Multilayered Polyurethane/Poly(vinyl alcohol) Nanofibrous Mats for Local Topotecan Delivery as a Potential Retinoblastoma Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Tri-Layered Nanofibrous Mats
2.3. Drug Loading and In Vitro Drug Release
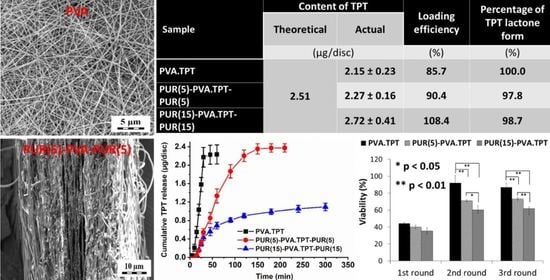
2.3.1. Determination of the TPT Form (Lactone/Carboxylate) and Total TPT Content in the Nanofibrous Mats
2.3.2. Determination of TPT Release Kinetics Using HPLC-FLD
2.4. In Vitro Cytotoxicity of the TPT-Loaded Nanofibrous Mats
3. Results and Discussion
3.1. Characterization of the Nanofibrous Mats
3.2. Drug Loading
3.3. In Vitro TPT Release Study
3.4. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Synthetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Talebian, S.; Foroughi, J.; Wade, S.J.; Vine, K.L.; Dolatshahi-Pirouz, A.; Mehrali, M.; Conde, J.; Wallace, G.G. Biopolymers for antitumor implantable drug delivery systems: Recent advances and future outlook. Adv. Mater. 2018, 30, 1706665. [Google Scholar] [CrossRef] [PubMed]
- Kopeckova, K.; Eckschlager, T.; Sirc, J.; Hobzova, R.; Plch, J.; Hrabeta, J.; Michalek, J. Nanodrugs used in cancer therapy. Biomed. Pap. 2019, 163, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Meinel, A.J.; Germershaus, O.; Luhmann, T.; Merkle, H.P.; Meinel, L. Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications. Eur. J. Pharm. Biopharm. 2012, 81, 1–13. [Google Scholar] [CrossRef]
- Fu, Y.; Li, X.; Ren, Z.; Mao, C.; Han, G. Multifunctional Electrospun Nanofibers for Enhancing Localized Cancer Treatment. Small 2018, 14, 1801183. [Google Scholar] [CrossRef]
- Poláková, L.; Širc, J.; Hobzová, R.; Cocârță, A.-I.; Heřmánková, E. Electrospun nanofibers for local anticancer therapy: Review of in vivo activity. Int. J. Pharm. 2019, 558, 268–283. [Google Scholar] [CrossRef]
- Tyo, K.; Minooei, F.; Curry, K.; NeCamp, S.; Graves, D.; Fried, J.; Steinbach-Rankins, J. Relating Advanced Electrospun Fiber Architectures to the Temporal Release of Active Agents to Meet the Needs of Next-Generation Intravaginal Delivery Applications. Pharmaceutics 2019, 11, 160. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Yu, D.; Wang, M.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1772. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, D.; Pannala, A.; Sandeman, S.; Lloyd, A. Sustained and targeted delivery of hydrophilic drug compounds: A review of existing and novel technologies from bench to bedside. J. Drug Deliv. Sci. Technol. 2022, 78, 103936. [Google Scholar] [CrossRef]
- Širc, J.; Hobzová, R.; Kostina, N.; Munzarová, M.; Juklíčková, M.; Lhotka, M.; Kubinová, Š.; Zajícová, A.; Michálek, J. Morphological Characterization of Nanofibers: Methods and Application in Practice. J. Nanomater. 2012, 2012, 121. [Google Scholar] [CrossRef]
- Hrib, J.; Sirc, J.; Hobzova, R.; Hampejsova, Z.; Bosakova, Z.; Munzarova, M.; Michalek, J. Nanofibers for drug delivery—Incorporation and release of model molecules, influence of molecular weight and polymer structure. Beilstein J. Nanotechnol. 2015, 6, 1939–1945. [Google Scholar] [CrossRef]
- Sirc, J.; Hampejsova, Z.; Trnovska, J.; Kozlik, P.; Hrib, J.; Hobzova, R.; Zajicova, A.; Holan, V.; Bosakova, Z. Cyclosporine A Loaded Electrospun Poly(D,L-Lactic Acid)/Poly(Ethylene Glycol) Nanofibers: Drug Carriers Utilizable in Local Immunosuppression. Pharm. Res. 2017, 34, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Plch, J.; Venclikova, K.; Janouskova, O.; Hrabeta, J.; Eckschlager, T.; Kopeckova, K.; Hampejsova, Z.; Bosakova, Z.; Sirc, J.; Hobzova, R. Paclitaxel-Loaded Polylactide/Polyethylene Glycol Fibers with Long-Term Antitumor Activity as a Potential Drug Carrier for Local Chemotherapy. Macromol. Biosci. 2018, 18, 1800011. [Google Scholar] [CrossRef]
- Hobzova, R.; Hampejsova, Z.; Cerna, T.; Hrabeta, J.; Venclikova, K.; Jedelska, J.; Bakowsky, U.; Bosakova, Z.; Lhotka, M.; Vaculin, S.; et al. Poly(d,l-lactide)/polyethylene glycol micro/nanofiber mats as paclitaxel-eluting carriers: Preparation and characterization of fibers, in vitro drug release, antiangiogenic activity and tumor recurrence prevention. Mater. Sci. Eng. C 2019, 98, 982–993. [Google Scholar] [CrossRef]
- Alhusein, N.; Blagbrough, I.S.; de Bank, P.A. Electrospun matrices for localised controlled drug delivery: Release of tetracycline hydrochloride from layers of polycaprolactone and poly(ethylene-co-vinyl acetate). Drug Deliv. Transl. Res. 2012, 2, 477–488. [Google Scholar] [CrossRef]
- Asgari, S.; Mohammadi Ziarani, G.; Badiei, A.; Pourjavadi, A.; Kiani, M. A smart tri-layered nanofibrous hydrogel thin film with controlled release of dual drugs for chemo-thermal therapy of breast cancer. J. Drug Deliv. Sci. Technol. 2022, 76, 103702. [Google Scholar] [CrossRef]
- Cortez Tornello, P.R.; Feresin, G.E.; Tapia, A.; Cuadrado, T.R.; Abraham, G.A. Multilayered electrospun nanofibrous scaffolds for tailored controlled release of embelin. Soft Mater. 2018, 16, 51–61. [Google Scholar] [CrossRef]
- Laha, A.; Sharma, C.S.; Majumdar, S. Sustained drug release from multi-layered sequentially crosslinked electrospun gelatin nanofiber mesh. Mater. Sci. Eng. C 2017, 76, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y.; Zhang, A.; Liu, W.; Wang, X.; Zhang, F.; Linhardt, R.J.; Lin, Z.; Sun, P. Sustained release of Ganoderma lucidum antitumor drugs using a sandwich structured material prepared by electrospinning. J. Drug Deliv. Sci. Technol. 2021, 64, 102627. [Google Scholar] [CrossRef]
- Rezk, A.I.; Rajan Unnithan, A.; Hee Park, C.; Sang Kim, C. Rational design of bone extracellular matrix mimicking tri-layered composite nanofibers for bone tissue regeneration. Chem. Eng. J. 2018, 350, 812–823. [Google Scholar] [CrossRef]
- Sebe, I.; Ostorházi, E.; Bodai, Z.; Eke, Z.; Szakács, J.; Kovács, N.K.; Zelkó, R. In vitro and in silico characterization of fibrous scaffolds comprising alternate colistin sulfate-loaded and heat-treated polyvinyl alcohol nanofibrous sheets. Int. J. Pharm. 2017, 523, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2020, 308, 125599. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Qi, Y.; Zhou, D.; Xie, Z.; Jing, X.; Chen, X.; Huang, Y. Time-programmed DCA and oxaliplatin release by multilayered nanofiber mats in prevention of local cancer recurrence following surgery. J. Control. Release 2016, 235, 125–133. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Zhang, Z.; Zhang, Y.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Use of asymmetric multilayer polylactide nanofiber mats in controlled release of drugs and prevention of liver cancer recurrence after surgery in mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1047–1056. [Google Scholar] [CrossRef]
- Falde, E.J.; Freedman, J.D.; Herrera, V.L.M.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Layered superhydrophobic meshes for controlled drug release. J. Control. Release 2015, 214, 23–29. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.; Zhu, Y.; Wang, Z.; Cao, H.; Li, X.; Yin, Y.; Ren, X.; Tian, Y.; Guo, Z.; et al. A Multilayer Nanofibrous Mat for the Topical Chemotherapy of the Positive Margin in Bladder Cancer. Tissue Eng. Part A 2022, 28, 958–967. [Google Scholar] [CrossRef]
- Available online: www.ema.europa.eu/en/documents/product-information/hycamtin-epar-product-information_en.pdf (accessed on 26 April 2023).
- Schaiquevich, P.; Carcaboso, A.M.; Buitrago, E.; Taich, P.; Opezzo, J.; Bramuglia, G.; Chantada, G.L. Ocular pharmacology of topotecan and its activity in retinoblastoma. Retina 2014, 34, 1719–1727. [Google Scholar] [CrossRef]
- Schaiquevich, P.; Francis, J.H.; Cancela, M.B.; Carcaboso, A.M.; Chantada, G.L.; Abramson, D.H. Treatment of Retinoblastoma: What Is the Latest and What Is the Future. Front. Oncol. 2022, 12, 822330. [Google Scholar] [CrossRef] [PubMed]
- Fassberg, J.; Stella, V.J. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J. Pharm. Sci. 1992, 81, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Underberg, W.J.M.; Goossen, R.M.J.; Smith, B.R.; Beijnen, J.H. Equilibrium kinetics of the new experimental anti-tumour compound SK&F 104864-A in aqueous solution. J. Pharm. Biomed. Anal. 1990, 8, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Herben, V.M.M.; ten Bokkel Huinink, W.W.; Beijnen, J.H. Clinical pharmacokinetics of topotecan. Clin. Pharmacokinet. 1996, 31, 85–102. [Google Scholar] [CrossRef]
- Burke, T.G.; Bom, D. Campthotecin Design and Delivery Approaches for Elevating Anti-Topoisomerase I Activities in Vivo. Ann. N. Y. Acad. Sci. 2006, 922, 36–45. [Google Scholar] [CrossRef]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hayes, M.E.; Connolly-Ingram, C.; Gabriel, B.S.; Hann, B.; Liu, B.; Park, J.W.; Hong, K. Development of a highly stable and targetable nanoliposomal formulation of topotecan. J. Control. Release 2010, 141, 13–21. [Google Scholar] [CrossRef]
- Lalloo, A.; Chao, P.; Hu, P.; Stein, S.; Sinko, P.J. Pharmacokinetic and pharmacodynamic evaluation of a novel in situ forming poly(ethylene glycol)-based hydrogel for the controlled delivery of the camptothecins. J. Control. Release 2006, 112, 333–342. [Google Scholar] [CrossRef]
- Souza, L.G.G.; Silva, E.J.J.; Martins, A.L.L.L.L.; Mota, M.F.F.; Braga, R.C.C.; Lima, E.M.M.; Valadares, M.C.C.; Taveira, S.F.F.; Marreto, R.N.N. Development of topotecan loaded lipid nanoparticles for chemical stabilization and prolonged release. Eur. J. Pharm. Biopharm. 2011, 79, 189–196. [Google Scholar] [CrossRef]
- Xing, R.; Mustapha, O.; Ali, T.; Rehman, M.; Zaidi, S.S.; Baseer, A.; Batool, S.; Mukhtiar, M.; Shafique, S.; Malik, M.; et al. Development, Characterization, and Evaluation of SLN-Loaded Thermoresponsive Hydrogel System of Topotecan as Biological Macromolecule for Colorectal Delivery. Biomed. Res. Int. 2021, 2021, 9968602. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, Y.; Zhao, Y.; Ren, M.; Li, Y.; Lu, G.; Wu, K.; He, S. Topotecan-loaded thermosensitive nanocargo for tumor therapy: In vitro and in vivo analyses. Int. J. Pharm. 2021, 606, 120871. [Google Scholar] [CrossRef]
- Chang, G.; Ci, T.; Yu, L.; Ding, J. Enhancement of the fraction of the active form of an antitumor drug topotecan via an injectable hydrogel. J. Control. Release 2011, 156, 21–27. [Google Scholar] [CrossRef]
- Huo, Y.; Wang, Q.; Liu, Y.; Wang, J.; Li, Q.; Li, Z.; Dong, Y.; Huang, Y.; Wang, L. A temperature-sensitive phase-change hydrogel of topotecan achieves a long-term sustained antitumor effect on retinoblastoma cells. Onco. Targets Ther. 2019, 12, 6069–6082. [Google Scholar] [CrossRef]
- Taich, P.; Moretton, M.A.; Del Sole, M.J.; Winter, U.; Bernabeu, E.; Croxatto, J.O.; Oppezzo, J.; Williams, G.; Chantada, G.L.; Chiappetta, D.A.; et al. Sustained-release hydrogels of topotecan for retinoblastoma. Colloids Surf. B Biointerfaces 2016, 146, 624–631. [Google Scholar] [CrossRef]
- Carcaboso, A.M.; Chiappetta, D.A.; Opezzo, J.A.W.W.; Höcht, C.; Fandiño, A.C.; Croxatto, J.O.; Rubio, M.C.; Sosnik, A.; Abramson, D.H.; Bramuglia, G.F.; et al. Episcleral Implants for Topotecan Delivery to the Posterior Segment of the Eye. Investig. Opthalmol. Vis. Sci. 2010, 51, 2126. [Google Scholar] [CrossRef]
- Cocarta, A.-I.; Hobzova, R.; Sirc, J.; Cerna, T.; Hrabeta, J.; Svojgr, K.; Pochop, P.; Kodetova, M.; Jedelska, J.; Bakowsky, U.; et al. Hydrogel implants for transscleral drug delivery for retinoblastoma treatment. Mater. Sci. Eng. C 2019, 103, 109799. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Sirc, J.; Kubinová, S.; Hobzova, R.; Stranska, D.; Kozlik, P.; Bosakova, Z.; Marekova, D.; Holan, V.; Sykova, E.; Michalek, J. Controlled gentamicin release from multi-layered electrospun nanofibrous structures of various thicknesses. Int. J. Nanomed. 2012, 7, 5315–5325. [Google Scholar] [CrossRef]
- Hobzova, R.; Kodetova, M.; Pochop, P.; Uhlik, J.; Dunovska, K.; Svojgr, K.; Hrabeta, J.; Feriancikova, B.; Cocarta, A.I.; Sirc, J. Hydrogel implants for transscleral diffusion delivery of topotecan: In vivo proof of concept in a rabbit eye model. Int. J. Pharm. 2021, 606, 120832. [Google Scholar] [CrossRef]
- Jirsák, O.; Sanetrník, F.; Lukáš, D.; Kotek, V.; Martinová, L.; Chaloupek, J. A Method of Nanofibres Production from a Polymer Solution Using Electrostatic Spinning and a Device for Carrying out the Method. U.S. Patent No. 7,585,437, 28 December 2006. [Google Scholar]
- Zeng, J.; Yang, L.; Liang, Q.; Zhang, X.; Guan, H.; Xu, X.; Chen, X.; Jing, X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J. Control. Release 2005, 105, 43–51. [Google Scholar] [CrossRef]
- Laurie, N.A.; Gray, J.K.; Zhang, J.; Leggas, M.; Relling, M.; Egorin, M.; Stewart, C.; Dyer, M.A. Topotecan combination chemotherapy in two new rodent models of retinoblastoma. Clin. Cancer Res. 2005, 11, 7569–7578. [Google Scholar] [CrossRef] [PubMed]
- Winter, U.; Mena, H.A.; Negrotto, S.; Arana, E.; Pascual-Pasto, G.; Laurent, V.; Suñol, M.; Chantada, G.L.; Carcaboso, A.M.; Schaiquevich, P. Schedule-dependent antiangiogenic and cytotoxic effects of chemotherapy on vascular endothelial and retinoblastoma cells. PLoS ONE 2016, 11, e0160094. [Google Scholar] [CrossRef] [PubMed]
- Winter, U.; Aschero, R.; Fuentes, F.; Buontempo, F.; Zugbi, S.; Sgroi, M.; Sampor, C.; Abramson, D.; Carcaboso, A.; Schaiquevich, P. Tridimensional Retinoblastoma Cultures as Vitreous Seeds Models for Live-Cell Imaging of Chemotherapy Penetration. Int. J. Mol. Sci. 2019, 20, 1077. [Google Scholar] [CrossRef] [PubMed]
- Rosing, H.; Doyle, E.; Davies, B.E.; Beijnen, J.H. High-performance liquid chromatographic determination of the novel antitumour drug topotecan and topotecan as the total of the lactone plus carboxylate forms, in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 1995, 668, 107–115. [Google Scholar] [CrossRef]
- Kodetova, M.; Hobzova, R.; Sirc, J.; Uhlik, J.; Dunovska, K.; Svojgr, K.; Cocarta, A.; Felsoova, A.; Slanar, O.; Sima, M.; et al. The Role of Cryotherapy in Vitreous Concentrations of Topotecan Delivered by Episcleral Hydrogel Implant. Pharmaceutics 2022, 14, 903. [Google Scholar] [CrossRef]







| Sample Code | Area Weight (g/m2) | Fiber Diameter 1 (nm) | |
|---|---|---|---|
| PVA | PUR | ||
| PVA | 5 | - | 168 ± 80 |
| PVA.TPT | 5 | - | 188 ± 56 |
| PUR(15) | - | 15 | 355 ± 137 |
| PUR(5)-PVA-PUR(5) | 5 | 5 | |
| PUR(15)-PVA-PUR(15) | 5 | 15 | |
| PUR(5)-PVA.TPT-PUR(5) | 5 | 5 | |
| PUR(15)-PVA.TPT-PUR(15) | 5 | 15 | |
| Sample | Release Medium/Time (h) | Content of TPT | LE 3 (%) | Percentage of TPT Lactone Form 4 (%) | |
|---|---|---|---|---|---|
| Theoretical 1 (µg/disc) | Real 2 (µg/disc) | ||||
| PVA.TPT | Methanol/24 h | 2.51 | 2.15 ± 0.23 | 85.7 | 100 0 |
| PUR(5)-PVA.TPT-PUR(5) | 2.27 ± 0.16 | 90.4 | 97.8 | ||
| PUR(15-PVA.TPT-PUR(15) | 2.72 ± 0.41 | 108.4 | 98.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobzova, R.; Sirc, J.; Shrestha, K.; Mudrova, B.; Bosakova, Z.; Slouf, M.; Munzarova, M.; Hrabeta, J.; Feglarova, T.; Cocarta, A.-I. Multilayered Polyurethane/Poly(vinyl alcohol) Nanofibrous Mats for Local Topotecan Delivery as a Potential Retinoblastoma Treatment. Pharmaceutics 2023, 15, 1398. https://doi.org/10.3390/pharmaceutics15051398
Hobzova R, Sirc J, Shrestha K, Mudrova B, Bosakova Z, Slouf M, Munzarova M, Hrabeta J, Feglarova T, Cocarta A-I. Multilayered Polyurethane/Poly(vinyl alcohol) Nanofibrous Mats for Local Topotecan Delivery as a Potential Retinoblastoma Treatment. Pharmaceutics. 2023; 15(5):1398. https://doi.org/10.3390/pharmaceutics15051398
Chicago/Turabian StyleHobzova, Radka, Jakub Sirc, Kusum Shrestha, Barbora Mudrova, Zuzana Bosakova, Miroslav Slouf, Marcela Munzarova, Jan Hrabeta, Tereza Feglarova, and Ana-Irina Cocarta. 2023. "Multilayered Polyurethane/Poly(vinyl alcohol) Nanofibrous Mats for Local Topotecan Delivery as a Potential Retinoblastoma Treatment" Pharmaceutics 15, no. 5: 1398. https://doi.org/10.3390/pharmaceutics15051398
APA StyleHobzova, R., Sirc, J., Shrestha, K., Mudrova, B., Bosakova, Z., Slouf, M., Munzarova, M., Hrabeta, J., Feglarova, T., & Cocarta, A.-I. (2023). Multilayered Polyurethane/Poly(vinyl alcohol) Nanofibrous Mats for Local Topotecan Delivery as a Potential Retinoblastoma Treatment. Pharmaceutics, 15(5), 1398. https://doi.org/10.3390/pharmaceutics15051398