Lamivudine and Emtricitabine Dosing Proposal for Children with HIV and Chronic Kidney Disease, Supported by Physiologically Based Pharmacokinetic Modelling
Abstract
1. Introduction
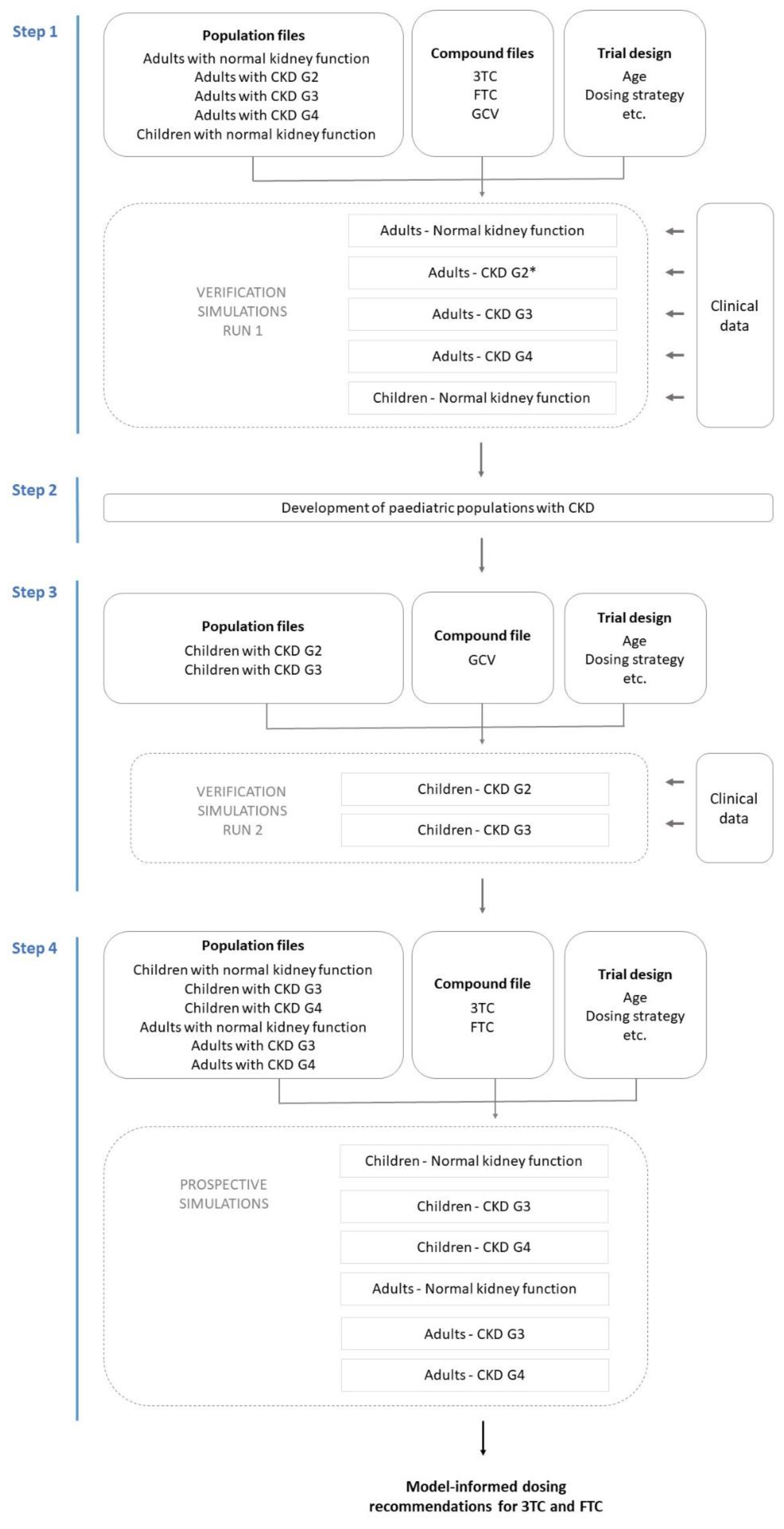
2. Materials and Methods
2.1. PBPK Model Verification Simulations—Run 1
2.1.1. Adult Population with Normal Kidney Function
2.1.2. Adult Populations with Various Degrees of CKD
2.1.3. Paediatric Population with Normal Kidney Function
2.2. Development of Paediatric CKD Population Models
2.3. PBPK Model Verification Simulations—Run 2
2.4. Prospective Simulation of 3TC and FTC PK in Paediatric CKD Populations
3. Results
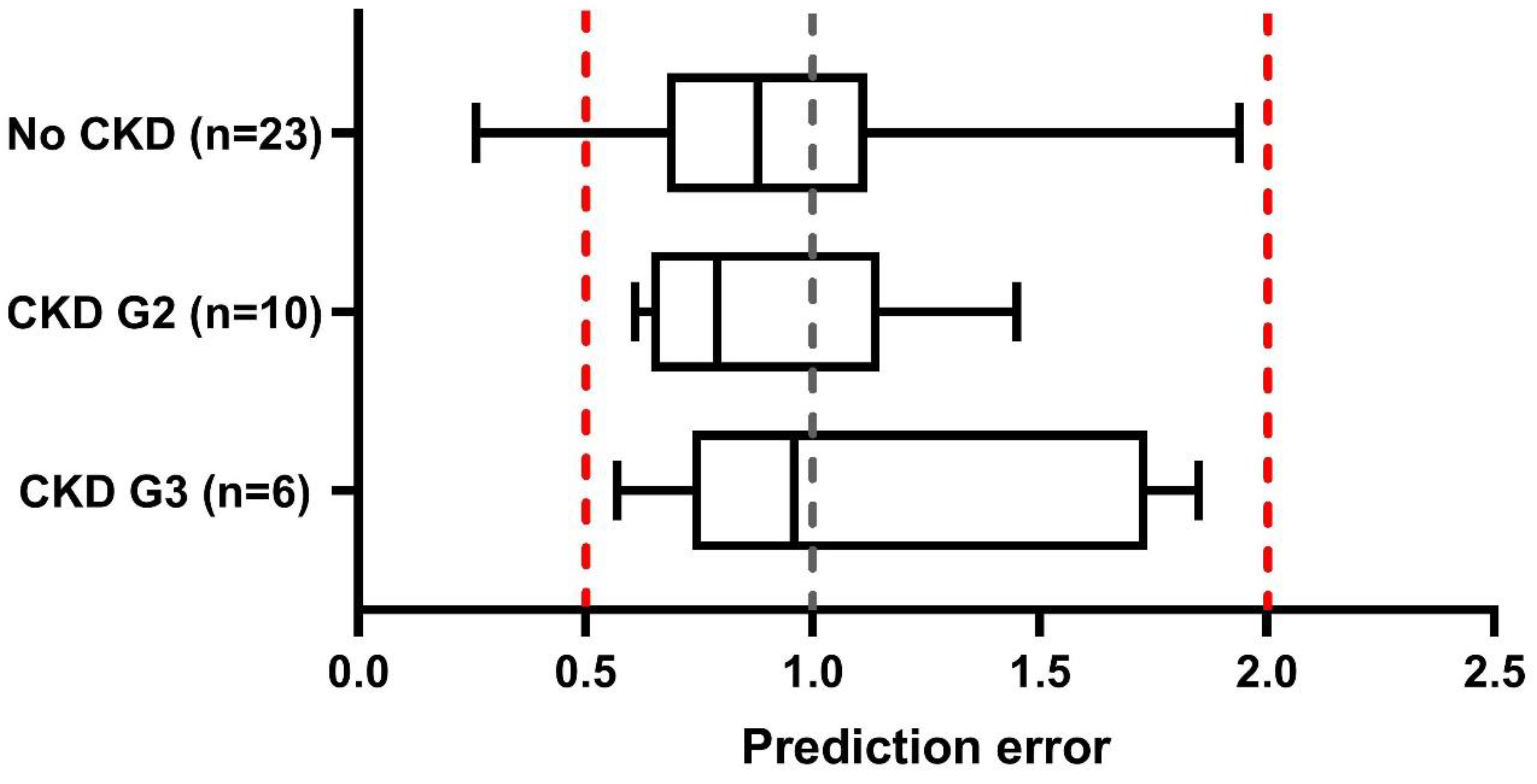
3.1. PBPK Model Verification Simulations—Run 1
3.2. Verification of Paediatric CKD Populations—Run 2
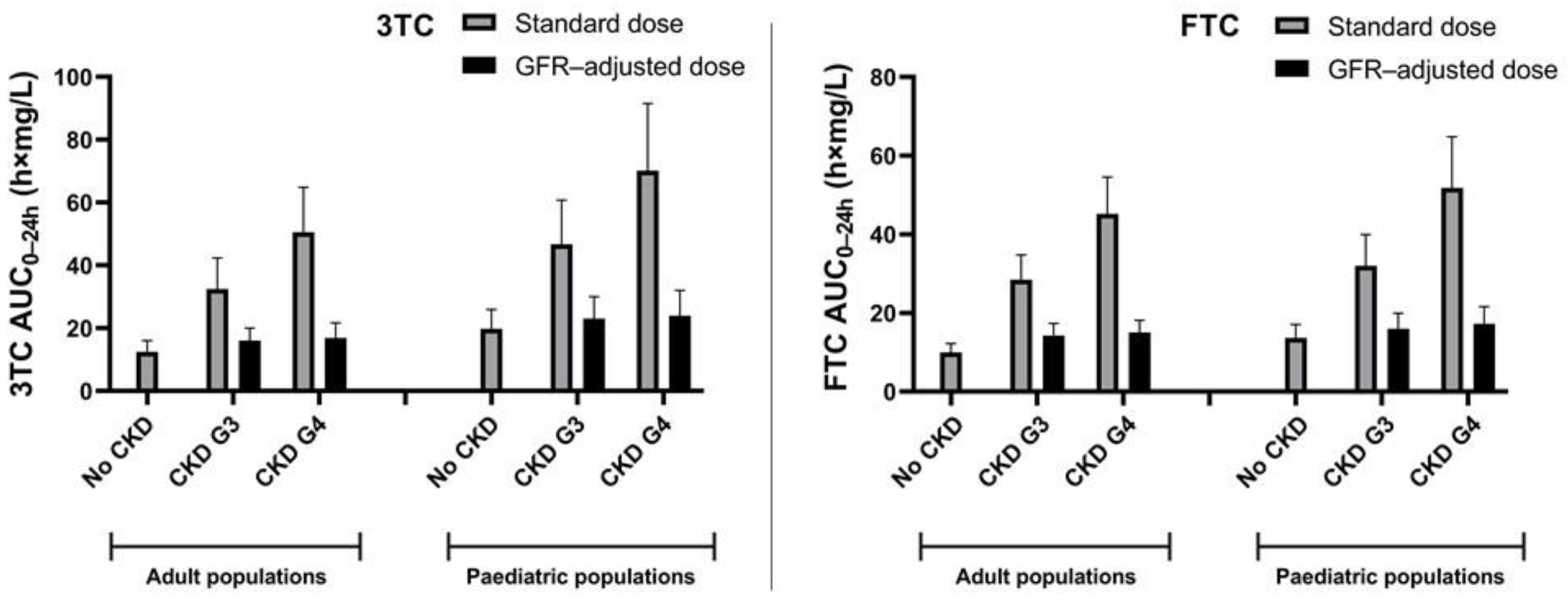
3.3. Prospective Simulations of 3TC and FTC PK in Paediatric CKD Populations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. UNAIDS Data. 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (accessed on 9 August 2022).
- UNAIDS; WHO. Summary of the Global HIV Epidemic, 2020; UNAIDS: Geneva, Switzerland; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Bryant, J.L.; Guda, P.R.; Asemu, G.; Subedi, R.; Ray, S.; Khalid, O.S.; Shukla, V.; Patel, D.; Davis, H.; Nimmagadda, V.K.C.; et al. Glomerular mitochondrial changes in HIV associated renal injury. Exp. Mol. Pathol. 2018, 104, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Fredrick, F.; Francis, J.M.; Ruggajo, P.J.; Maro, E.E. Renal abnormalities among HIV infected children at Muhimbili National Hospital (MNH)-Dar es Salaam, Tanzania. BMC Nephrol. 2016, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Maggi, P.; Bartolozzi, D.; Bonfanti, P.; Calza, L.; Cherubini, C.; Di Biagio, A.; Marcotullio, S.; Montella, F.; Montinaro, V.; Mussini, C.; et al. Renal complications in HIV disease: Between present and future. AIDS Rev. 2012, 14, 37–53. [Google Scholar]
- Jindal, A.K.; Tiewsoh, K.; Pilania, R.K. A review of renal disease in children with HIV infection. Infect. Dis. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for kidney function and disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef]
- Ray, P.E.; Rakusan, T.; Loechelt, B.J.; Selby, D.M.; Liu, X.H.; Chandra, R.S. Human immunodeficiency virus (HIV)-associated nephropathy in children from the Washington, DC area: 12 years’ experience. InSeminars Nephrol. 1998, 18, 396–405. [Google Scholar]
- Beng, H.; Rakhmanina, N.; Moudgil, A.; Tuchman, S.; Ahn, S.Y.; Griffith, C.; Mims, M.M.; Ray, P.E. HIV-Associated CKDs in Children and Adolescents. Kidney Int. Rep. 2020, 5, 2292–2300. [Google Scholar] [CrossRef]
- UNAIDS Data 2020; UNAIDS: Geneva, Switzerland, 2020.
- New Report Reveals Stark Inequalities in Access to HIV Prevention and Treatment Services for Children—Partners Call for Urgent Action. Available online: https://www.unaids.org/en/keywords/children (accessed on 4 December 2022).
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. 2021. Available online: https://www.who.int/publications/i/item/9789240031593 (accessed on 2 December 2022).
- The 2021 Optimal Formulary and Limited-Use List for Antiretroviral Drugs for Children; World Health Organization: Geneva, Switzerland, 2021; Available online: http://www.who.int/hiv/pub/research-dev-toolkit-paediatric-arv-drug-formulation/en/ (accessed on 12 March 2022).
- Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/PedARV_GL.pdf (accessed on 2 December 2022).
- Muller, F.; Konig, J.; Hoier, E.; Mandery, K.; Fromm, M.F. Role of organic cation transporter OCT2 and multidrug and toxin extrusion proteins MATE1 and MATE2-K for transport and drug interactions of the antiviral lamivudine. Biochem. Pharmacol. 2013, 86, 808–815. [Google Scholar] [CrossRef]
- Emtriva. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021500s010,021896s004lbl.pdf (accessed on 22 January 2022).
- Healthcare, V. 131-Pristine-3TC-pm. Available online: https://viivhealthcare.com/content/dam/cf-viiv/viiv-healthcare/en_CA/pdf/3tc.pdf (accessed on 22 January 2022).
- Pradhan, S.; Duffull, S.B.; Walker, R.J.; Wright, D.F.B. The intact nephron hypothesis as a model for renal drug handling. Eur. J. Clin. Pharmacol. 2019, 75, 147–156. [Google Scholar] [CrossRef]
- Johnson, M.A.; Verpooten, G.A.; Daniel, M.J.; Plumb, R.; Moss, J.; Van Caesbroeck, D.; De Broe, M.E. Single dose pharmacokinetics of lamivudine in subjects with impaired renal function and the effect of haemodialysis. Br. J. Clin. Pharmacol. 1998, 46, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Guidelines Version 11.1. Available online: https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf (accessed on 3 January 2023).
- Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/antiretroviral-dosing-recommendations-patients-renal-or-hepatic (accessed on 22 July 2022).
- Schijvens, A.M.; de Wildt, S.N.; Schreuder, M.F. Pharmacokinetics in children with chronic kidney disease. Pediatr. Nephrol. 2020, 35, 1153–1172. [Google Scholar] [CrossRef]
- Jamei, M.; Marciniak, S.; Feng, K.; Barnett, A.; Tucker, G.; Rostami-Hodjegan, A. The Simcyp population-based ADME simulator. Expert Opin. Drug Metab. Toxicol. 2009, 5, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Fischetti, B.; Cha, A.; Taft, D.R. Using PBPK Modeling to Predict Drug Exposure and Support Dosage Adjustments in Patients with Renal Impairment: An Example with Lamivudine. Curr. Drug Discov. Technol. 2020, 17, 387–396. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Mendes, M.; Hirt, D.; Urien, S.; Valade, E.; Bouazza, N.; Foissac, F.; Blanche, S.; Treluyer, J.M.; Benaboud, S. Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women. Br. J. Clin. Pharmacol. 2015, 80, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.H.; Hsu, V.; Zhao, P.; Zhang, L.; Giacomini, K.M.; Huang, S.M. PBPK Modeling of the Effect of Reduced Kidney Function on the Pharmacokinetics of Drugs Excreted Renally by Organic Anion Transporters. Clin. Pharmacol. Ther. 2018, 103, 485–492. [Google Scholar] [CrossRef]
- Hsu, V.; Vieira, M.D.L.T.; Zhao, P.; Zhang, L.; Zheng, J.H.; Nordmark, A.; Berglund, E.G.; Giacomini, K.M.; Huang, S.M. Towards quantitation of the effects of renal impairment and probenecid inhibition on kidney uptake and efflux transporters, using physiologically based pharmacokinetic modelling and simulations. Clin. Pharmacokinet. 2014, 53, 283–293. [Google Scholar] [CrossRef]
- Heald, A.E.; Hsyu, P.H.; Yuen, G.J.; Robinson, P.; Mydlow, P.; Bartlett, J.A. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob. Agents Chemother. 1996, 40, 1514–1519. [Google Scholar] [CrossRef]
- Wang, L.H.; Begley, J.; St Claire, R.L., 3rd; Harris, J.; Wakeford, C.; Rousseau, F.S. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res. Hum. Retrovir. 2004, 20, 1173–1182. [Google Scholar] [CrossRef]
- Czock, D.; Scholle, C.; Rasche, F.M.; Schaarschmidt, D.; Keller, F. Pharmacokinetics of valganciclovir and ganciclovir in renal impairment. Clin. Pharmacol. Ther. 2002, 72, 142–150. [Google Scholar] [CrossRef]
- Burger, D.M.; Verweel, G.; Rakhmanina, N.; Verwey-Van Wissen, C.P.; La Porte, C.J.; Bergshoeff, A.S.; Lyall, H.; Hartwig, N.G.; Green, H.; Soldin, S.; et al. Age-dependent pharmacokinetics of lamivudine in HIV-infected children. Clin. Pharmacol. Ther. 2007, 81, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.H.; Cotton, M.F.; Rodriguez, C.A.; McGrath, E.J.; Helström, E.; Liberty, A.; Natukunda, E.; Kosalaraksa, P.; Chokephaibulkit, K.; Maxwell, H.; et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide in adolescents and children with HIV: Week 48 results of a single-arm, open-label, multicentre, phase 2/3 trial. Lancet Child Adolesc. Health 2021, 5, 642–651. [Google Scholar] [CrossRef]
- Wang, L.H.; Wiznia, A.A.; Rathore, M.H.; Chittick, G.E.; Bakshi, S.S.; Emmanuel, P.J.; Flynn, P.M. Pharmacokinetics and safety of single oral doses of emtricitabine in human immunodeficiency virus-infected children. Antimicrob. Agents Chemother. 2004, 48, 183–191. [Google Scholar] [CrossRef]
- Vaudry, W.; Ettenger, R.; Jara, P.; Varela-Fascinetto, G.; Bouw, M.R.; Ives, J.; Walker, R. Valganciclovir dosing according to body surface area and renal function in pediatric solid organ transplant recipients. Am. J. Transplant. 2009, 9, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Salem, F.; Small, B.G.; Johnson, T.N. Development and application of a pediatric mechanistic kidney model. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Yi, S.; Rhee, S.J.; Lee, H.A.; Kim, Y.; Yu, K.S.; Chung, J.Y. Development of a physiologically-based pharmacokinetic model for cyclosporine in Asian children with renal impairment. Transl. Clin. Pharmacol. 2019, 27, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, R.; Chen, Y.; You, X.; Huang, P.; Lin, C. Physiologically Based Pharmacokinetic Modeling and Dose Adjustment of Teicoplanin in Pediatric Patients with Renal Impairment. J. Clin. Pharmacol. 2021, 62, 620–630. [Google Scholar] [CrossRef]
- Ke, C.; You, X.; Lin, C.; Chen, J.; Guo, G.; Wu, W.; Ye, L.; Huang, P. Development of Physiologically Based Pharmacokinetic Model for Pregabalin to Predict the Pharmacokinetics in Pediatric Patients with Renal Impairment and Adjust Dosage Regimens: PBPK Model of Pregabalin in Pediatric Patients with Renal Impairment. J. Pharm. Sci. 2021, 111, 542–551. [Google Scholar] [CrossRef]
- Zhou, J.; You, X.; Ke, M.; Ye, L.; Wu, W.; Huang, P.; Lin, C. Dosage Adjustment for Ceftazidime in Pediatric Patients with Renal Impairment Using Physiologically Based Pharmacokinetic Modeling. J. Pharm. Sci. 2021, 110, 1853–1862. [Google Scholar] [CrossRef]
- Ye, L.; Ke, M.; You, X.; Huang, P.; Lin, C. A Physiologically Based Pharmacokinetic Model of Ertapenem in Pediatric Patients with Renal Impairment. J. Pharm. Sci. 2020, 109, 2909–2918. [Google Scholar] [CrossRef]
- Zino, L.; Jacobs, T.G.; Nieuwenstein, T.; Grintjes, K.; Colbers, A.; Burger, D.M. Model-informed intermittent tenofovir disoproxil fumarate and emtricitabine dosing for HIV pre-exposure prophylaxis in subjects with renal impairment: A case report. Aids 2023, 37, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; You, X.; Zhou, J.; Wu, C.; Ke, M.; Wu, W.; Huang, P.; Lin, C. Physiologically based pharmacokinetic modeling of daptomycin dose optimization in pediatric patients with renal impairment. Front. Pharmacol. 2022, 13, 838599. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.J.F.; Upadhyay, P.J.; Hagedoorn, N.N.; Mathôt, R.A.A.; Moll, H.A.; van der Flier, M.; Schreuder, M.F.; Brüggemann, R.J.; Knibbe, C.A.; de Wildt, S.N. Current Ceftriaxone Dose Recommendations are Adequate for Most Critically Ill Children: Results of a Population Pharmacokinetic Modeling and Simulation Study. Clin. Pharmacokinet. 2021, 60, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Bazzoli, C.; Jullien, V.; Le Tiec, C.; Rey, E.; Mentré, F.; Taburet, A.M. Intracellular Pharmacokinetics of Antiretroviral Drugs in HIV-Infected Patients, and their Correlation with Drug Action. Clin. Pharmacokinet. 2010, 49, 17–45. [Google Scholar] [CrossRef]
- Pluda, J.M.; Cooley, T.P.; Montaner, J.S.; Shay, L.E.; Reinhalter, N.E.; Warthan, S.N.; Ruedy, J.; Hirst, H.M.; Vicary, C.A.; Quinn, J.B.; et al. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in patients with advanced human immunodeficiency virus infection. J. Infect. Dis. 1995, 171, 1438–1447. [Google Scholar] [CrossRef]
- Mounzer, K.; Brunet, L.; Wyatt, C.M.; Fusco, J.S.; Vannappagari, V.; Tenorio, A.R.; Shaefer, M.S.; Ragone, L.; Hsu, R.K.; Fusco, G.P. To dose-adjust or not to dose-adjust: Lamivudine dose in kidney impairment. Aids 2021, 35, 1201–1208. [Google Scholar] [CrossRef]
- Wood, B.R.; Pozniak, A.L. Dosing lamivudine or emtricitabine in renal impairment: New data confirm it’s time for updated guidance! Aids 2021, 35, 1305–1307. [Google Scholar] [CrossRef]
- Moore, K.H.; Yuen, G.J.; Raasch, R.H.; Eron, J.J.; Martin, D.; Mydlow, P.K.; Hussey, E.K. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin. Pharmacol. Ther. 1996, 59, 550–558. [Google Scholar] [CrossRef]
- Anderson, G.D. Children versus adults: Pharmacokinetic and adverse-effect differences. Epilepsia 2002, 43 (Suppl. S3), 53–59. [Google Scholar] [CrossRef]
- Jacobs, T.G.; Schouwenburg, S.; Penazzato, M.; Archary, M.; Ruel, T.D.; van den Anker, J.; Burger, D.M.; Cressey, T.R.; Abrams, E.J.; Lyall, H.; et al. What babies need: Accelerating access to current and novel antiretroviral drugs in neonates through pharmacokinetic studies. Lancet HIV 2022, 9, e649–e657. [Google Scholar] [CrossRef]
- Verbeeck, R.K.; Musuamba, F.T. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur. J. Clin. Pharmacol. 2009, 65, 757–773. [Google Scholar] [CrossRef]
- Evers, R.; Piquette-Miller, M.; Polli, J.W.; Russel, F.G.M.; Sprowl, J.A.; Tohyama, K.; Ware, J.A.; de Wildt, S.N.; Xie, W.; Brouwer, K.L.R. Disease-Associated Changes in Drug Transporters May Impact the Pharmacokinetics and/or Toxicity of Drugs: A White Paper from the International Transporter Consortium. Clin. Pharmacol. Ther. 2018, 104, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Prasad, B.; Neuhoff, S.; Yoshida, K.; Leeder, J.S.; Mukherjee, D.; Taskar, K.; Varma, M.V.S.; Zhang, X.; Yang, X.; et al. Clinical Implications of Altered Drug Transporter Abundance/Function and PBPK Modeling in Specific Populations: An ITC Perspective. Clin. Pharmacol. Ther. 2022, 112, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Drugbank. Emtricitabine. Available online: https://go.drugbank.com/drugs/DB00879 (accessed on 14 March 2023).
- Drugbank. Lamivudine. Available online: https://go.drugbank.com/drugs/DB00709 (accessed on 14 March 2023).
- Seifert, S.M.; Castillo-Mancilla, J.R.; Erlandson, K.M.; Anderson, P.L. Inflammation and pharmacokinetics: Potential implications for HIV-infection. Expert Opin. Drug Metab. Toxicol. 2017, 13, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.G.; Kim, R.B.; Wood, A.J.; Stein, C.M. Molecular basis of ethnic differences in drug disposition and response. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 815–850. [Google Scholar] [CrossRef]

| Adult CKD Models | Paediatric CKD Models | |
|---|---|---|
| CLfilt | GFR cut-off values (i.e., 30–50 mL/min/1.73 m2) were set to reflect the GFR in the various CKD populations *. | In order to define various paediatric CKD populations, we pragmatically included scaling factors in the validated Simcyp® default GFR equation to create paediatric CKD population models with specified GFR ranges. |
| CLtub | Kidney size is adjusted for each CKD group, by reducing kidney size baseline (the equation offset), body weight and body height coefficients *. | Paediatric individual kidney size calculation is solely based on body weight. Hence, we have reduced kidney size in paediatric populations by calculating the ratio for body weight coefficients (CKD/no CKD) in adults and incorporated these ratios in the equation determining the paediatric kidney size. |
| For 3TC and GCV, drug-specific PTCPGK reductions in specific CKD populations (defined differently than in this study) have been set and verified previously [25,27]. The established correlations between GFR and PTCPGK were used to determine PTCPGK values for the adult CKD populations in this study. In the absence of quantitative data for FTC model parameterization, it was assumed that the PTCPGK for the different CKD groups was similar for FTC as for 3TC. | PTCPGK for GCV, 3TC, and FTC was reduced according to the reduction applied in the adult CKD population models. | |
| CLadd | There is no CLadd component in the GCV compound model and hence CLadd is not adjusted for CKD populations. For 3TC, CLadd reductions in specific CKD populations (defined differently than in this study) have been set and verified previously [25]. The established correlations between GFR and CLadd were used to determine CLadd values for the adult CKD populations in this study. The effect of CKD on CLadd was assumed to be similar for FTC and 3TC. Hence, a similar proportional reduction was used for FTC CLadd as compared to the 3TC CLadd reductions. | CLadd for 3TC and FTC was reduced according to the reduction applied in the adult CKD population models. A CLadd component was not included in the GCV model and hence not adjusted in case of CKD. |
| Drug and Formulation | Age of Simulated Subjects | Population and Dose (Standard Dose or GFR-Adjusted Dose) | ||
|---|---|---|---|---|
| No CKD | CKD G3 (GFR 30–50 mL/min/1.73 m2) | CKD G4 (GFR 15–30 mL/min/1.73 m2) | ||
| 3TC Dispersible tablets/oral solution | 3 mo to <2 yrs | Standard dose: 5 mg/kg/12 h | ||
| Standard dose | 2.5 mg/kg/12 h | 2.5 mg/kg LD, 1.88 mg/kg/12 h MD | ||
| 2 to <6 yrs | Standard dose: 10 mg/kg/24 h | |||
| Standard dose | 5 mg/kg/24 h | 5 mg/kg LD, 3.25 mg/kg/24 h MD | ||
| 3TC Film-coated tablets | 6 to <12 yrs | Standard dose: 300 mg/24 h | ||
| Standard dose | 150 mg/24 h | 150 mg LD, 100 mg/24 h MD | ||
| 12 to <15 yrs | Standard dose: 300 mg/24 h | |||
| Standard dose | 150 mg/24 h | 150 mg LD, 100 mg/24 h MD | ||
| ≥15 yrs | Standard dose: 300 mg/24 h | |||
| Standard dose | 150 mg/24 h | 150 mg LD, 100 mg/24 h MD | ||
| FTC Oral solution | 3 mo to <2 yrs | Standard dose: 6 mg/kg/24 h | ||
| Standard dose | 3 mg/kg/24 h # | 2 mg/kg/24 h # | ||
| 2 to <6 yrs | Standard dose: 6 mg/kg/24 h | |||
| Standard dose | 3 mg/kg/24 h # | 2 mg/kg/24 h # | ||
| FTC Capsules | 6 to <12 yrs | Standard dose: 200 mg/24 h | ||
| Standard dose | 200 mg/48 h # | 200 mg/72 h # | ||
| 12 to <15 yrs | Standard dose: 200 mg/24 h | |||
| Standard dose | 200 mg/48 h # | 200 mg/72 h # | ||
| ≥18 yrs | Standard dose: 200 mg/24 h | |||
| Standard dose | 200 mg/48 h | 200 mg/72 h | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, T.G.; de Hoop-Sommen, M.A.; Nieuwenstein, T.; van der Heijden, J.E.M.; de Wildt, S.N.; Burger, D.M.; Colbers, A.; Freriksen, J.J.M. Lamivudine and Emtricitabine Dosing Proposal for Children with HIV and Chronic Kidney Disease, Supported by Physiologically Based Pharmacokinetic Modelling. Pharmaceutics 2023, 15, 1424. https://doi.org/10.3390/pharmaceutics15051424
Jacobs TG, de Hoop-Sommen MA, Nieuwenstein T, van der Heijden JEM, de Wildt SN, Burger DM, Colbers A, Freriksen JJM. Lamivudine and Emtricitabine Dosing Proposal for Children with HIV and Chronic Kidney Disease, Supported by Physiologically Based Pharmacokinetic Modelling. Pharmaceutics. 2023; 15(5):1424. https://doi.org/10.3390/pharmaceutics15051424
Chicago/Turabian StyleJacobs, Tom G., Marika A. de Hoop-Sommen, Thomas Nieuwenstein, Joyce E. M. van der Heijden, Saskia N. de Wildt, David M. Burger, Angela Colbers, and Jolien J. M. Freriksen. 2023. "Lamivudine and Emtricitabine Dosing Proposal for Children with HIV and Chronic Kidney Disease, Supported by Physiologically Based Pharmacokinetic Modelling" Pharmaceutics 15, no. 5: 1424. https://doi.org/10.3390/pharmaceutics15051424
APA StyleJacobs, T. G., de Hoop-Sommen, M. A., Nieuwenstein, T., van der Heijden, J. E. M., de Wildt, S. N., Burger, D. M., Colbers, A., & Freriksen, J. J. M. (2023). Lamivudine and Emtricitabine Dosing Proposal for Children with HIV and Chronic Kidney Disease, Supported by Physiologically Based Pharmacokinetic Modelling. Pharmaceutics, 15(5), 1424. https://doi.org/10.3390/pharmaceutics15051424









