Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Compression Mixtures Preparation
2.2.2. Production of Tablets
2.2.3. Carvedilol Release Profiles
2.2.4. LOESS Modelling and Analysis of Dissolution Data in Excel
2.2.5. Carvedilol Release Profile Comparison Using Similarity Factor (f2)
2.2.6. Determination of Particle Size Distribution
3. Results and Discussion
3.1. Carvedilol Release Results, LOESS Modelling, and Carvedilol Release Profile Comparison
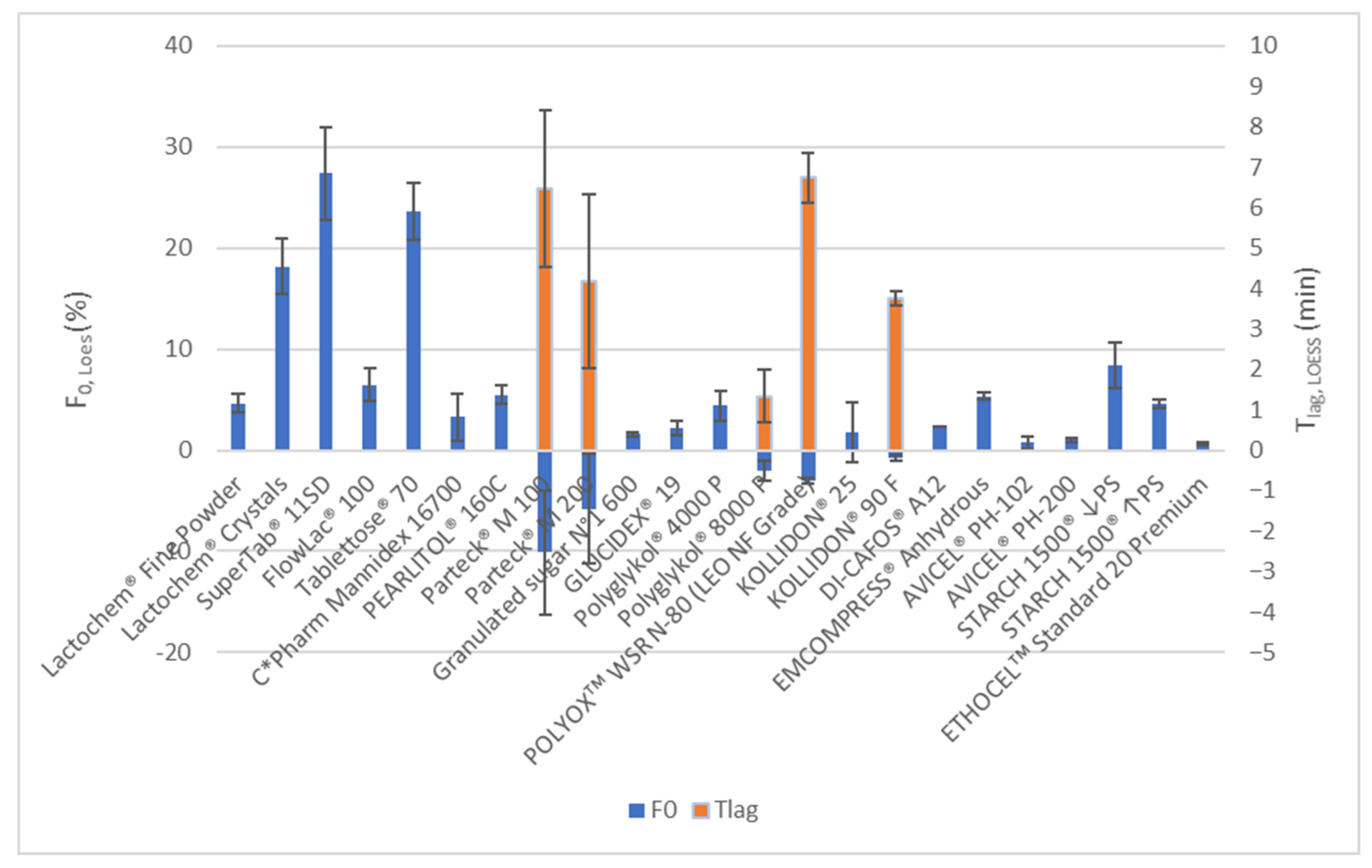
3.1.1. Overview
3.1.2. Formulations Using Soluble Selected Excipients
PEG/PEO and PVP
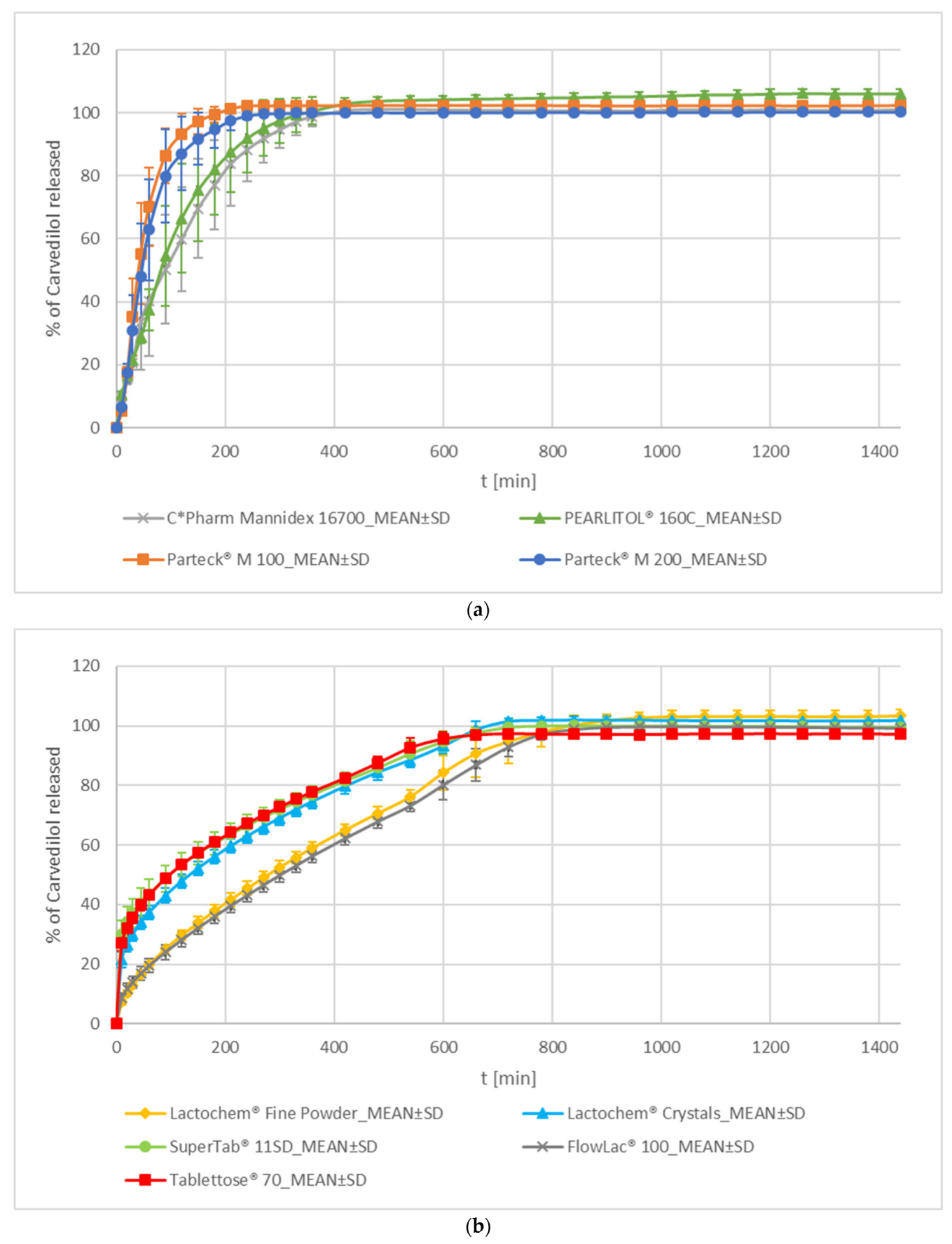
Mannitol and Lactose
- Mannitol
- 2.
- Lactose
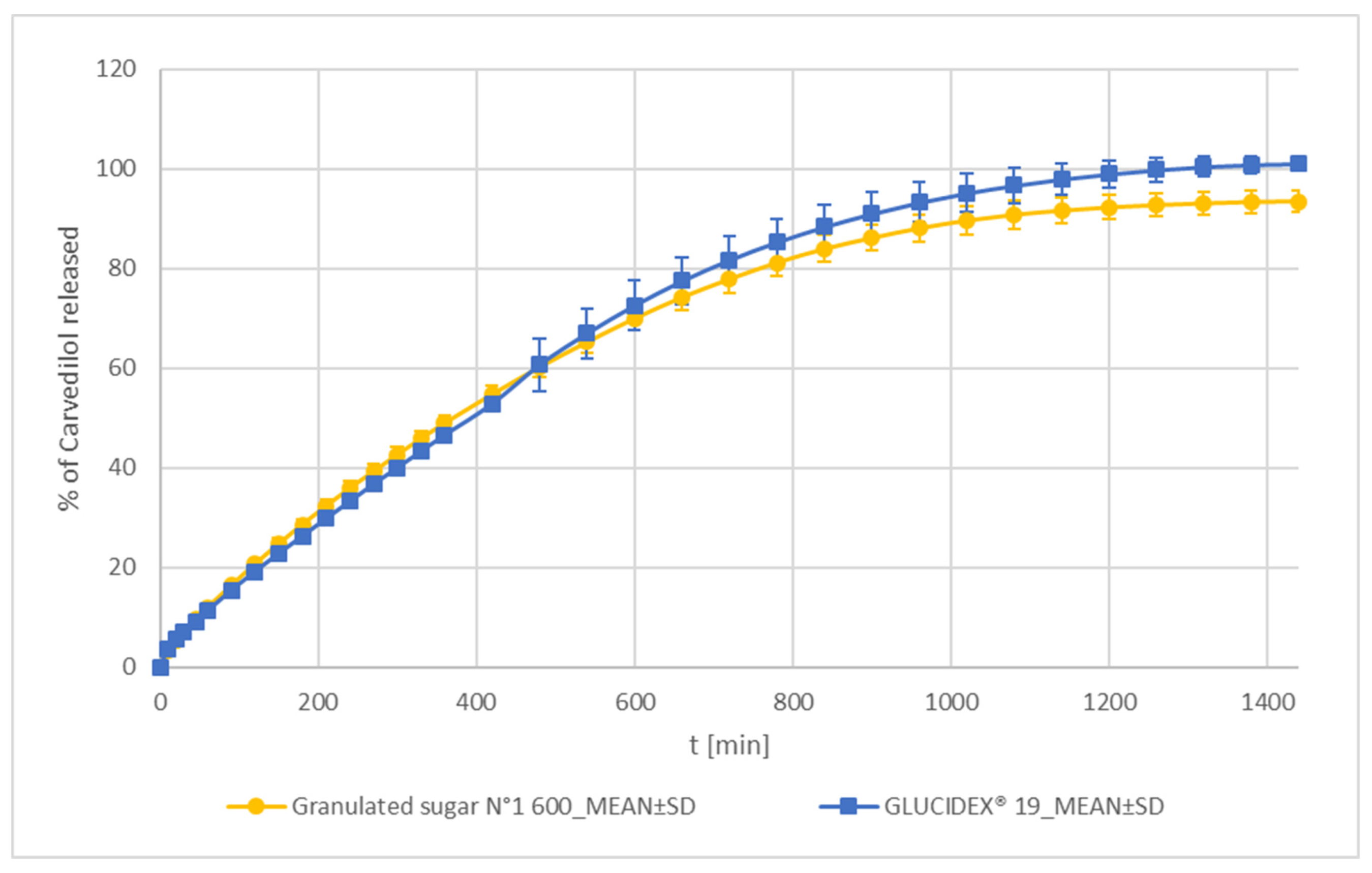
Sucrose and Maltodextrin
3.1.3. Formulations Using Insoluble Excipients
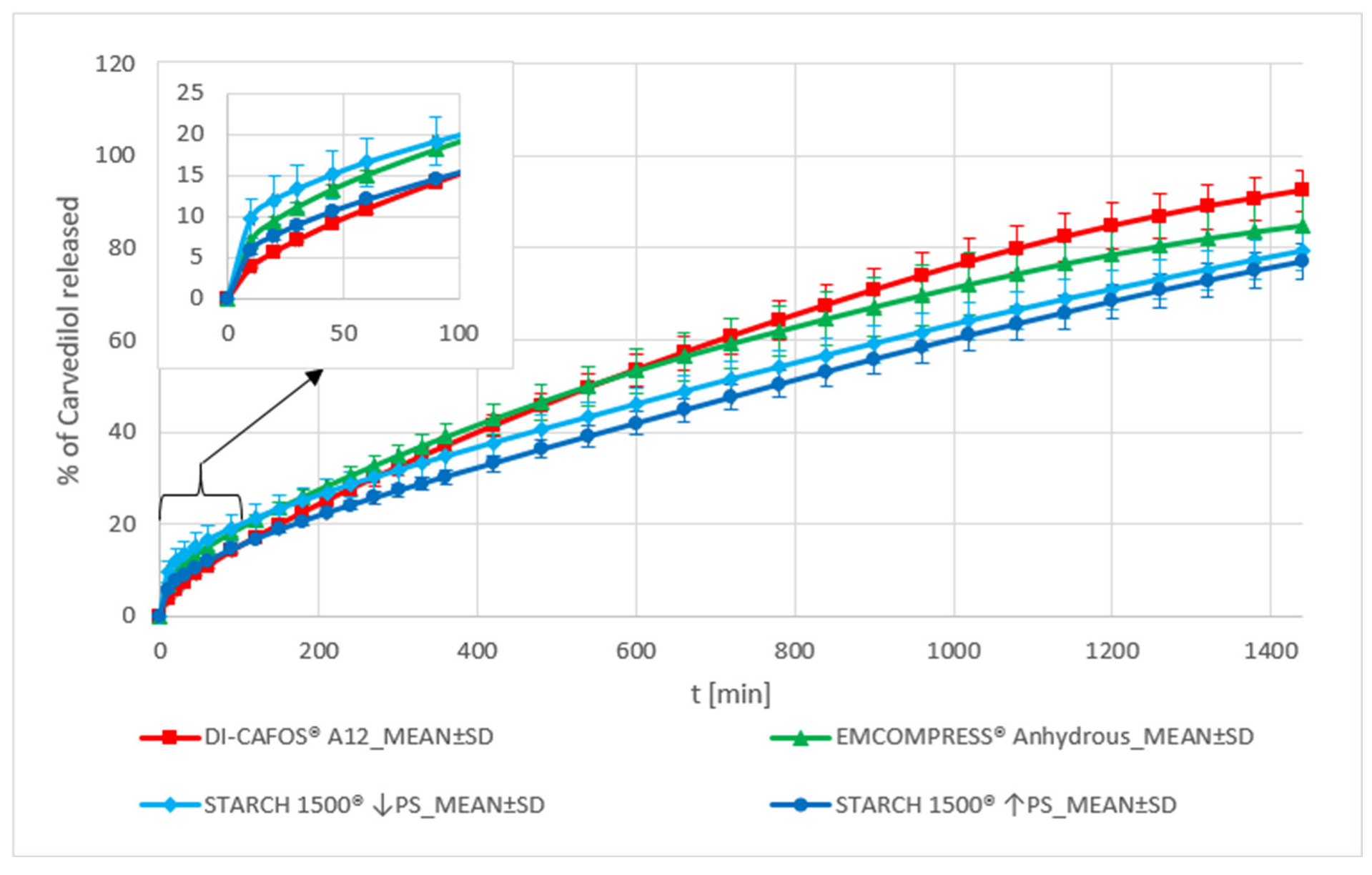
Dibasic Calcium Phosphate and Pregelatinized Starch
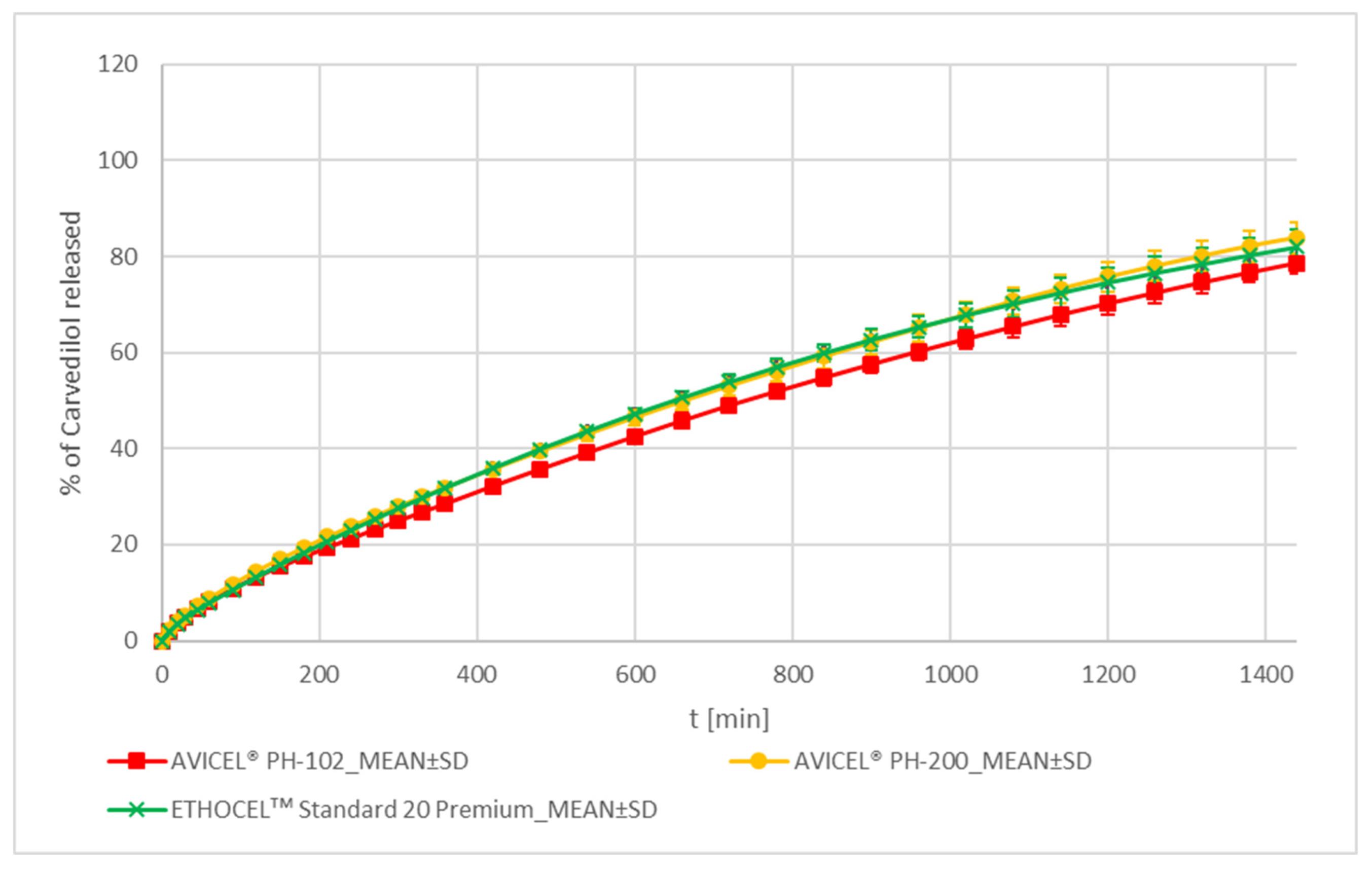
MCC and EC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- Timmins, P.; Pygall, S.R.; Melia, C.D. Hydrophilic Matrix Dosage Forms: Definitions, General Attributes, and the Evolution of Clinical Utilization. In Hydrophilic Matrix Tablets for Oral Controlled Release; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–15. [Google Scholar]
- Levina, M.; Rajabi-Siahboomi, A.R. An Industrial Perspective on Hydrophilic Matrix Tablets Based on Hyproxypropyl Methylcellulose (Hypromellose). In Hydrophilic Matrix Tablets for Oral Controlled Release; Springer: Berlin/Heidelberg, Germany, 2014; pp. 53–85. [Google Scholar]
- Ford, J.L. Design and evaluation of hydroxypropyl methylcellulose matrix tablets for oral controlled release: A historical perspective. In Hydrophilic Matrix Tablets for Oral Controlled Release; Springer: Berlin/Heidelberg, Germany, 2014; pp. 17–51. [Google Scholar]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose–A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control Release 2020, 324, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Melia, C.D.; Timmins, P. Natural polysaccharides in hydrophilic matrices. In Hydrophilic Matrix Tablets for Oral Controlled Release; Springer: Berlin/Heidelberg, Germany, 2014; pp. 87–122. [Google Scholar]
- Martin, L.M.; Rajabi-Siahboomi, A.R. Applications of polyethylene oxide (POLYOX) in hydrophilic matrices. In Hydrophilic Matrix Tablets for Oral Controlled Release; Springer: Berlin/Heidelberg, Germany, 2014; pp. 123–141. [Google Scholar]
- Ford, J.L.; Rubinstein, M.H.; McCaul, F.; Hogan, J.E.; Edgar, P.J. Importance of drug type, tablet shape and added diluents on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Int. J. Pharm. 1987, 40, 223–234. [Google Scholar] [CrossRef]
- Gao, P.; Nixon, P.R.; Skoug, J.W. Diffusion in HPMC gels. II. Prediction of drug release rates from hydrophilic matrix extended-release dosage forms. Pharm. Res. 1995, 12, 965–971. [Google Scholar] [CrossRef]
- Sung, K.; Nixon, P.R.; Skoug, J.W.; Ju, T.R.; Gao, P.; Topp, E.; Patel, M. Effect of formulation variables on drug and polymer release from HPMC-based matrix tablets. Int. J. Pharm. 1996, 142, 53–60. [Google Scholar] [CrossRef]
- Vargas, C.I.; Ghaly, E.S. Kinetic release of theophylline from hydrophilic swellable matrices. Drug Dev. Ind. Pharm. 1999, 25, 1045–1050. [Google Scholar] [CrossRef]
- Lotfipour, F.; Nokhodchi, A.; Saeedi, M.; Norouzi-Sani, S.; Sharbafi, J.; Siahi-Shadbad, M. The effect of hydrophilic and lipophilic polymers and fillers on the release rate of atenolol from HPMC matrices. Il Farmaco 2004, 59, 819–825. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Hassan-Zadeh, D.; Monajjem-Zadeh, F.; Taghi-Zadeh, N. Effect of various surfactants and their concentration on controlled release of captopril from polymeric matrices. Acta Pharm. 2008, 58, 151–162. [Google Scholar] [CrossRef]
- Tajarobi, F.; Abrahmsén-Alami, S.; Carlsson, A.S.; Larsson, A. Simultaneous probing of swelling, erosion and dissolution by NMR-microimaging—Effect of solubility of additives on HPMC matrix tablets. Eur. J. Pharm. Sci. 2009, 37, 89–97. [Google Scholar] [CrossRef]
- Tajarobi, F.; Abrahmsén-Alami, S.; Hansen, M.; Larsson, A. The impact of dose and solubility of additives on the release from HPMC matrix tablets—Identifying critical conditions. Pharm. Res. 2009, 26, 1496–1503. [Google Scholar] [CrossRef]
- Tukaram, B.N.; Rajagopalan, I.V.; Shartchandra, P.S.I. The effects of lactose, microcrystalline cellulose and dicalcium phosphate on swelling and erosion of compressed HPMC matrix tablets: Texture analyzer. Iran. J. Pharm. Res. IJPR 2010, 9, 349. [Google Scholar]
- Maghsoodi, M.; Barghi, L. Polymer percolation threshold in multi-component HPMC matrices tablets. Adv. Pharm. Bull. 2011, 1, 27. [Google Scholar] [PubMed]
- Lapidus, H.; Lordi, N. Drug release from compressed hydrophilic matrices. J. Pharm. Sci. 1968, 57, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Alderman, D. A review of cellulose ethers in hydrophilic matrices for oral controlled-release dosage forms. Int. J. Pharm. Tech. Prod. Mfr. 1984, 5, 1–9. [Google Scholar]
- Jamzad, S.; Tutunji, L.; Fassihi, R. Analysis of macromolecular changes and drug release from hydrophilic matrix systems. Int. J. Pharm. 2005, 292, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Sunada, H. Influence of formulation change on drug release kinetics from hydroxypropylmethylcellulose matrix tablets. Chem. Pharm. Bull. 1995, 43, 483–487. [Google Scholar] [CrossRef]
- Levina, M.; Rajabi-Siahboomi, A.R. The influence of excipients on drug release from hydroxypropyl methylcellulose matrices. J. Pharm. Sci. 2004, 93, 2746–2754. [Google Scholar] [CrossRef]
- Williams, H.D.; Ward, R.; Culy, A.; Hardy, I.J.; Melia, C.D. Designing HPMC matrices with improved resistance to dissolved sugar. Int. J. Pharm. 2010, 401, 51–59. [Google Scholar] [CrossRef]
- Michailova, V.; Titeva, S.; Kotsilkova, R.; Krusteva, E.; Minkov, E. Influence of hydrogel structure on the processes of water penetration and drug release from mixed hydroxypropylmethyl cellulose/thermally pregelatinized waxy maize starch hydrophilic matrices. Int. J. Pharm. 2001, 222, 7–17. [Google Scholar] [CrossRef]
- Hanselmann, R.; Burchard, W.; Ehrat, M.; Widmer, H. Structural properties of fractionated starch polymers and their dependence on the dissolution process. Macromolecules 1996, 29, 3277–3282. [Google Scholar] [CrossRef]
- Vandecruys, R.P.G.; Jans, E.M.J. Pregelatinized Starch in a Controlled Release Formulation. 2003. Available online: https://patents.google.com/patent/US6667060B1/en (accessed on 5 April 2023).
- Wong, E.B. Partially Pregelatinized Starch as an Excipient in HPMC Matrices; University of Nottingham: Nottingham, UK, 2009. [Google Scholar]
- Elgaied-Lamouchi, D.; Descamps, N.; Lefevre, P.; Rambur, I.; Pierquin, J.-Y.; Siepmann, F.; Siepmann, J.; Muschert, S. Starch-based controlled release matrix tablets: Impact of the type of starch. J. Drug Deliv. Sci. Technol. 2021, 61, 102152. [Google Scholar] [CrossRef]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Floating matrix tablets based on low density foam powder: Effects of formulation and processing parameters on drug release. Eur. J. Pharm. Sci. 2003, 18, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Gusler, G.; Berner, B.; Chau, M.; Padua, A. Optimal Polymer Mixtures for Gastric Retentive Tablets. 2004. Available online: https://patents.google.com/patent/US6723340B2/en (accessed on 5 April 2023).
- Liu, Q.; Fassihi, R. Zero-order delivery of a highly soluble, low dose drug alfuzosin hydrochloride via gastro-retentive system. Int. J. Pharm. 2008, 348, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hardy, I.J.; Windberg-Baarup, A.; Neri, C.; Byway, P.V.; Booth, S.W.; Fitzpatrick, S. Modulation of drug release kinetics from hydroxypropyl methyl cellulose matrix tablets using polyvinyl pyrrolidone. Int. J. Pharm. 2007, 337, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Tenero, D.M.; Henderson, L.S.; Baidoo, C.A.; Harter, A.H.; Campanile, A.M.; Danoff, T.M.; Boyle, D. Pharmacokinetic properties of a new controlled-release formulation of carvedilol. Am. J. Cardiol. 2006, 98, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Carter, N.J.; Keating, G.M. Controlled-release carvedilol. Am. J. Cardiovasc. Drugs 2008, 8, 271–282. [Google Scholar] [CrossRef]
- Košir, D.; Ojsteršek, T.; Baumgartner, S.; Vrečer, F. A study of critical functionality-related characteristics of HPMC for sustained-release tablets. Pharm. Dev. Technol. 2018, 23, 865–873. [Google Scholar] [CrossRef]
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Devlin, S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- LOESS Smoothing in Excel. Available online: https://peltiertech.com/loess-smoothing-in-excel/ (accessed on 11 December 2021).
- LOESS (aka LOWESS). Available online: https://www.itl.nist.gov/div898/handbook/pmd/section1/pmd144.htm (accessed on 11 December 2021).
- Example of LOESS Computations. Available online: https://www.itl.nist.gov/div898/handbook/pmd/section1/dep/dep144.htm (accessed on 11 December 2021).
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.-P. In vitro dissolution profile comparison—Statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Your Universally Applicable Polymer: POLYGLYKOLS®—POLYETHYLENE GLYCOLS. Available online: https://www.essentialingredients.com/pdf/ClariantPolyglykolsBrochure.pdf (accessed on 8 October 2022).
- POLYOX™. Versatile Water-Soluble Resins for Industrial Applications. Available online: https://cms.chempoint.com/ic/getattachment/949bd073-4a8c-4820-846b-e149bc45e27b/attachment.aspx (accessed on 8 October 2022).
- Bühler, V. Soluble polyvinylpyrrolidone (povidone). In Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; pp. 5–124. [Google Scholar]
- Kurakula, M.; Rao, G.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Chang, S.-Y.; Dun, J.; Sun, W.-J.; Wang, K.; Tajarobi, P.; Boissier, C.; Sun, C.C. Comparative analyses of flow and compaction properties of diverse mannitol and lactose grades. Int. J. Pharm. 2018, 546, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wewers, M.; Czyz, S.; Finke, J.H.; John, E.; Van Eerdenbrugh, B.; Juhnke, M.; Bunjes, H.; Kwade, A. Influence of formulation parameters on redispersibility of naproxen nanoparticles from granules produced in a fluidized bed process. Pharmaceutics 2020, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, A.; Hofland, G.W.; Witkamp, G.-J. Properties of sugar, polyol, and polysaccharide water–ethanol solutions. J. Chem. Eng. Data 2007, 52, 1838–1842. [Google Scholar] [CrossRef]
- Newell, H.E.; Buckton, G.; Butler, D.A.; Thielmann, F.; Williams, D.R. The use of inverse phase gas chromatography to measure the surface energy of crystalline, amorphous, and recently milled lactose. Pharm. Res. 2001, 18, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, F.; Burnett, D.J.; Heng, J.Y. Determination of the surface energy distributions of different processed lactose. Drug Dev. Ind. Pharm. 2007, 33, 1240–1253. [Google Scholar] [CrossRef]
- Willart, J.; Dujardin, N.; Dudognon, E.; Danède, F.; Descamps, M. Amorphization of sugar hydrates upon milling. Carbohydr. Res. 2010, 345, 1613–1616. [Google Scholar] [CrossRef]
- Pazesh, S.; Gråsjö, J.; Berggren, J.; Alderborn, G. Comminution-amorphisation relationships during ball milling of lactose at different milling conditions. Int. J. Pharm. 2017, 528, 215–227. [Google Scholar] [CrossRef]
- Tejedor, M.B.; Pazesh, S.; Nordgren, N.; Schuleit, M.; Rutland, M.W.; Alderborn, G.; Millqvist-Fureby, A. Milling induced amorphisation and recrystallization of α-lactose monohydrate. Int. J. Pharm. 2018, 537, 140–147. [Google Scholar] [CrossRef]
- Lamešić, D.; Planinšek, O.; Lavrič, Z.; Ilić, I. Spherical agglomerates of lactose with enhanced mechanical properties. Int. J. Pharm. 2017, 516, 247–257. [Google Scholar] [CrossRef]
- ROQUETTE. PRODUCT SPECIFICATION SHEET. GLUCIDEX® IT 19—MALTODEXTRIN. Available online: https://media.knowde.com/image/upload/v1581768774/production/Collateral/94760/unnamed.pdf (accessed on 4 April 2023).
- Kennedy, J.; Knill, C.; Taylor, D. Maltodextrins. In Handbook of Starch Hydrolysis Products and Their Derivatives; Springer: Berlin/Heidelberg, Germany, 1995; pp. 65–82. [Google Scholar]
- Dokic, P.; Jakovljevic, J.; Dokic-Baucal, L. Molecular characteristics of maltodextrins and rheological behaviour of diluted and concentrated solutions. Colloids Surf. Physicochem. Eng. Aspects 1998, 141, 435–440. [Google Scholar] [CrossRef]
- Avaltroni, F.; Bouquerand, P.; Normand, V. Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions. Carbohydr. Polym. 2004, 58, 323–334. [Google Scholar] [CrossRef]
- Siemons, I.; Politiek, R.; Boom, R.; Van der Sman, R.; Schutyser, M. Dextrose equivalence of maltodextrins determines particle morphology development during single sessile droplet drying. Food Res. Int. 2020, 131, 108988. [Google Scholar] [CrossRef] [PubMed]
- Lerk, C.; Bolhuis, G.; De Boer, A. Effect of microcrystalline cellulose on liquid penetration in and disintegration of directly compressed tablets. J. Pharm. Sci. 1979, 68, 205–211. [Google Scholar] [CrossRef]


| Ingredient | Functionality of Ingredient | The Theoretical Amount of Ingredients in a Tablet (mg) | Theoretical w/w % of Ingredients in a Tablet | Additional Info |
|---|---|---|---|---|
| Carvedilol (free base) 1 (Ph. Eur.: Carvedilol) | Drug substance 1 | 64.8 | 10.00 | see Table S9 |
| METHOCELᵀᴹ K15M Premium (HPMC 2208 with nominal viscosity 17,700 mPa·s; Ph. Eur.: Hypromellose) | Hydrophilic matrix forming agent | 97.2 | 15.00 | FRC data from CoA 2: viscosity 21,762 mPa·s 3, % of methoxy-groups (-OCH3, %MeO) 23.6% 4, % of hydroxypropoxygroups (-OCH2CH(OH)CH3, %HP) 9.6% 4; see Table S9 |
| See Table 2 | Filler/Bulking agent/Carvedilol release modifier | 475.632 | 73.40 | see Table S9 |
| AEROSIL® 200 Pharma (Colloidal silicon dioxide; Ph. Eur.: Silica, Colloidal Anhydrous) | Glidant | 1.944 | 0.30 | / |
| Magnesium stearate EUR PHAR Vegetable (Ph. Eur.: Magnesium stearate) | Lubricant | 8.424 | 1.30 | see Table S9 |
| Total | 648.0 | 100.0 | / |
| (a) | ||||
| Excipient Generic Name | Selected Excipient’s Marketed Product Name | Name Abbreviation | Manufacturer | Additional Info 1 |
| Polyethylene Glycol (Ph. Eur.: Macrogols) & Polyethylene Oxide (Ph. Eur.: Macrogols, High-Molecular-Mass) | Polyglykol® 4000 P | PEG 4k | Clariant Produkte (Deutschland) GmbH, Frankfurt, Germany | M = 4017 g/mol (CoA) |
| Polyglykol® 8000 P | PEG 8k | M = 8026 g/mol (CoA) | ||
| POLYOXᵀᴹ WSR N-80 (LEO NF Grade) | PEO | DUPONT, Nutrition and Biosciences (Freienbach, Switzerland) GmbH | nominal M of 200,000 g/mol; | |
| Povidone | KOLLIDON® 25 | PVP K25 | BASF SE, Ludwigshafen, Germany | K value = 24.7 (CoA) |
| KOLLIDON® 90 F | PVP K90 | K value = 92.4 (CoA) | ||
| Mannitol | C*Pharm Mannidex 16700 | MAN_C_1 | Cargill S.r.l., Milan, Italy | crystalline D-mannitol, sorbitol content = 0.6% (CoA) |
| PEARLITOL® 160C | MAN_C_2 | ROQUETTE Frères, Lestrem, France | crystalline D-mannitol, sorbitol content = 0.7% (CoA) | |
| Parteck® M 100 | MAN_SD_1 | Merck KGaA, Darmstadt, Germany | spray-dried D-mannitol, sorbitol content = 1.3% (CoA) | |
| Parteck® M 200 | MAN_SD_2 | spray-dried D-mannitol, sorbitol content = 1.3% (CoA) | ||
| Lactose Monohydrate | Lactochem® Crystals | LAC_M | DFE Pharma GmbH and Co. KG, Goch, Germany | crystalline lactose monohydrate |
| Lactochem® Fine Powder | LAC_C | milled lactose monohydrate | ||
| SuperTab® 11SD | LAC_SD_1 | spray-dried lactose monohydrate | ||
| FlowLac® 100 | LAC_SD_2 | MEGGLE GmbH and Co. KG, Wasserburg, Germany | spray-dried lactose monohydrate | |
| Tablettose® 70 | LAC_AG | agglomerated lactose monohydrate | ||
| Sucrose | Granulated sugar N°1 600 | SUC | Tereos, Moussy-le-Vieux, France | crystalline sucrose |
| Maltodextrin | GLUCIDEX® 19 | MD_SD | ROQUETTE Frères, France | a spray-dried mixture of glucose, disaccharides, and polysaccharides |
| (b) | ||||
| Excipient Generic Name | Selected Excipient’s Marketed Product Name | Name Abbreviation | Manufacturer | Additional Info 1 |
| Anhydrous Dibasic Calcium Phosphate (Ph. Eur.: Calcium Hydrogen Phosphate) | DI-CAFOS® A12 | DCP_1 | Chemische Fabrik Budenheim KG, Budenheim, Germany | anhydrous DCP |
| EMCOMPRESS® Anhydrous | DCP_2 | JRS Pharma GmbH and Co. KG, Rosenberg, Germany | anhydrous DCP | |
| Microcrystalline Cellulose (Ph. Eur.: Cellulose, Microcrystalline) | AVICEL® PH-102 | MCC 101 | DuPont Nutrition Ireland, Little Island, Ireland | nominal particle size of app. 100 µm |
| AVICEL® PH-200 | MCC 200 | nominal particle size of app. 180 µm | ||
| Ethylcellulose | ETHOCELᵀᴹ Standard 20 Premium | EC | DOW, Specialty Electronic Materials Switzerland GmbH, Horgen, Switzerland | FRC data from CoA: viscosity 20.6 mPa·s, ethoxyl content (assay) 48.7% |
| Pregelatinized Starch | STARCH 1500® sample with smaller particle size (↓PS) | PS_1 | Colorcon Inc., Harleysville, PA, USA | sieve analysis: 99.3% through 100 mesh and 46.9% through 270 mesh (CoA) |
| STARCH 1500® sample with larger particle size (↑PS) | PS_2 | sieve analysis: 93.5% through 100 mesh and 31.9% through 270 mesh (CoA) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojsteršek, T.; Hudovornik, G.; Vrečer, F. Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets. Pharmaceutics 2023, 15, 1525. https://doi.org/10.3390/pharmaceutics15051525
Ojsteršek T, Hudovornik G, Vrečer F. Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets. Pharmaceutics. 2023; 15(5):1525. https://doi.org/10.3390/pharmaceutics15051525
Chicago/Turabian StyleOjsteršek, Tadej, Grega Hudovornik, and Franc Vrečer. 2023. "Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets" Pharmaceutics 15, no. 5: 1525. https://doi.org/10.3390/pharmaceutics15051525
APA StyleOjsteršek, T., Hudovornik, G., & Vrečer, F. (2023). Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets. Pharmaceutics, 15(5), 1525. https://doi.org/10.3390/pharmaceutics15051525





