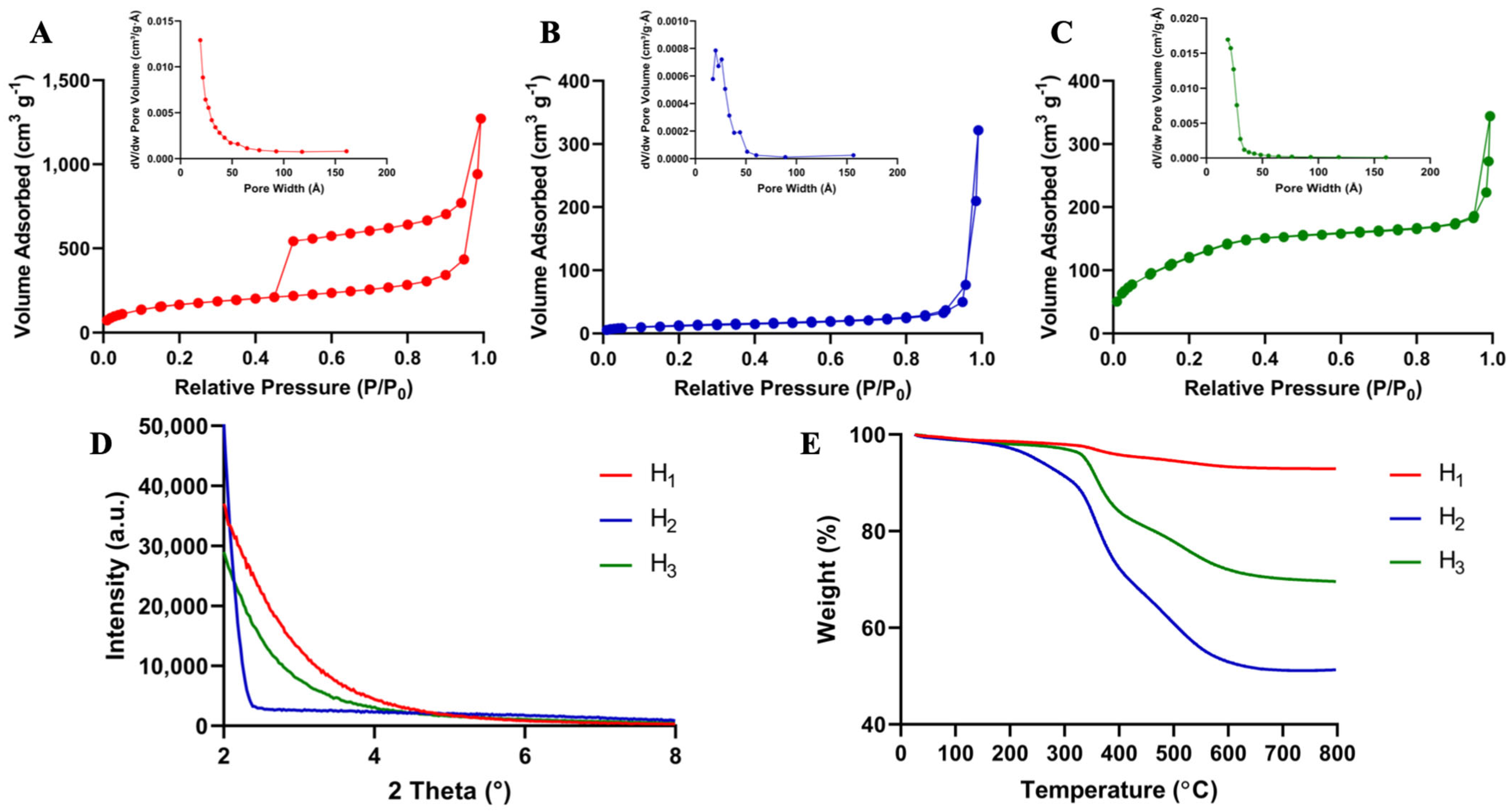
3.1. Synthesis and Characterization of Layer-by-Layer HMSNPs
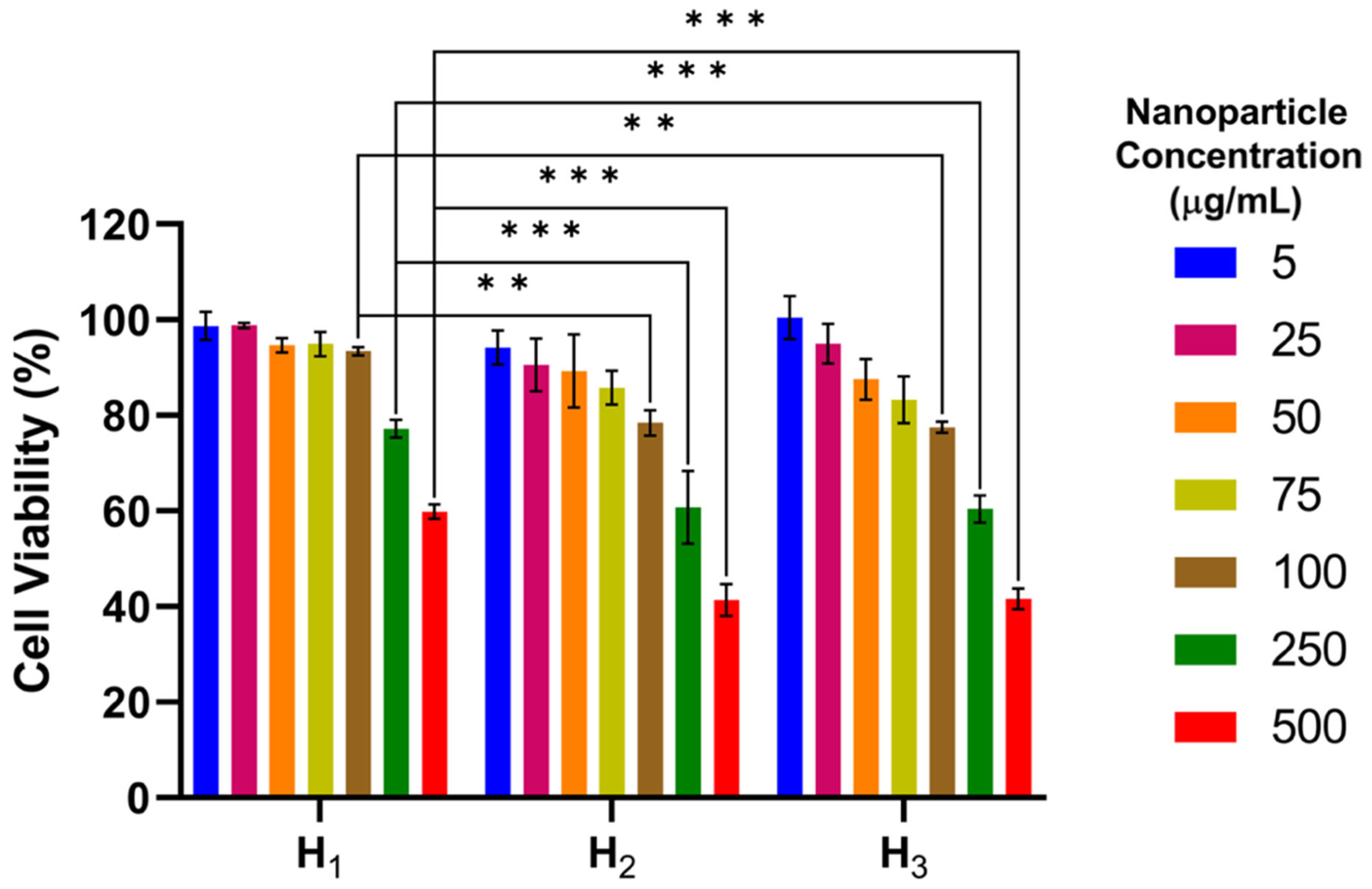
3.2. In Vitro Cytotoxicity
3.3. Evaluation of In Vitro Degradation Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Liang, T.; Ma, Q. Layer-by-Layer assembled nano-drug delivery systems for cancer treatment. Drug Deliv. 2021, 28, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, N.; Correa, S.; Hao, L.; Wang, W.; Straehla, J.P.; Bhatia, S.N.; Hammond, P.T. Theranostic Layer-by-Layer Nanoparticles for Simultaneous Tumor Detection and Gene Silencing. Angew. Chem. Int. Ed. Engl. 2020, 59, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, S.; Tatykhanova, G.; Bakranov, N.; Tursunova, R. Layer-by-Layer Thin Films and Coatings Containing Metal Nanoparticles in Catalysis. In Thin Film Processes—Artifacts on Surface Phenomena and Technological Facets; Thirumalai, J., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Shaabani, E.; Sharifiaghdam, M.; De Keersmaecker, H.; De Rycke, R.; De Smedt, S.; Faridi-Majidi, R.; Braeckmans, K.; Fraire, J.C. Layer by Layer Assembled Chitosan-Coated Gold Nanoparticles for Enhanced siRNA Delivery and Silencing. Int. J. Mol. Sci. 2021, 22, 831. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef]
- Poon, Z.; Chang, D.; Zhao, X.; Hammond, P.T. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano 2011, 5, 4284–4292. [Google Scholar] [CrossRef]
- Kong, S.M.; Costa, D.F.; Jagielska, A.; Van Vliet, K.J.; Hammond, P.T. Stiffness of targeted layer-by-layer nanoparticles impacts elimination half-life, tumor accumulation and tumor penetration. Proc. Natl. Acad. Sci. USA 2021, 118, e2104826118. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: Advances, challenges, and outlook. Int. J. Nanomed. 2017, 1, 87–110. [Google Scholar] [CrossRef]
- Finnie, K.S.; Waller, D.J.; Perret, F.L.; Krause-Heuer, A.M.; Lin, H.Q.; Hanna, J.V.; Barbe, G.J. Biodegradability of sol-gel silica microparticles for drug delivery. J. Sol.-Gel. Sci. Technol. 2009, 49, 12–18. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Quignard, S.; Masse, S.; Laurent, G.; Coradin, T. Introduction of disulfide bridges within silica nanoparticles to control their intra-cellular degradation. Chem. Commun. 2013, 49, 3410–3412. [Google Scholar] [CrossRef]
- Hadipour Moghaddam, S.P.; Saikia, J.; Ghandehari, H. Redox-responsive polysulfide-based biodegradable organosilica nanoparticles for delivery of bioactive agents. ACS Appl. Mater. Interfaces 2017, 25, 21133–21146. [Google Scholar] [CrossRef] [PubMed]
- Maggini, L.; Cabrera, I.; Ruiz-Carretero, A.; Prasetyanto, E.A.; Robinet, E.; De Cola, L. Breakable mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 7240–7247. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.; Cattoën, X.; Man, M.W.C.; Gallud, A.; Raehm, L.; Trens, P.; Maynadier, M.; Durand, J.-O. Biodegradable Ethylene-Bis(Propyl)Disulfide-Based Periodic Mesoporous Organosilica Nanorods and Nanospheres for Efficient In-Vitro Drug Delivery. Adv. Mater. 2014, 26, 6174–6180. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Redox-responsive mesoporous silica nanoparticles for cancer treatment: Recent updates. Nanomaterials 2021, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [PubMed]
- Hadipour Moghaddam, S.P.; Yazdimamaghani, M.; Ghandehari, H. Glutathione-sensitive hollow mesoporous silica nanoparticles for controlled drug delivery. J. Control. Release 2018, 282, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Chiu, P.-J.; Vetrivel, S.; Chiang, A.S.T.; Kao, H.-M. Synthesis and characterization of cubic periodic mesoporous organosilicas with a high loading of disulfide groups. New J. Chem. 2011, 35, 489–494. [Google Scholar] [CrossRef]
- Bunker, B.C. Molecular mechanisms for corrosion of silica and silicate glasses. J. Non-Cryst. Solids. 1994, 179, 300–308. [Google Scholar] [CrossRef]
- Weber, J.S.; Gibney, G.; Sullivan, R.J.; Sosman, J.A.; Slingluff, C.L.; Lawrence, D.P.; Logan, T.F.; Schuchter, L.M.; Nair, S.; Fecher, L.; et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): An open-label, randomised, phase 2 trial. Lancet Oncol. 2016, 17, 943–955. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Zhai, Z.; Yang, L.; Tang, X.; Zhao, L.; Xu, K.; Zhong, W. Double-crosslinked nanocomposite hydrogels for temporal control of drug dosing in combination therapy. Acta Biomater. 2020, 16, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Ye, A.S.; Gardino, A.K.; Heijink, A.M.; Sorger, P.K.; MacBeath, G.; Yaffe, M.B. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell 2012, 149, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Peppas, N.A. Chronobiology, drug delivery, and chronotherapeutics. Adv. Drug Deliv. Rev. 2007, 59, 828–851. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, H.; Liu, Z.; Chen, B. Effects of major parameters of nanoparticles on their physical and chemical properties and recent application of nanodrug delivery system in targeted chemotherapy. Int. J. Nanomed. 2017, 12, 8483–8493. [Google Scholar] [CrossRef]
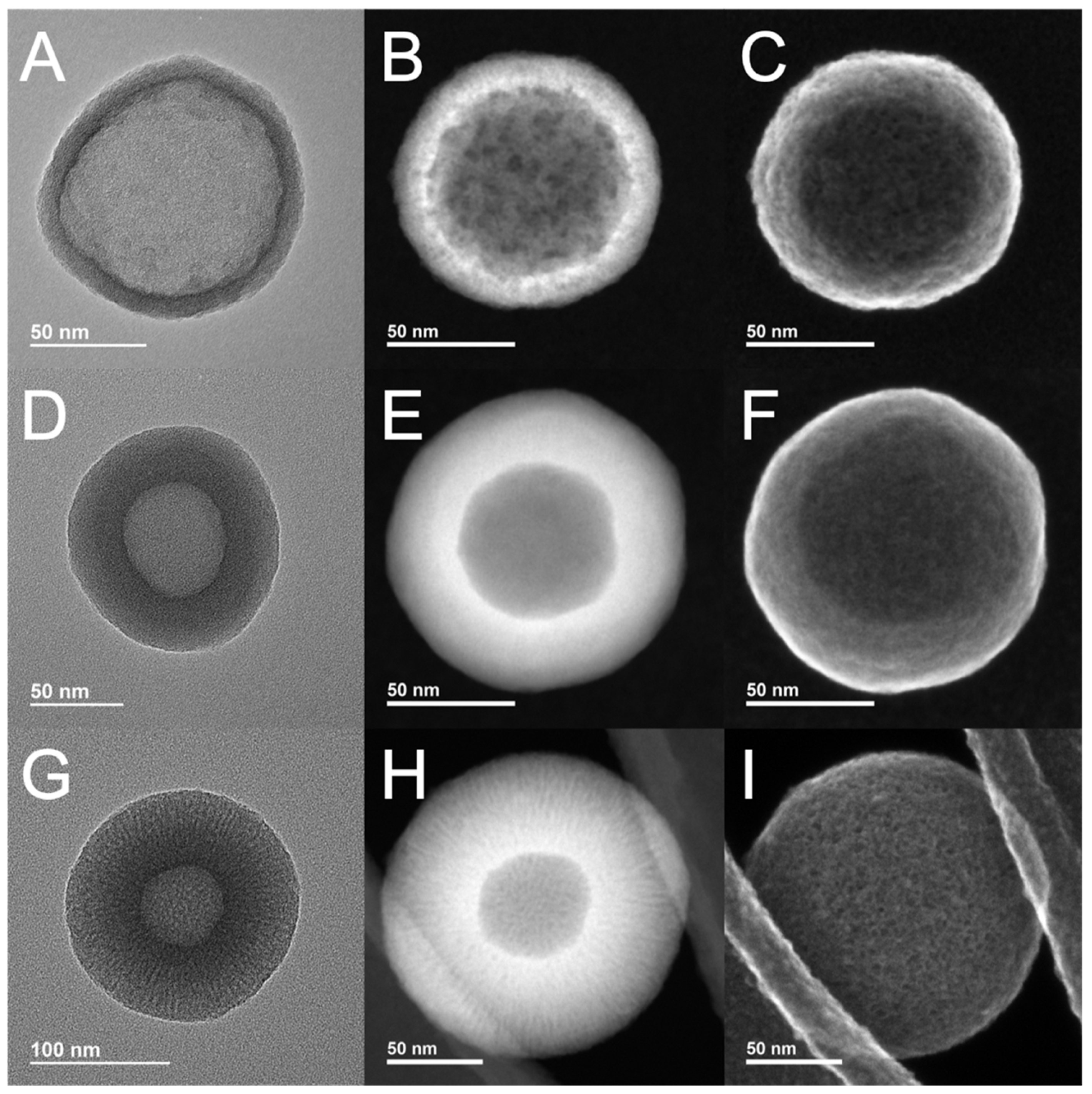



| Nanoparticle | Diameter ± SD Measured by TEM (nm) | Shell Thickness ± SD Measured by TEM (nm) | Hydrodynamic Size ± SD Measured by DLS (nm) | PdI | Zeta Potential ± SD Measured by DLS (mV) |
|---|---|---|---|---|---|
| H1 | 106 ± 12 | 10 ± 1 | 132 ± 41 | 0.09 | −16 ± 3 |
| H2 | 131 ± 14 | 33 ± 4 | 196 ± 56 | 0.07 | −30 ± 4 |
| H3 | 171 ± 8 | 55 ± 2 | 224 ± 76 | 0.10 | −22 ± 3 |
| Nanoparticle | Silicon to Sulfur Composition Percentages (Atom %) | |
|---|---|---|
| Si | S | |
| H1 | 94.24 | 5.76 |
| H2 | 65.29 | 34.71 |
| H3 | 81.11 | 18.89 |
| Nanoparticle | BET Surface Area (m2 g−1) | Total Volume in Pores (cm3 g−1) | Total Area in Pores (m2 g−1) | Pore Diameter (nm) |
|---|---|---|---|---|
| H1 | 602 | 0.44 | 307 | 5.7 |
| H2 | 41 | 0.49 | 36 | 3.9 |
| H3 | 465 | 0.47 | 292 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunberger, J.W.; Ghandehari, H. Layer-by-Layer Hollow Mesoporous Silica Nanoparticles with Tunable Degradation Profile. Pharmaceutics 2023, 15, 832. https://doi.org/10.3390/pharmaceutics15030832
Grunberger JW, Ghandehari H. Layer-by-Layer Hollow Mesoporous Silica Nanoparticles with Tunable Degradation Profile. Pharmaceutics. 2023; 15(3):832. https://doi.org/10.3390/pharmaceutics15030832
Chicago/Turabian StyleGrunberger, Jason William, and Hamidreza Ghandehari. 2023. "Layer-by-Layer Hollow Mesoporous Silica Nanoparticles with Tunable Degradation Profile" Pharmaceutics 15, no. 3: 832. https://doi.org/10.3390/pharmaceutics15030832
APA StyleGrunberger, J. W., & Ghandehari, H. (2023). Layer-by-Layer Hollow Mesoporous Silica Nanoparticles with Tunable Degradation Profile. Pharmaceutics, 15(3), 832. https://doi.org/10.3390/pharmaceutics15030832






