Permeation of Phytochemicals of Selected Psychoactive Medicinal Plants across Excised Sheep Respiratory and Olfactory Epithelial Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Plant Material
2.2. Preparation of Crude Extracts and Isolation of Phytochemicals
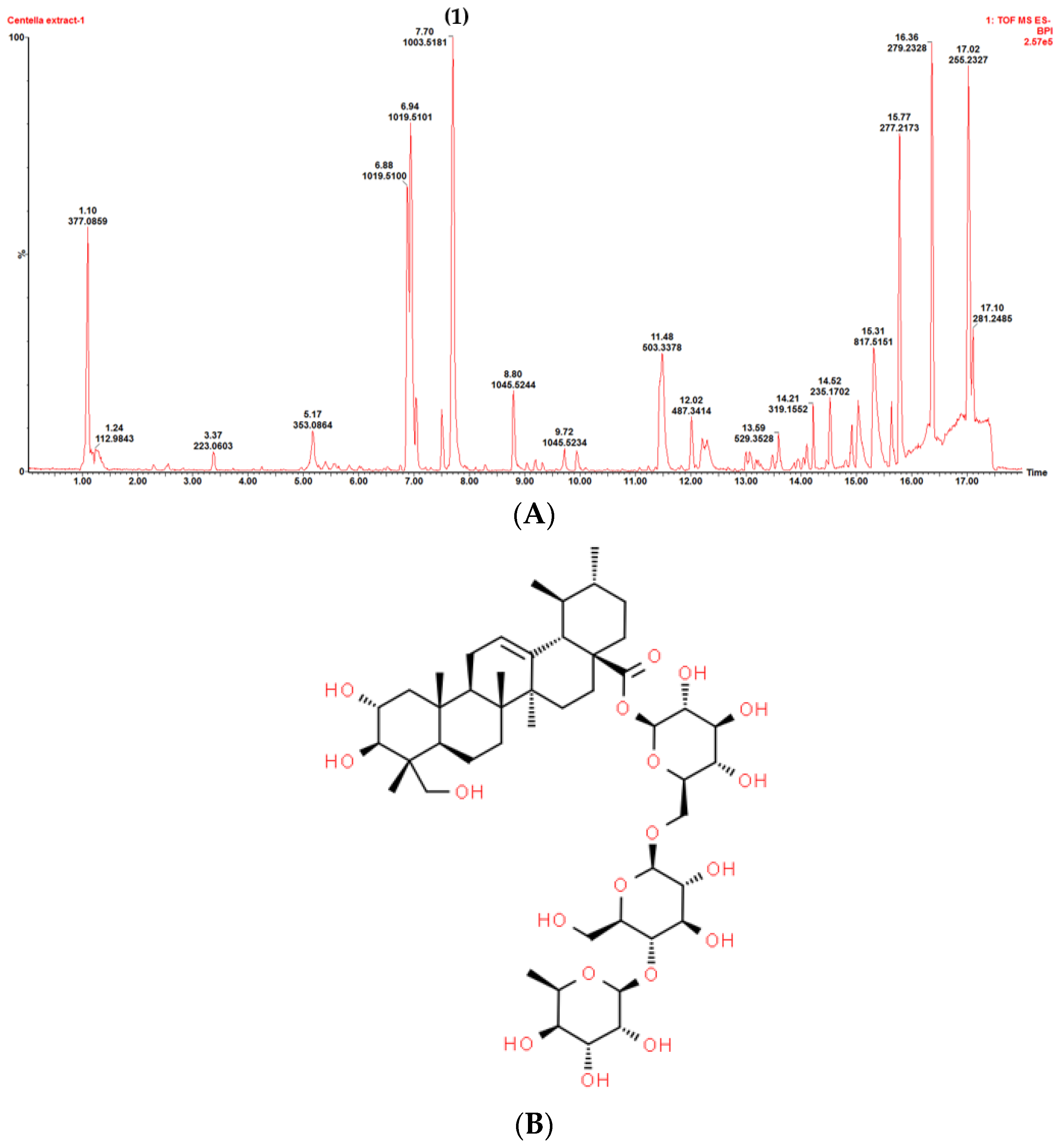
2.2.1. Centella asiatica
2.2.2. Mesembryanthemum tortuosum
2.3. Analytical Methods
2.3.1. Fluorescence Spectroscopy
2.3.2. Ultra-Performance Liquid Chromatographic Linked to Mass Spectrometry (UPLC-MS) for Analysis of Phytocomponents (Alone and in the Crude Extract)
2.4. Tissue Preparation for Permeation Studies
2.5. Assessment of Excised Nasal Tissue Integrity by Permeation of an Exclusion Marker (Lucifer Yellow)
2.6. Ex Vivo Permeation Studies with Plant Crude Extracts and Isolated Phytocompounds
2.7. Histological Examination of Excised Sheep Nasal Epithelial Tissues
2.8. Data Processing and Analysis
3. Results
3.1. Validation of Analytical Methods
3.2. Plant Crude Extract Characterization
3.3. Assessment of Excised Nasal Tissue Integrity by Permeation of an Exclusion Marker (Lucifer Yellow)
3.4. Ex Vivo Permeation Studies with Plant Crude Extracts and Isolated Phytocompounds
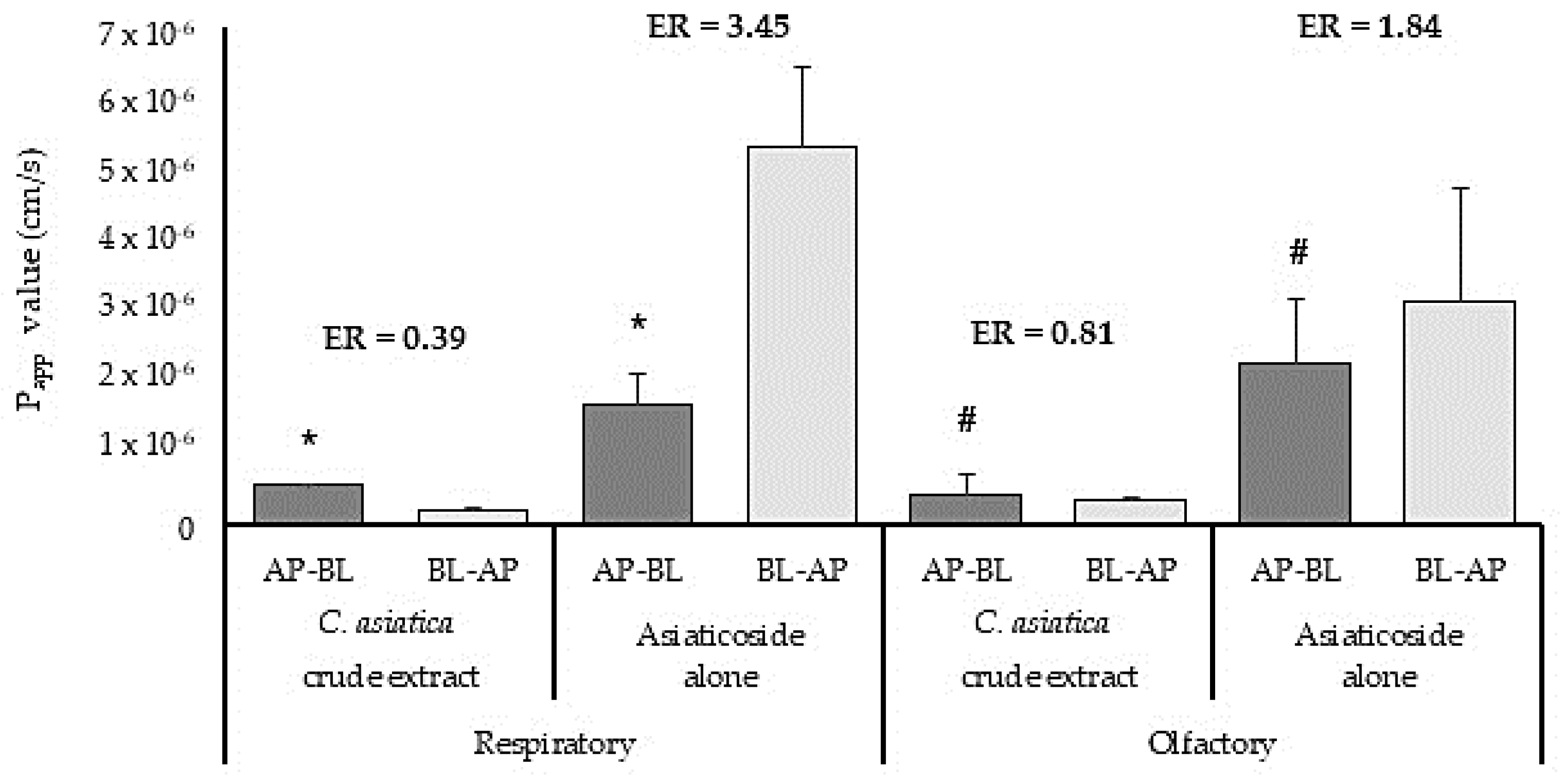
3.4.1. Ex Vivo Permeation Studies with Centella asiatica Crude Extract and Isolated Phytocompound, Asiaticoside
3.4.2. Ex Vivo Permeation Studies with Mesembryanthemum tortuosum Crude Extract and Isolated Phytocompound, Mesembrine
3.5. Histological Examination of Excised Sheep Nasal Epithelial Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartos, C.; Szabó-Révész, P.; Horváth, T.; Varga, P.; Ambrus, R. Comparison of Modern in Vitro Permeability Methods with the Aim of Investigation Nasal Dosage Forms. Pharmaceutics 2021, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Alves, G.; Fonseca, C.; Carona, A.; Bicker, J.; Falcão, A.; Fortuna, A. Is Intranasal Administration an Opportunity for Direct Brain Delivery of Lacosamide? Eur. J. Pharm. Sci. 2021, 157, 105632. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Fortuna, A.; Alves, G.; Falcão, A. Nose-to-Brain Delivery of Natural Compounds for the Treatment of Central Nervous System Disorders. Curr. Pharm. Des. 2020, 26, 594–619. [Google Scholar] [CrossRef] [PubMed]
- Maphanga, V.B.; Skalicka-Woźniak, K.; Budzynska, B.; Enslin, G.M.; Viljoen, A.M. Screening Selected Medicinal Plants for Potential Anxiolytic Activity Using an in Vivo Zebrafish Model. Psychopharmacology 2020, 237, 3641–3652. [Google Scholar] [CrossRef]
- Fennell, C.W.; Lindsey, K.L.; McGaw, L.J.; Sparg, S.G.; Stafford, G.I.; Elgorashi, E.E.; Grace, O.M.; Van Staden, J. Assessing African Medicinal Plants for Efficacy and Safety: Pharmacological Screening and Toxicology. J. Ethnopharmacol. 2004, 94, 205–217. [Google Scholar] [CrossRef]
- Stafford, G.I.; Pedersen, M.E.; van Staden, J.; Jäger, A.K. Review on Plants with CNS-Effects Used in Traditional South African Medicine against Mental Diseases. J. Ethnopharmacol. 2008, 119, 513–537. [Google Scholar] [CrossRef]
- Sobiecki, J.F. A Preliminary Inventory of Plants Used for Psychoactive Purposes in Southern African Healing Traditions. Trans. R. Soc. South Afr. 2002, 57, 1–24. [Google Scholar] [CrossRef]
- Wanasuntronwong, A.; Tantisira, M.H.; Tantisira, B.; Watanabe, H. Anxiolytic Effects of Standardized Extract of Centella asiatica (ECa 233) after Chronic Immobilization Stress in Mice. J. Ethnopharmacol. 2012, 143, 579–585. [Google Scholar] [CrossRef]
- Puttarak, P.; Dilokthornsakul, P.; Saokaew, S.; Dhippayom, T.; Kongkaew, C.; Sruamsiri, R.; Chuthaputti, A.; Chaiyakunapruk, N. Effects of Centella asiatica (L.) Urb. on Cognitive Function and Mood Related Outcomes: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 10646. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Y.N.; Chen, S.W.; Wang, W.J.; Xu, N.; Cui, S.; Liu, X.H.; Zhang, H.; Liu, Y.N.; Liu, S.; et al. Antidepressant-like Effect of Asiaticoside in Mice. Pharm. Biochem. Behav. 2008, 89, 444–449. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.L.; Mu, R.H.; Wu, Y.J.; Liu, B.B.; Geng, D.; Liu, Q.; Yi, L.T. Hippocampal BDNF Signaling Restored with Chronic Asiaticoside Treatment in Depression-like Mice. Brain Res. Bull. 2015, 114, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, T.; Guo, Y.; Xu, Y. Asiaticoside Produces an Antidepressant-like Effect in a Chronic Unpredictable Mild Stress Model of Depression in Mice, Involving Reversion of Inflammation and the PKA/PCREB/BDNF Signaling Pathway. Mol. Med. Rep. 2020, 22, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Nurlaily, A.; Noor Baitee, A.; Musalmah, M. Comparative Antioxidant and Anti-Inflammatory Activity of Different Extracts of Centella asiatica (L.) Urban and Its Active Compounds, Asiaticoside and Madecassoside. Med. Health 2012, 7, 62–72. [Google Scholar]
- Wanasuntronwong, A.; Wanakhachornkrai, O.; Phongphanphanee, P.; Isa, T.; Tantisira, B.; Tantisira, M.H. Modulation of Neuronal Activity on Intercalated Neurons of Amygdala Might Underlie Anxiolytic Activity of a Standardized Extract of Centella asiatica ECa233. Evid. Based Complement. Altern. Med. 2018, 2018, 3853147. [Google Scholar] [CrossRef] [PubMed]
- Subaraja, M.; Vanisree, A.J. The Novel Phytocomponent Asiaticoside-D Isolated from Centella asiatica Exhibits Monoamine Oxidase-B Inhibiting Potential in the Rotenone Degenerated Cerebral Ganglions of Lumbricus Terrestris. Phytomedicine 2019, 58, 152833. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Wang, W.J.; Li, W.J.; Wang, R.; Li, Y.L.; Huang, Y.N.; Liang, X. Anxiolytic-like Effect of Asiaticoside in Mice. Pharm. Biochem. Behav. 2006, 85, 339–344. [Google Scholar] [CrossRef]
- Gericke, N.; Viljoen, A.M. Sceletium-A Review Update. J. Ethnopharmacol. 2008, 119, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Young, L.C.; Viljoen, A.M.; Gericke, N.P. Pharmacological Actions of the South African Medicinal and Functional Food Plant Sceletium tortuosum and Its Principal Alkaloids. J. Ethnopharmacol. 2011, 137, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.M.; Jourdan, M.K.; Fountain, E.M.; Ali, Z.; Abe, N.; Khan, I.A.; Sufka, K.J. The Effects of Sceletium tortuosum (L.) N.E. Br. Extract Fraction in the Chick Anxiety-Depression Model. J. Ethnopharmacol. 2016, 193, 329–332. [Google Scholar] [CrossRef]
- Coetzee, D.D.; López, V.; Smith, C. High-Mesembrine Sceletium Extract (TrimesemineTM) Is a Monoamine Releasing Agent, Rather than Only a Selective Serotonin Reuptake Inhibitor. J. Ethnopharmacol. 2016, 177, 111–116. [Google Scholar] [CrossRef]
- Loria, M.J.; Ali, Z.; Abe, N.; Sufka, K.J.; Khan, I.A. Effects of Sceletium tortuosum in Rats. J. Ethnopharmacol. 2014, 155, 731–735. [Google Scholar] [CrossRef]
- Terburg, D.; Syal, S.; Rosenberger, L.A.; Heany, S.; Phillips, N.; Gericke, N.; Stein, D.J.; Van Honk, J. Acute Effects of Sceletium tortuosum (Zembrin), a Dual 5-HT Reuptake and PDE4 Inhibitor, in the Human Amygdala and Its Connection to the Hypothalamus. Neuropsychopharmacology 2013, 38, 2708–2716. [Google Scholar] [CrossRef]
- Nell, H.; Siebert, M.; Chellan, P.; Gericke, N. A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of Extract Sceletium tortuosum (Zembrin) in Healthy Adults. J. Altern. Complement. Med. 2013, 19, 898–904. [Google Scholar] [CrossRef]
- Murbach, T.S.; Hirka, G.; Szakonyiné, I.P.; Gericke, N.; Endres, J.R. A Toxicological Safety Assessment of a Standardized Extract of Sceletium tortuosum (Zembrin®) in Rats. Food Chem. Toxicol. 2014, 74, 190–199. [Google Scholar] [CrossRef]
- Maphanga, V.B.; Skalicka-Wozniak, K.; Budzynska, B.; Skiba, A.; Chen, W.; Agoni, C.; Enslin, G.M.; Viljoen, A.M. Mesembryanthemum tortuosum, L. Alkaloids Modify Anxiety-like Behaviour in a Zebrafish Model. J. Ethnopharmacol. 2022, 290, 115068. [Google Scholar] [CrossRef]
- Gericke, J.; Lekhooa, M.; Steyn, S.F.; Viljoen, A.M.; Harvey, B.H. An Acute Dose-Ranging Evaluation of the Antidepressant Properties of Sceletium tortuosum (Zembrin®) versus Escitalopram in the Flinders Sensitive Line Rat. J. Ethnopharmacol. 2022, 284, 114550. [Google Scholar] [CrossRef]
- Swart, A.C.; Smith, C. Modulation of Glucocorticoid, Mineralocorticoid and Androgen Production in H295 Cells by TrimesemineTM, a Mesembrine-Rich Sceletium Extract. J. Ethnopharmacol. 2016, 177, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Smith, C. The Effects of Sceletium tortuosum in an in Vivo Model of Psychological Stress. J. Ethnopharmacol. 2011, 133, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Shikanga, E.A.; Hamman, J.H.; Chen, W.; Combrinck, S.; Gericke, N.; Viljoen, A.M. In Vitro Permeation of Mesembrine Alkaloids from Sceletium tortuosum across Porcine Buccal, Sublingual, and Intestinal Mucosa. Planta Med. 2012, 78, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Is Nose-to-Brain Transport of Drugs in Man a Reality? J. Pharm. Pharmacol. 2004, 56, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hu, K.; Jiang, X. From Nose to Brain: Understanding Transport Capacity and Transport Rate of Drugs. Expert Opin. Drug Deliv. 2008, 5, 1159–1168. [Google Scholar] [CrossRef]
- Guennoun, R.; Fréchou, M.; Gaignard, P.; Liere, P.; Slama, A.; Schumacher, M.; Denier, C.; Mattern, C. Intranasal Administration of Progesterone: A Potential Efficient Route of Delivery for Cerebroprotection after Acute Brain Injuries. Neuropharmacology 2019, 145, 283–291. [Google Scholar] [CrossRef]
- Sosnik, A. Tissue-Based in Vitro and Ex Vivo Models for Nasal Permeability Studies. In Concepts and Models for Drug Permeability Studies; Sarmento, B., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 237–254. [Google Scholar]
- Shikanga, E.A.; Viljoen, A.; Combrinck, S.; Marston, A. Isolation of Sceletium Alkaloids by High-Speed Countercurrent Chromatography. Phytochem. Lett. 2011, 4, 190–193. [Google Scholar] [CrossRef]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of Permeability-Related Hurdles in Oral Delivery of Curcumin Using the Caco-2 Cell Model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 7 March 2022).
- ICH. Validation of Analytical Procedures Q2(R2). Available online: https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf (accessed on 28 July 2022).
- Zhang, H.; Zhuo, H.; Zhang, F. The Treatment Efficacy of Asiaticoside in Brain Glioma. Int. J. Clin. Exp. Med. 2016, 9, 20983–20989. [Google Scholar]
- Karasulu, E.; Yavaşoǧlu, A.; Evrenşanal, Z.; Uyanikgil, Y.; Karasulu, H.Y. Permeation Studies and Histological Examination of Sheep Nasal Mucosa Following Administration of Different Nasal Formulations with or without Absorption Enhancers. Drug Deliv. 2008, 15, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gerber, W.; Steyn, D.; Kotzé, A.; Svitina, H.; Weldon, C.; Hamman, J. Capsaicin and Piperine as Functional Excipients for Improved Drug Delivery across Nasal Epithelial Models. Planta Med. 2019, 85, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Gao, Y.; Nie, S.; Pan, W. The Permeation of Nalmefene Hydrochloride across Different Regions of Ovine Nasal Mucosa. Chem. Pharm. Bull 2006, 54, 1722–1724. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Picchini, U.; Beck, B.; Van Gelder, J.; Delbar, N.; DeGaetano, A. A General Approach to the Apparent Permeability Index. J Pharm. Pharm. 2008, 35, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, H.; Sun, F.; Sun, S.; Zhu, Z.; Chai, Y. Biopharmaceutical and Pharmacokinetic Characterization of Asiatic Acid in Centella asiatica as Determined by a Sensitive and Robust HPLC-MS Method. J. Ethnopharmacol. 2015, 163, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Gerber, W.; Svitina, H.; Steyn, D.; Peterson, B.; Kotzé, A.; Weldon, C.; Hamman, J.H. Comparison of RPMI 2650 Cell Layers and Excised Sheep Nasal Epithelial Tissues in Terms of Nasal Drug Delivery and Immunocytochemistry Properties. J. Pharm. Toxicol. Methods 2022, 113, 107131. [Google Scholar] [CrossRef]
- Hengjumrut, P.; Anukunwithaya, T.; Tantisira, M.H.; Tantisira, B.; Khemawoot, P. Comparative PK between Madecassoside + Asiaticoside Presented in C Asiatica Extract + Pure Compound given Seperately in Rats. Xenobiotica 2018, 48, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Haasbroek-Pheiffer, A.; Viljoen, A.; Steenekamp, J.; Chen, W.; Hamman, J. An Ex Vivo Investigation on Drug Permeability of Sheep Nasal Epithelial Tissue Membranes from the Respiratory and Olfactory Regions. Curr. Drug Deliv. 2022; ahead of print. [Google Scholar] [CrossRef]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.H.; Zhao, J.; Zhang, W.D.; Ma, B.L. Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Gizurarson, S. Anatomical and Histological Factors Affecting Intranasal Drug and Vaccine Delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Bobade, V.; Bodhankar, S.L.; Aswar, U.; Mohan, V.; Thakurdesai, P. Prophylactic Effects of Asiaticoside-Based Standardized Extract of Centella asiatica (L.) Urban Leaves on Experimental Migraine: Involvement of 5HT1A/1B Receptors. Chin. J. Nat. Med. 2015, 13, 274–0282. [Google Scholar] [CrossRef]




| Analyte | R2 | Regression Equation | LOD (µg/mL) | LOQ (µg/mL) | Accuracy | Precision (%RSD) | |
|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||||
| ▪ Asiaticoside | 0.9959 | Y = 262X + 52 | 0.13 | 0.40 | 96.7% | 1.81% | 4.74% |
| ▪ Lucifer yellow | 0.998 | Y = 229,4576,058X | 0.012 | 0.036 | 99.2% | 1.93% | 1.42% |
| ▪ Mesembrenol | 0.993 | Y = 2970X + 510 | 0.32 | 0.98 | 101.8% | 0.96% | 2.84% |
| ▪ Mesembranol | 0.989 | Y = 4108X + 1067 | 0.23 | 0.71 | 98.7% | 0.87% | 1.59% |
| ▪ Mesembrenone | 0.995 | Y = 2760X − 214 | 0.36 | 1.09 | 100.3% | 0.44% | 2.05% |
| ▪ Mesembrine | 0.993 | Y = 4270X − 52 | 0.24 | 0.73 | 98.9% | 1.40% | 3.27% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haasbroek-Pheiffer, A.; Viljoen, A.; Steenekamp, J.; Chen, W.; Hamman, J. Permeation of Phytochemicals of Selected Psychoactive Medicinal Plants across Excised Sheep Respiratory and Olfactory Epithelial Tissues. Pharmaceutics 2023, 15, 1423. https://doi.org/10.3390/pharmaceutics15051423
Haasbroek-Pheiffer A, Viljoen A, Steenekamp J, Chen W, Hamman J. Permeation of Phytochemicals of Selected Psychoactive Medicinal Plants across Excised Sheep Respiratory and Olfactory Epithelial Tissues. Pharmaceutics. 2023; 15(5):1423. https://doi.org/10.3390/pharmaceutics15051423
Chicago/Turabian StyleHaasbroek-Pheiffer, Anja, Alvaro Viljoen, Jan Steenekamp, Weiyang Chen, and Josias Hamman. 2023. "Permeation of Phytochemicals of Selected Psychoactive Medicinal Plants across Excised Sheep Respiratory and Olfactory Epithelial Tissues" Pharmaceutics 15, no. 5: 1423. https://doi.org/10.3390/pharmaceutics15051423
APA StyleHaasbroek-Pheiffer, A., Viljoen, A., Steenekamp, J., Chen, W., & Hamman, J. (2023). Permeation of Phytochemicals of Selected Psychoactive Medicinal Plants across Excised Sheep Respiratory and Olfactory Epithelial Tissues. Pharmaceutics, 15(5), 1423. https://doi.org/10.3390/pharmaceutics15051423








