Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Main EDCs
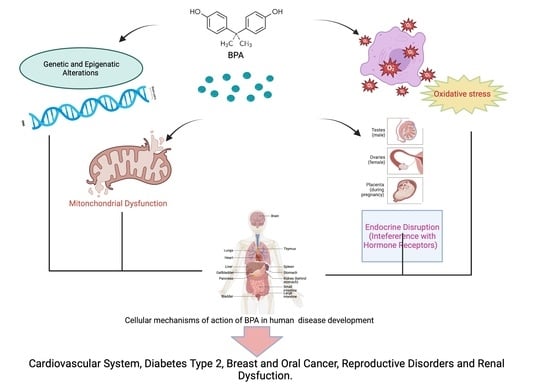
3.1. Bisphenol A
3.2. Other EDCs
Phthalates
3.3. Dioxins
4. Common Clinical Uses of Bisphenol A
4.1. Dentistry
4.2. Orthopaedics
4.3. Bisphenol in the Industrial Uses
5. Bisphenol A Effects on Human Health
5.1. Bisphenol A Effects on Immune System
5.2. Bisphenol A and Type 2 Diabetes Mellitus
5.3. Cardiovascular Toxicity of Bisphenol A
5.4. BPA and Cancer
5.5. Bisphenol A and Infertility
5.6. BPA and Polycystic Ovary Syndrome (PCOS)
5.7. BPA and the Gut
5.8. BPA and Thyroid Dysfunction
5.9. BPA and Renal Dysfunction/Nephrotoxicity
6. BPA and MSCs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Endocrine disrupting chemicals and breast cancer: A systematic review of epidemiological studies. Crit. Rev. Food Sci. 2022, 62, 6549–6576. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.J.A. The endocrine disrupters: A major medical challenge. Food Chem. Toxicol. 2002, 40, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Cargnelutti, F.; Di Nisio, A.; Pallotti, F.; Sabovic, I.; Spaziani, M.; Tarsitano, M.G.; Paoli, D.; Foresta, C. Effects of endocrine disruptors on fetal testis development, male puberty, and transition age. Endocrine 2021, 72, 591–595. [Google Scholar] [CrossRef]
- Duenas-Moreno, J.; Mora, A.; Cervantes-Aviles, P.; Mahlknecht, J. Groundwater contamination pathways of phthalates and bisphenol A: Origin, characteristics, transport, and fate—A review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef]
- Penserini, L.; Cantoni, B.; Gabrielli, M.; Sezenna, E.; Saponaro, S.; Antonelli, M. An integrated human health risk assessment framework for alkylphenols due to drinking water and crops’ food consumption. Chemosphere 2023, in press. [Google Scholar] [CrossRef]
- Alberghini, L.; Truant, A.; Santonicola, S.; Colavita, G.; Giaccone, V. Microplastics in Fish and Fishery Products and Risks for Human Health: A Review. Int. J. Environ. Res. Public Health 2022, 20, 789. [Google Scholar] [CrossRef]
- Kolan, A.S.; Hall, J.M. Association of Preterm Birth and Exposure to Endocrine Disrupting Chemicals. Int. J. Mol. Sci. 2023, 24, 1952. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Araiza, V.; Mendoza, M.S.; Castro, K.E.N.; Cruz, S.M.; Rueda, K.C.; de Leon, C.T.G.; Morales Montor, J. Bisphenol A, an endocrine-disruptor compund, that modulates the immune response to infections. Front. Biosci. 2021, 26, 346–362. [Google Scholar] [CrossRef]
- Rathee, M.; Malik, P.; Singh, J. Bisphenol A in dental sealants and its estrogen like effect. Ind. J Endocrinol. Metab. 2012, 16, 339–342. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L.; Mark-Lee, W.F. A Review on the Effects of Bisphenol A and Its Derivatives on Skeletal Health. Int. J. Med. Sci. 2018, 15, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Weizhen, Z.; Xiaowei, Z.; Peng, G.; Ning, W.; Zini, L.; Jian, H.; Zheng, Z. Distribution and risk assessment of phthalates in water and sediment of the Pearl River Delta. Environ. Sci. Pollut. Res. Int. 2020, 27, 12550–12565. [Google Scholar] [CrossRef]
- Huang, P.C.; Tsai, C.H.; Liang, W.Y.; Li, S.S.; Huang, H.B.; Kuo, P.L. Early Phthalates Exposure in Pregnant Women Is Associated with Alteration of Thyroid Hormones. PLoS ONE 2016, 11, e0159398. [Google Scholar] [CrossRef] [Green Version]
- Philippat, C.; Bennett, D.H.; Krakowiak, P.; Rose, M.; Hwang, H.M.; Hertz-Picciotto, I. Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study. Environ. Health-Glob. 2015, 14, 56. [Google Scholar] [CrossRef] [Green Version]
- Van Gerwen, M.; Vasan, V.; Genden, E.; Saul, S.R. Human 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure and thyroid cancer risk. Toxicology 2023, 488, 153474. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, S.; Guo, X.L.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Z.; Lyu, B.; Li, J.; Zhao, Y.; Wu, Y. Polychlorinated dibenzo-p-dioxins and dibenzofurans and dioxin-like polychlorinated biphenyls in human milk from national human breast milk monitoring in 2016–2019 in China. Sci. Total Environ. 2023, 872, 162243. [Google Scholar] [CrossRef]
- Dioxins and their effects on human health. Saudi Med. J. 1999, 20, 652–653.
- Milbrath, M.O.; Wenger, Y.; Chang, C.W.; Emond, C.; Garabrant, D.; Gillespie, B.W.; Jolliet, O. Apparent Half-Lives of Dioxins, Furans, and Polychlorinated Biphenyls as a Function of Age, Body Fat, Smoking Status, and Breast-Feeding. Environ. Health Perspect. 2009, 117, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, N.; Pasalic, D.; Ferencak, G.; Grskovic, B.; Rukavina, A.S. Dioxins and Human Toxicity. Arh. Hig. Rada Toksiko. 2010, 61, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halperin, W.; Vogt, R.; Sweeney, M.H.; Shopp, G.; Fingerhut, M.; Petersen, M. Immunological markers among workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup. Environ. Med. 1998, 55, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiden, T.K.; Carvan, M.J., 3rd; Hutz, R.J. Inhibition of follicular development, vitellogenesis, and serum 17beta-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006, 90, 490–499. [Google Scholar] [CrossRef] [Green Version]
- Ishimura, R.; Kawakami, T.; Ohsako, S.; Nohara, K.; Tohyama, C. Suppressive effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on vascular remodeling that takes place in the normal labyrinth zone of rat placenta during late gestation. Toxicol. Sci. 2006, 91, 265–274. [Google Scholar] [CrossRef]
- Schnorr, T.M.; Lawson, C.C.; Whelan, E.A.; Dankovic, D.A.; Deddens, J.A.; Piacitelli, L.A.; Reefhuis, J.; Sweeney, M.H.; Connally, L.B.; Fingerhut, M.A. Spontaneous abortion, sex ratio, and paternal occupational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Environ. Health Perspect. 2001, 109, 1127–1132. [Google Scholar] [CrossRef]
- Kitajima, M.; Khan, K.N.; Fujishita, A.; Masuzaki, H.; Koji, T.; Ishimaru, T. Expression of the arylhydrocarbon receptor in the peri-implantation period of the mouse uterus and the impact of dioxin on mouse implantation. Arch. Histol. Cytol. 2004, 67, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Lopes-Rocha, L.; Ribeiro-Goncalves, L.; Henriques, B.; Ozcan, M.; Tiritan, M.E.; Souza, J.C.M. An integrative review on the toxicity of Bisphenol A (BPA) released from resin composites used in dentistry. J. Biomed. Mater. Research. Part B Appl. Biomater. 2021, 109, 1942–1952. [Google Scholar] [CrossRef]
- Dursun, E.; Fron-Chabouis, H.; Attal, J.P.; Raskin, A. Bisphenol A Release: Survey of the Composition of Dental Composite Resins. Open Dent. J. 2016, 10, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Bationo, R.; Jordana, F.; Boileau, M.J.; Colat-Parros, J. Release of monomers from orthodontic adhesives. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 491–498. [Google Scholar] [CrossRef]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Tichy, A.; Simkova, M.; Vrbova, R.; Roubickova, A.; Duskova, M.; Bradna, P. Bisphenol A Release from Dental Composites and Resin-Modified Glass Ionomers under Two Polymerization Conditions. Polymers 2022, 14, 46. [Google Scholar] [CrossRef]
- Jepson, N.J.A.; McGill, J.T.; McCabe, J.F. Influence of dietary simulating solvents on the viscoelasticity of temporary soft lining materials. J. Prosthet. Dent. 2000, 83, 25–31. [Google Scholar] [CrossRef]
- Gupta, S.K.; Saxena, P.; Pant, V.A.; Pant, A.B. Release and toxicity of dental resin composite. Toxicol. Int. 2012, 19, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Pinera, A.R.; Duran, C.; Lopez, B.; Saez, I.; Correia, E.; Alvarez, L. Instrumented lumbar arthrodesis in elderly patients: Prospective study using cannulated cemented pedicle screw instrumentation. Eur. Spine J. 2011, 20, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Wahnert, D.; Raschke, M.J.; Fuchs, T. Cement augmentation of the navigated iliosacral screw in the treatment of insufficiency fractures of the sacrum: A new method using modified implants. Int. Orthop. 2013, 37, 1147–1150. [Google Scholar] [CrossRef] [Green Version]
- Dubory, A.; Bachy, M.; Bouloussa, H.; Courvoisier, A.; Morel, B.; Vialle, R. Screw augmentation for spinopelvic fixation in neuromuscular spine deformities: Technical note. Eur. Spine J. 2015, 24, 2580–2587. [Google Scholar] [CrossRef]
- Bae, H.; Hatten, H.P., Jr.; Linovitz, R.; Tahernia, A.D.; Schaufele, M.K.; McCollom, V.; Gilula, L.; Maurer, P.; Benyamin, R.; Mathis, J.M.; et al. A prospective randomized FDA-IDE trial comparing Cortoss with PMMA for vertebroplasty: A comparative effectiveness research study with 24-month follow-up. Spine 2012, 37, 544–550. [Google Scholar] [CrossRef]
- Rollinghoff, M.; Siewe, J.; Eysel, P.; Delank, K.S. Pulmonary cement embolism after augmentation of pedicle screws with bone cement. Acta Orthop. Belg. 2010, 76, 269–273. [Google Scholar]
- Nikafshar, S.; Wang, J.; Dunne, K.; Sangthonganotai, P.; Nejad, M. Choosing the Right Lignin to Fully Replace Bisphenol A in Epoxy Resin Formulation. ChemSusChem 2021, 14, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Q.; Liu, X.Q.; Jiang, Y.H.; Tang, Z.B.; Zhang, C.Z.; Zhu, J. Bio-based epoxy resin from itaconic acid and its thermosets cured with anhydride and comonomers. Green Chem. 2013, 15, 245–254. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karabela, M.M.; Varkopoulou, E.A.; Sideridou, I.D. Cure Kinetics Study of Two Epoxy Systems with Fourier Tranform Infrared Spectroscopy (FTIR) and Differential Scanning Calorimetry (DSC). J. Macromol. Sci. A 2012, 49, 630–638. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Teixeira Mendonça, R. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Campesi, I.; Marino, M.; Montella, A.; Pais, S.; Franconi, F. Sex Differences in Estrogen Receptor alpha and beta Levels and Activation Status in LPS-Stimulated Human Macrophages. J. Cell. Physiol. 2017, 232, 340–345. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhao, J.; Qiao, Z. Estrogen, but not testosterone, down-regulates cytokine production in nicotine-induced murine macrophage. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 311–316. [Google Scholar] [CrossRef]
- Yamashita, U.; Sugiura, T.; Yoshida, Y.; Kuroda, E. Effect of endocrine disrupters on macrophage functions in vitro. J. UOEH 2005, 27, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Liu, T.; Uemura, Y.; Jiao, S.; Wang, D.; Lin, Z.; Narita, Y.; Suzuki, M.; Hirosawa, N.; Ichihara, Y.; et al. Bisphenol A in combination with TNF-alpha selectively induces Th2 cell-promoting dendritic cells in vitro with an estrogen-like activity. Cell. Mol. Immunol. 2010, 7, 227–234. [Google Scholar] [CrossRef]
- Ratajczak-Wrona, W.; Rusak, M.; Nowak, K.; Dabrowska, M.; Radziwon, P.; Jablonska, E. Effect of bisphenol A on human neutrophils immunophenotype. Sci. Rep. 2020, 10, 3083. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Adachi, R.; Kusui, K.; Hirayama, A.; Kasahara, T.; Suzuki, K. Bisphenol A significantly enhances the neutrophilic differentiation of promyelocytic HL-60 cells. Int. Immunopharmacol. 2003, 3, 1601–1608. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Salem, M.L.; Hossain, M.S.; Nomoto, K. Mediation of the immunomodulatory effect of beta-estradiol on inflammatory responses by inhibition of recruitment and activation of inflammatory cells and their gene expression of TNF-alpha and IFN-gamma. Int. Arch. Allergy Imm. 2000, 121, 235–245. [Google Scholar] [CrossRef]
- Tian, X.L.; Takamoto, M.; Sugane, K. Bisphenol A promotes IL-4 production by Th2 cells. Int. Arch. Allergy Imm. 2003, 132, 240–247. [Google Scholar] [CrossRef]
- Zerdan, M.B.; Moussa, S.; Atoui, A.; Assi, H.I. Mechanisms of Immunotoxicity: Stressors and Evaluators. Int. J. Mol. Sci. 2021, 22, 8242. [Google Scholar] [CrossRef]
- Yoshino, S.; Yamaki, K.; Li, X.J.; Sai, T.; Yanagisawa, R.; Takano, H.; Taneda, S.; Hayashi, H.; Mori, Y. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology 2004, 112, 489–495. [Google Scholar] [CrossRef]
- Yoshino, S.; Yamaki, K.; Yanagisawa, R.; Takano, H.; Hayashi, H.; Mori, Y. Effects of bisphenol A on antigen-specific antibody production, proliferative responses of lymphoid cells, and TH1 and TH2 immune responses in mice. Brit. J. Pharmacol. 2003, 138, 1271–1276. [Google Scholar] [CrossRef] [Green Version]
- Nowak, K.; Jablonska, E.; Ratajczak-Wrona, W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ. Int. 2019, 125, 350–364. [Google Scholar] [CrossRef]
- Soriano, S.; Alonso-Magdalena, P.; Garcia-Arevalo, M.; Novials, A.; Muhammed, S.J.; Salehi, A.; Gustafsson, J.A.; Quesada, I.; Nadal, A. Rapid Insulinotropic Action of Low Doses of Bisphenol-A on Mouse and Human Islets of Langerhans: Role of Estrogen Receptor beta. PLoS ONE 2012, 7, e31109. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, T.M.; Alonso-Magdalena, P.; Vieira, E.; Amaral, M.E.C.; Cederroth, C.R.; Nef, S.; Quesada, I.; Carneiro, E.M.; Nadal, A. Short-Term Treatment with Bisphenol-A Leads to Metabolic Abnormalities in Adult Male Mice. PLoS ONE 2012, 7, e33814. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N.P. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Ropero, A.B.; Carrera, M.P.; Cederroth, C.R.; Baquie, M.; Gauthier, B.R.; Nef, S.; Stefani, E.; Nadal, A. Pancreatic Insulin Content Regulation by the Estrogen Receptor ER alpha. PLoS ONE 2008, 3, e2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzzin, J.; Petersen, R.; Meugnier, E.; Madsen, L.; Lock, E.J.; Lillefosse, H.; Ma, T.; Pesenti, S.; Sonne, S.B.; Marstrand, T.T.; et al. Persistent Organic Pollutant Exposure Leads to Insulin Resistance Syndrome. Environ. Health Perspect. 2010, 118, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.F.; Shan, C.; Wang, Y.; Qian, L.L.; Jia, D.D.; Zhang, Y.F.; Hao, X.D.; Xu, H.M. Cardiovascular toxicity and mechanism of bisphenol A and emerging risk of bisphenol S. Sci. Total Environ. 2020, 723, 137952. [Google Scholar] [CrossRef]
- Moreman, J.; Takesono, A.; Trznadel, M.; Winter, M.J.; Perry, A.; Wood, M.E.; Rogers, N.J.; Kudoh, T.; Tyler, C.R. Estrogenic Mechanisms and Cardiac Responses Following Early Life Exposure to Bisphenol A (BPA) and Its Metabolite 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) in Zebrafish. Environ. Sci. Technol. 2018, 52, 6656–6665. [Google Scholar] [CrossRef] [Green Version]
- Posnack, N.G.; Brooks, D.; Chandra, A.; Jaimes, R.; Sarvazyan, N.; Kay, M. Physiological response of cardiac tissue to bisphenol a: Alterations in ventricular pressure and contractility. Am. J. Physiol.-Heart C 2015, 309, H267–H275. [Google Scholar] [CrossRef] [Green Version]
- Belcher, S.M.; Chen, Y.; Yan, S.; Wang, H.S. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology 2012, 153, 712–720. [Google Scholar] [CrossRef]
- Marmugi, A.; Lasserre, F.; Beuzelin, D.; Ducheix, S.; Huc, L.; Polizzi, A.; Chetivaux, M.; Pineau, T.; Martin, P.; Guillou, H.; et al. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 2014, 325, 133–143. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, M.K.; Kang, G.H.; Lee, K.J.; Choi, S.H.; Lim, S.; Oh, B.C.; Park, D.J.; Park, K.S.; Jang, H.C.; et al. Chronic Exposure to Bisphenol A can Accelerate Atherosclerosis in High-Fat-Fed Apolipoprotein E Knockout Mice. Cardiovasc. Toxicol. 2014, 14, 120–128. [Google Scholar] [CrossRef]
- Winz, C.; Suh, N. Understanding the Mechanistic Link between Bisphenol A and Cancer Stem Cells: A Cancer Prevention Perspective. J. Cancer Prev. 2021, 26, 18–24. [Google Scholar] [CrossRef]
- Dairkee, S.H.; Luciani-Torres, M.G.; Moore, D.H.; Goodson, W.H. Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis 2013, 34, 703–712. [Google Scholar] [CrossRef]
- Goodson, W.H.; Luciani, M.G.; Sayeed, S.A.; Jaffee, I.M.; Moore, D.H.; Dairkee, S.H. Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women. Carcinogenesis 2011, 32, 1724–1733. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Gwon, D.; Kim, J.A.; Choi, H.; Jang, C.Y. Bisphenol A disrupts mitotic progression via disturbing spindle attachment to kinetochore and centriole duplication in cancer cell lines. Toxicol Vitr. 2019, 59, 115–125. [Google Scholar] [CrossRef]
- Chen, Y.K.; Tan, Y.Y.; Yao, M.; Lin, H.C.; Tsai, M.H.; Li, Y.Y.; Hsu, Y.J.; Huang, T.T.; Chang, C.W.; Cheng, C.M.; et al. Bisphenol A-induced DNA damages promote to lymphoma progression in human lymphoblastoid cells through aberrant CTNNB1 signaling pathway. iScience 2021, 24, 102888. [Google Scholar] [CrossRef]
- Atlas, E.; Dimitrova, V. Bisphenol S and Bisphenol A disrupt morphogenesis of MCF-12A human mammary epithelial cells. Sci. Rep.-UK 2019, 9, 16005. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.G.; Correia, J.; Adiga, D.; Rai, P.S.; Dsouza, H.S.; Chakrabarty, S.; Kabekkodu, S.P. A comprehensive review on the carcinogenic potential of bisphenol A: Clues and evidences. Environ. Sci. Poll. Res. 2021, 28, 19643–19663. [Google Scholar] [CrossRef]
- Lee, J.H.; Yi, S.K.; Kim, S.Y.; Kim, J.S.; Son, S.A.; Jeong, S.H.; Kim, J.B. Salivary bisphenol A levels and their association with composite resin restoration. Chemosphere 2017, 172, 46–51. [Google Scholar] [CrossRef]
- Emfietzoglou, R.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Could the endocrine disruptor bisphenol-A be implicated in the pathogenesis of oral and oropharyngeal cancer? Metabolic considerations and future directions. Metab. Clin. Exp. 2019, 91, 61–69. [Google Scholar] [CrossRef]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjanen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Valimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Makela, S.; Kujari, H.; Gustafsson, J.A.; Laine, M. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colella, G.; Izzo, G.; Carinci, F.; Campisi, G.; Lo Muzio, L.; D’Amato, S.; Mazzotta, M.; Cannavale, R.; Ferrara, D.; Minucci, S. Expression of sexual hormones receptors in oral squamous cell carcinoma. Int. J. Immunopathol. Pharmacol. 2011, 24, 129–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, T.F.A.; Oliveira, S.R.; Mayra da Silva, J.; Fernandes de Oliveira, A.L.; de Lourdes Cardeal, Z.; Menezes, H.C.; Gomes, J.M.; Campolina-Silva, G.H.; Oliveira, C.A.; Macari, S.; et al. Effects of high-dose bisphenol A on the mouse oral mucosa: A possible link with oral cancers. Environ. Pollut. 2021, 286, 117296. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Vaccari, M.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Barcellos-Hoff, M.H.; Brown, D.G.; Chapellier, M.; Christopher, J.; Curran, C.S.; et al. The effect of environmental chemicals on the tumor microenviroment. Carcinogenesis 2015, 36, S160–S183. [Google Scholar] [CrossRef] [Green Version]
- Nomiri, S.; Hoshyar, R.; Ambrosino, C.; Tyler, C.R.; Mansouri, B. A mini review of bisphenol A (BPA) effects on cancer-related cellular signaling pathways. Environ. Sci. Pollut. Res. Int. 2019, 26, 8459–8467. [Google Scholar] [CrossRef]
- Ptak, A.; Gregoraszczuk, E.L. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol. Lett. 2012, 210, 332–337. [Google Scholar] [CrossRef]
- Song, H.X.; Zhang, T.; Yang, P.; Li, M.H.; Yang, Y.H.; Wang, Y.Y.; Du, J.; Pan, K.J.; Zhang, K. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERR gamma signals. Toxicol In Vitro 2015, 30, 521–528. [Google Scholar] [CrossRef]
- Wang, P.; Luo, C.H.; Li, Q.Y.; Chen, S.; Hue, Y. Mitochondrion-mediated apoptosis is involved in reproductive damage caused by BPA in male rats. Environ. Toxicol. Pharmacol. 2014, 38, 1025–1033. [Google Scholar] [CrossRef]
- Yin, L.; Dai, Y.L.; Cui, Z.H.; Jiang, X.; Liu, W.B.; Han, F.; Ao, L.; Cao, J.; Liu, J.Y. The regulation of cellular apoptosis by the ROS-triggered PERK/EIF2 alpha chop pathway plays a vital role in bisphenol A-induced male reproductive toxicity. Toxicol. Appl. Pharm. 2017, 314, 98–108. [Google Scholar] [CrossRef]
- Othman, A.I.; Edrees, G.M.; El-Missiry, M.A.; Ali, D.A.; Aboel-Nour, M.; Dabdoub, B.R. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health 2016, 32, 1537–1549. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef]
- Hiura, H.; Obata, Y.; Komiyama, J.; Shirai, M.; Kono, T. Oocyte growth-dependent progression of maternal imprinting in mice. Genes Cells 2006, 11, 353–361. [Google Scholar] [CrossRef]
- Robertson, K.D. DNA methylation, methyltransferases, and cancer. Oncogene 2001, 20, 3139–3155. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.H.; Zhang, X.F.; Chen, B.; Pan, B.; Zhang, L.J.; Li, L.; Sun, X.F.; Shi, Q.H.; Shen, W. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem. Cell Biol. 2012, 137, 249–259. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Qin, X.S.; Zhou, Y.; Zhang, X.F.; Wang, L.Q.; De Felici, M.; Chen, H.; Qin, G.Q.; Shen, W. Di-(2-ethylhexyl) Phthalate and Bisphenol a Exposure Impairs Mouse Primordial Follicle Assembly In Vitro. Environ. Mol. Mutagen. 2014, 55, 343–353. [Google Scholar] [CrossRef]
- Can, A.; Semiz, O.; Cinar, O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol. Hum. Reprod. 2005, 11, 389–396. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Lu, J.; Zhang, Y.Z.; Zhang, M.; Liu, T.; Qu, X.L. Effect of Bisphenol A on invasion ability of human trophoblastic cell line BeWo. Int. J. Clin. Exp. Pathol. 2015, 8, 14355–14364. [Google Scholar]
- Morice, L.; Benaitreau, D.; Dieudonne, M.N.; Morvan, C.; Serazin, V.; de Mazancourt, P.; Pecquery, R.; Dos Santos, E. Antiproliferative and proapoptotic effects of bisphenol A on human trophoblastic JEG-3 cells. Reprod. Toxicol. 2011, 32, 69–76. [Google Scholar] [CrossRef]
- Konieczna, A.; Rutkowska, A.; Rachon, D. Health risk of exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar]
- Rutkowska, A.; Rachon, D. Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS). Gynecol.Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2014, 30, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.C.; Tavares, S.C.; Garcia, H.V.; Cucielo, M.S.; Dos Santos, S.A.A.; Aal, M.C.E.; de Golim, M.A.; Justulin, L.A., Jr.; Ribeiro, A.O.; Deffune, E.; et al. Bisphenol A and 2,3,7,8-tetrachlorodibenzo-p-dioxin at non-cytotoxic doses alter the differentiation potential and cell function of rat adipose-stem cells. Environ. Toxicol. 2022, 37, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Qiu, L.; Zhu, J.J.; Sun, Q.; Qu, W.; Yu, Y.F.; Zhao, Z.G.; Yu, Y.F.; Shao, G.Y. Environmental contaminant BPA causes intestinal damage by disrupting cellular repair and injury homeostasis in vivo and in vitro. Biomed. Pharmacother. 2021, 137, 111270. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocr. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef] [PubMed]
- Heimeier, R.A.; Das, B.; Buchholz, D.R.; Shi, Y.B. The Xenoestrogen Bisphenol a Inhibits Postembryonic Vertebrate Development by Antagonizing Gene Regulation by Thyroid Hormone. Endocrinology 2009, 150, 2964–2973. [Google Scholar] [CrossRef] [Green Version]
- Zoeller, R.T. Environmental chemicals as thyroid hormone analogues: New studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol. Cell. Endocrinol. 2005, 242, 10–15. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, Y.J. Bisphenols and Thyroid Hormone. Endocrinol. Metab. 2019, 34, 340–348. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, Y.; Tae, K.; Bae, G.U.; Park, J.Y.; Cho, Y.H.; Yang, M. Proteomic Biomarkers for Bisphenol A-Early Exposure and Women’s Thyroid Cancer. Cancer Res. Treat. 2018, 50, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, H.; Zhang, J.; Gao, X.; Jin, H.; Liu, R.; Zhang, Z.; Zhang, X.; Wang, X.; Qu, P.; et al. Bisphenol A at a human exposed level can promote epithelial-mesenchymal transition in papillary thyroid carcinoma harbouring BRAF(V600E) mutation. J. Cell. Mol. Med. 2021, 25, 1739–1749. [Google Scholar] [CrossRef]
- Moreno-Gomez-Toledano, R.; Arenas, M.I.; Velez-Velez, E.; Coll, E.; Quiroga, B.; Bover, J.; Bosch, R.J. Bisphenol a Exposure and Kidney Diseases: Systematic Review, Meta-Analysis, and NHANES 03-16 Study. Biomolecules 2021, 11, 1046. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxidative Med. Cell. Longev. 2018, 2018, 3082438. [Google Scholar] [CrossRef] [Green Version]
- Kobroob, A.; Peerapanyasut, W.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules 2021, 11, 655. [Google Scholar] [CrossRef]
- Hu, J.B.; Wang, Y.; Xiang, X.J.; Peng, C.; Gao, R.F.; Goswami, R.; Zhou, H.; Zhang, Y.; Zhen, Q.N.; Cheng, Q.F.; et al. Serum bisphenol A as a predictor of chronic kidney disease progression in primary hypertension: A 6-year prospective study. J. Hypertens. 2016, 34, 332–337. [Google Scholar] [CrossRef]
- Fonticoli, L.; Della Rocca, Y.; Rajan, T.S.; Murmura, G.; Trubiani, O.; Oliva, S.; Pizzicannella, J.; Marconi, G.D.; Diomede, F. A Narrative Review: Gingival Stem Cells as a Limitless Reservoir for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 4135. [Google Scholar] [CrossRef]
- Marconi, G.D.; Porcheri, C.; Trubiani, O.; Mitsiadis, T.A. Three-Dimensional Culture Systems for Dissecting Notch Signalling in Health and Disease. Int. J. Mol. Sci. 2021, 22, 12473. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef]
- Cohen, I.C.; Cohenour, E.R.; Harnett, K.G.; Schuh, S.M. BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity. Int. J. Mol. Sci. 2021, 22, 5363. [Google Scholar] [CrossRef]
- Salehpour, A.; Shidfar, F.; Hedayati, M.; Farshad, A.A.; Tehrani, A.N.; Mohammadi, S. Molecular mechanisms of vitamin D plus Bisphenol A effects on adipogenesis in human adipose-derived mesenchymal stem cells. Diabetol. Metab. Syndr. 2021, 13, 41. [Google Scholar] [CrossRef]
- Desai, M.; Ferrini, M.G.; Jellyman, J.K.; Han, G.; Ross, M.G. In vivo and in vitro bisphenol A exposure effects on adiposity. J. Dev. Orig. Health Dis. 2018, 9, 678–687. [Google Scholar] [CrossRef]
- Dong, H.; Yao, X.; Liu, S.; Yin, N.; Faiola, F. Non-cytotoxic nanomolar concentrations of bisphenol A induce human mesenchymal stem cell adipogenesis and osteogenesis. Ecotoxicol. Environ. Saf. 2018, 164, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Biemann, R.; Fischer, B.; Navarrete Santos, A. Adipogenic effects of a combination of the endocrine-disrupting compounds bisphenol A, diethylhexylphthalate, and tributyltin. Obes. Facts 2014, 7, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, A.; Shidfar, F.; Hedayati, M.; Tehrani, A.N.; Farshad, A.A.; Mohammadi, S. Bisphenol A enhances adipogenic signaling pathways in human mesenchymal stem cells. Genes Environ. 2020, 42, 13. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Garcia, R.; Kirchner, S.; Li, X.; Janesick, A.; Casey, S.C.; Chow, C.; Blumberg, B. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ. Health Perspect. 2012, 120, 984–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junge, K.M.; Leppert, B.; Jahreis, S.; Wissenbach, D.K.; Feltens, R.; Grutzmann, K.; Thurmann, L.; Bauer, T.; Ishaque, N.; Schick, M.; et al. MEST mediates the impact of prenatal bisphenol A exposure on long-term body weight development. Clin. Epigenetics 2018, 10, 58. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Rivera, F.J.; Guerrero-Bosagna, C. Bisphenol-A and metabolic diseases: Epigenetic, developmental and transgenerational basis. Environ. Epigenetics 2016, 2, dvw022. [Google Scholar] [CrossRef] [Green Version]
- Soltani, A.; Abroun, S.; Abbasnejadshani, F.; Gholampour, M.A. Effects of bone marrow-derived mesenchymal stem cells exposed to endocrine-disrupting chemicals on the differentiation of umbilical cord blood hematopoietic stem cells. Environ. Sci. Pollut. Res. Int. 2022, 29, 39903–39913. [Google Scholar] [CrossRef]
- Fouad, H.; Faruk, E.M.; Alasmari, W.A.; Nadwa, E.H.; Ebrahim, U.F.A. Structural and chemical role of mesenchymal stem cells and resveratrol in regulation of apoptotic-induced genes in Bisphenol-A induced uterine damage in adult female albino rats. Tissue Cell 2021, 70, 101502. [Google Scholar] [CrossRef]
- Harnett, K.G.; Chin, A.; Schuh, S.M. BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells. Ecotoxicol. Environ. Saf. 2021, 216, 112210. [Google Scholar] [CrossRef]
- Leem, Y.H.; Oh, S.; Kang, H.J.; Kim, J.H.; Yoon, J.; Chang, J.S. BPA-toxicity via superoxide anion overload and a deficit in beta-catenin signaling in human bone mesenchymal stem cells. Environ. Toxicol. 2017, 32, 344–352. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Rocca, Y.; Traini, E.M.; Diomede, F.; Fonticoli, L.; Trubiani, O.; Paganelli, A.; Pizzicannella, J.; Marconi, G.D. Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions. Pharmaceutics 2023, 15, 908. https://doi.org/10.3390/pharmaceutics15030908
Della Rocca Y, Traini EM, Diomede F, Fonticoli L, Trubiani O, Paganelli A, Pizzicannella J, Marconi GD. Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions. Pharmaceutics. 2023; 15(3):908. https://doi.org/10.3390/pharmaceutics15030908
Chicago/Turabian StyleDella Rocca, Ylenia, Enrico Matteo Traini, Francesca Diomede, Luigia Fonticoli, Oriana Trubiani, Alessia Paganelli, Jacopo Pizzicannella, and Guya Diletta Marconi. 2023. "Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions" Pharmaceutics 15, no. 3: 908. https://doi.org/10.3390/pharmaceutics15030908
APA StyleDella Rocca, Y., Traini, E. M., Diomede, F., Fonticoli, L., Trubiani, O., Paganelli, A., Pizzicannella, J., & Marconi, G. D. (2023). Current Evidence on Bisphenol A Exposure and the Molecular Mechanism Involved in Related Pathological Conditions. Pharmaceutics, 15(3), 908. https://doi.org/10.3390/pharmaceutics15030908