Pullulan/Poly(vinyl alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pristine P/PVA Hydrogels
2.3. Preparation of Calendula Extract—Loaded P/PVA Hydrogels
2.4. Characterization of Calendula officinalis Extract
2.4.1. Identification of Phenolic and Triterpenic Compounds by HPLC-ESI MS
2.4.2. Total Phenolic Content (TPC)
2.4.3. Total Flavonoid Content (TFC)
2.4.4. Total Triterpenes Content (TTPC)
2.5. Characterization of Unloaded and Calendula-Extract-Loaded P/PVA Hydrogels
2.5.1. Chemical Composition
2.5.2. Morphology and Porosity
2.5.3. Swelling Ratio
2.5.4. Mechanical Properties and Bioadhesiveness
2.6. Loading Capacity and Calendula Extract Release from Hydrogels
2.7. Antioxidant Activity
2.8. Antibacterial Activity
2.9. Cytotoxic Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Calendula officinalis Extract
3.2. Synthesis of P/PVA Hydrogels
3.3. Loading of P/PVA Hydrogels
3.3.1. FT-IR Spectra
3.3.2. Morphology and Pore Size
3.3.3. Swelling Behavior
3.3.4. Mechanical Properties and Bioadhesiveness
3.4. Release Kinetic of Calendula officinalis from P/PVA Hydrogels
3.5. Antioxidant Activity
3.6. Antibacterial Activity
3.7. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Divyashri, G.; Badhe, R.V.; Sadanandan, B.; Vijayalakshmi, V.; Kumari, M.; Ashrit, P.; Bijukumar, D.; Mathew, M.T.; Shetty, K.; Raghu, A.V. Applications of hydrogel-based delivery systems in wound care and treatment: An up-to-date review. Polym. Adv. Technol. 2022, 33, 2025–2043. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, Z.; Liu, L.; Li, M.; Xu, B.; Yu, D.; Qi, D.; Wu, J. Multifunctional fibrous wound dressings for refractory wound healing. J. Polym. Sci. 2022, 60, 2191–2212. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. An Up-to-Date Review of Biomaterials Application in Wound Management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhang, L.; Ou, R.; Xu, Y.; Xu, L.; Zhan, X.-Y.; Li, D. Polysaccharide-based hydrogels for wound dressing: Design considerations and clinical applications. Front. Bioeng. Biotechnol. 2022, 10, 845735. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.K.; Gaba, A. Phyto-Extracts in Wound Healing. J. Pharm. Pharm. Sci. 2013, 16, 760–820. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, M.A.; Khatoon, F.; Rizvi, M.A.; Zafaryab, M. Ethyl acetate Salix alba leaves extract loaded Chitosan based hydrogel film for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2015, 26, 1452–1464. [Google Scholar] [CrossRef]
- Imtiaz, N.; Niazi, M.B.K.; Fasim, F.; Khan, B.A.; Bano, S.A.; Shah, G.M.; Badshah, M.; Menaa, F.; Uzair, B. Fabrication of an Original Transparent PVA/Gelatin Hydrogel: In Vitro Antimicrobial Activity against Skin Pathogens. Int. J. Polym. Sci. 2019, 2019, 7651810. [Google Scholar] [CrossRef] [Green Version]
- Ditta, L.A.; Rao, E.; Provenzano, F.; Sanchez, J.L.; Santonocito, R.; Passantino, R.; Costa, M.A.; Sabatino, M.A.; Dispenza, C.; Giacomazza, D.; et al. Agarose/κ-carrageenan-based hydrogel film enriched with natural plant extracts for the treatment of cutaneous wounds. Int. J. Biol. Macromol. 2020, 164, 2818–2830. [Google Scholar] [CrossRef]
- Khan, B.A.; Khan, A.; Khan, M.K.; Braga, V.A. Preparation and properties of High sheared Poly(Vinyl Alcohol)/Chitosan blended Hydrogels films with Lawsonia inermis extract as wound dressing. J. Drug Deliv. Sci. Technol. 2021, 61, 102227. [Google Scholar] [CrossRef]
- Ali, A.; Garg, P.; Goyal, R.; Kaur, G.; Li, X.; Negi, P.; Valis, M.; Kuca, K.; Kulshrestha, S. A Novel Herbal Hydrogel Formulation of Moringa oleifera for Wound Healing. Plants 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Lodhi, M.; Afzal, A.; Rehan, Z.A.; Mehmood, M.; Javed, T.; Shabbir, R.; Siuta, D.; Althobaiti, F.; Dessok, E.S. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels 2021, 7, 107. [Google Scholar] [CrossRef]
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.K.; Achim, M.; Mirel, S. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242. [Google Scholar] [CrossRef]
- Rathod, L.; Bhowmick, S.; Patel, P.; Sawant, K. Calendula flower extract loaded PVA hydrogel sheet for wound management: Optimization, characterization and in-vivo study. J. Drug Deliv. Sci. Technol. 2022, 68, 103035. [Google Scholar] [CrossRef]
- Tajik, F.; Eslahi, N.; Rashidi, A.; Rad, M.M. Hybrid antibacterial hydrogels based on PVP and keratin incorporated with lavender extract. J. Polym. Res. 2021, 28, 316. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Kishi, A.; Kageura, T.; Matsuda, H. Medicinal fowers. III. Marigold. (1): Hypoglycemic, gastric, emptying inhibitory, and gastroprotective principles and new oleanane-type triterpene oligoglycosides, calendasaponins A, B, C, and D, from Egyptian Calendula offcinalis. Chem. Pharm. Bull. 2001, 49, 863–870. [Google Scholar] [CrossRef] [Green Version]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of calendula officinalis—A review. Indian J. Pharm. Sci. 2015, 5, 172–185. [Google Scholar]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable wound dressing based on carboxymethyl chitosan triple-network hydrogel for effective wound antibacterial and hemostasis. Int. J. Biol. Macromol. 2023, 225, 1235–1245. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef]
- HMPC. European Union Herbal Monograph on Calendula officinalis L., Flos. 2018, London. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-calendula-officinalis-l-flos-revision-1_en.pdf (accessed on 4 May 2023).
- Jiménez, R.A.; Millán, D.; Suesca, E.; Sosnik, A.; Fontanilla, M.R. Controlled release of an extract of Calendula officinalis, flowers from a system based on the incorporation of gelatin-collagen microparticles into collagen I scaffolds: Design and in vitro performance. Drug Deliv. Transl. Res. 2015, 5, 209–218. [Google Scholar] [CrossRef]
- Lahmar, A.; Rjab, M.; Sioud, F.; Selmi, M.; Salek, A.; Kilani-Jaziri, S.; Ghedira, L.C. Design of 3D hybrid plant extract/marine and bovine collagen matrixes as potential dermal scaffolds for skin wound. Sci. World J. 2022, 2022, 8788061. [Google Scholar] [CrossRef] [PubMed]
- Rad Pedram, Z.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.d.M.C.; Bandeira, E.d.S.; Gomes, M.F.; Lynch, D.G.; Bastos, G.N.T.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide Hydrogel Containing Calendula Extract as a Wound Healing Bandage: In Vivo Test. Int. J. Mol. Sci. 2023, 24, 3806. [Google Scholar] [CrossRef]
- Kharat, Z.; Goushki, M.A.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO nanofibers containing Calendula officinalis extract: Preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int. J. Pharm. 2021, 609, 121132. [Google Scholar] [CrossRef]
- Kozlowska, J.; Tylkowski, B.; Stachowiak, N.; Prus-Walendziak, W. Controlling the Skin Barrier Quality through the Application of Polymeric Films Containing Microspheres with Encapsulated Plant Extract. Processes 2020, 8, 530. [Google Scholar] [CrossRef]
- Shafeie, N.; Naini, A.T.; Jahromi, H.K. Comparison of different concentrations of calendula officinalis gel on cutaneous wound healing. Biomed. Pharmacol. J. 2015, 8, 979–992. [Google Scholar] [CrossRef]
- Aduba, D.C., Jr.; Yang, H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering 2017, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Alhaique, F.; Casadei, M.A.; Cencetti, C.; Coviello, T.; Meo, C.D.; Matricardi, P.; Montanari, E.; Pacelli, S.; Paolicelli, P. From macro to nano polysaccharide hydrogels: An opportunity for the delivery of drugs. J. Drug Deliv. Sci. Technol. 2016, 32, 88–99. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Danti, S.; De Clerck, K.; Lazzeri, A.; Morganti, P. Pullulan for Advanced Sustainable Body- and Skin-Contact Applications. J. Funct. Biomater. 2020, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Samoila, I.; Dinescu, S.; Gradisteanu Pircalabioru, G.; Marutescu, L.; Fundueanu, G.; Aflori, M.; Constantin, M. Pullulan/Poly(Vinyl Alcohol) Composite Hydrogels for Adipose Tissue Engineering. Materials 2019, 12, 3220. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu’s reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Rigane, G.; Salem, R.B.; Sayadi, S.; Bouaziz, M. Phenolic composition, isolation, and structure of a new deoxyloganic acid derivative from Dhokar and Gemri-Dhokar olive cultivars. J. Food Sci. 2011, 76, C965–C973. [Google Scholar] [CrossRef]
- Pedrosa, A.M.; de Castro, W.V.; Fonseca Castro, A.H.; Duarte-Almeida, J.M. Validated spectrophotometric method for quantification of total triterpenes in plant matrices. DARU J. Pharm. Sci. 2020, 28, 281–286. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Moshayedi, S.; Sarpoolaky, H.; Khavandi, A. Fabrication, swelling behavior, and water absorption kinetics of genipin-crosslinked gelatin–chitosan hydrogels. Polym. Eng. Sci. 2021, 61, 3094–3103. [Google Scholar] [CrossRef]
- Johnson, M.; Fathima, M.S.A. Spectroscopic studies on pouzolzia wightii benn. Int. J. Pharm. Pharm. Sci. 2018, 10, 124–132. [Google Scholar]
- Ayyanahalli Matta, B.K.; Kumar, S.; Mehta, C.H.; Nayak, U.Y.; Rodriguez, P.G. Comparative Evaluation of the Effectiveness of a Combination of Absorbable Gelatin Sponge and Calendula officinalis with Absorbable Gelatin Sponge Used Alone as a Hemostatic Agent—An In-Vitro Study. Dent. J. 2022, 10, 76. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable device. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Velicković, J.M.; Dimitrijević, D.S.; Mitić, S.S.; Mitić, M.N.; Kostić, D.A. The determination of the phenolic composition, antioxidative activity and heavy metals in the extracts of Calendula officinalis L. Adv. Technol. 2014, 3, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Sabir, S.M.; Khan, M.F.; Rocha, J.B.T.; Boligon, A.A.; Athayde, M.L. Phenolic profile, antioxidant activities and genotoxic evaluations of calendula officinalis. J. Food Biochem. 2015, 39, 316–324. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmed, S. Eco-friendly natural extract loaded antioxidative chitosan/polyvinyl alcohol based active films for food packaging. Heliyon 2021, 7, e06550. [Google Scholar]
- ASTM E2315-16; Standard Guide for Assessment of Antimicrobial Activity Using a Time-Kill Procedure. ASTM International: West Conshohocken, PA, USA, 2016.
- Szopa, A.; Klimek-Szczykutowicz, M.; Jafernik, K.; Koc, K.; Ekiert, H. Pot marigold (Calendula officinalis L.)—A position in classical phytotherapy and newly documented activities. Acta Sci. Pol. Hortorum Cultus 2020, 19, 47–61. [Google Scholar] [CrossRef]
- Budan, A.; Bellenot, D.; Freuze, I.; Gillmann, L.; Chicoteau, P.; Richomme, P.; Guilet, D. Potential of extracts from Saponaria officinalis and Calendula officinalis to modulate in vitro rumen fermentation with respect to their content in saponins. Biosci. Biotechnol. Biochem. 2014, 78, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. Int. J. Mol. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olennikov, D.N.; Kashchenko, N.I. New Isorhamnetin Glycosides and other Phenolic Compounds from Calendula officinalis. Chem. Nat. Compd. 2013, 49, 833–840. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I. Componential Profile and Amylase Inhibiting Activity of Phenolic Compounds from Calendula officinalis L. Leaves. Sci. World J. 2014, 2014, 654193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigane, G.; Ben Younes, S.; Ghazghazi, H.; Ben Salem, R. Investigation into the biological activities and chemical composition of Calendula officinalis L. growing in Tunisia. Int. Food Res. J. 2013, 20, 3001–3007. [Google Scholar]
- Cordova, C.A.S.; Siqueira, I.R.; Netto, C.A.; Yunes, R.A.; Volpato, A.M.; Filho, V.C.; Curi-Pedrosa, R.; Creczynski-Pasa, T.B. Protective properties of butanolic extract of the Calendula officinalis L. (marigold) against lipid peroxidation of rat liver microsomes and action as free radical scavenger. Redox Rep. 2002, 7, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Andersen, F.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Final report of the Cosmetic Ingredient Review Expert Panel amended safety assessment of Calendula officinalis-derived cosmetic ingredients. Int. J. Toxicol. 2010, 29, 221S–243S. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; Garcia, P.A.; Castro, M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragueto Escher, G.; Cordoso Borges, L.D.C.; Sousa Santos, J.; Mendanha Cruz, T.; Boscacci Marques, M.; Vieira do Carmo, M.A.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; Wen, M.; et al. From the Field to the Plot: Phytochemical and Functional Analyses of Calendula officinalis L. Flower for Incorporation in an Organic Yogurt. Antioxidants 2019, 8, 559. [Google Scholar] [CrossRef] [Green Version]
- Faustino, M.V.; Pinto, D.C.; Gonçalves, M.J.; Salgueiro, L.; Silveira, P.; Silva, A.M.; Calendula, L. species polyphenolic profile and in vitro antifungal activity. J. Funct. Food 2018, 45, 254–267. [Google Scholar] [CrossRef]
- Grati, W.; Samet, S.; Bouzayani, B.; Ayachi, A.; Treilhou, M.; Tene, N.; Mezghani-Jarraya, R. HESI-MS/MS Analysis of Phenolic Compounds from Calendula aegyptiaca Fruits Extracts and Evaluation of Their Antioxidant Activities. Molecules 2022, 27, 2314. [Google Scholar] [CrossRef]
- Mroczek, A.; Kapusta, I.; Stochmal, A.; Janiszowaska, W. MS/MS and UPLC-MS profiling of triterpenoid saponins from leaves and roots of four red beet (Beta vulgaris L.) cultivars. Phytochem. Lett. 2019, 30, 333–337. [Google Scholar] [CrossRef]
- Pizza, C.; Zhong-Liang, Z.; de Tommasi, N. Plant metabolites. Triterpenoid saponins from Calendula arvensis. J. Nat. Prod. 1987, 50, 927–931. [Google Scholar] [CrossRef]
- Orhan, D.D.; Hartevioğlu, A.; Küpeli, E.; Yesilada, E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Cakır, O.; Bensari, S.; Yılmaz, M.A.; Gallo, M.; Montesano, D. A Comparative Bio-Evaluation and Chemical Profiles of Calendula officinalis L. Extracts Prepared via Different Extraction Techniques. Appl. Sci. 2020, 10, 5920. [Google Scholar] [CrossRef]
- Ćetković, G.S.; Đilas, S.M.; Čanadanović-Brunet, J.M.; Tumbas, V.T. Thin-Layer chromatography analysis and scavenging activity of marigold (Calendula officinalis L.) extracts. Acta Period. Technol. 2003, 34, 93–102. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Preethi, K.C.; Kuttan, G.; Kuttan, R. Antioxidant Potential of an Extract of Calendula officinalis. Flowers In Vitro and In Vivo. Pharm. Biol. 2006, 44, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Dulong, V.; Forbice, R.; Condamine, E.; Le Cerf, D.; Picton, L. Pullulan–STMP hydrogels: A way to correlate crosslinking mechanism, structure and physicochemical properties. Polym. Bull. 2011, 67, 455–466. [Google Scholar] [CrossRef]
- Batain, F.; Crescencio, K.; Alves, T.; Souza, J.F.; Amaral, V.; Castro, J.; Santos, C.; Jozala, A.; Lopes, L.; Chaud, M. Medicinal plant extract associated with bacterial cellulose membrane: Antibacterial activity and physicochemical properties. Arch. Pharm. Pharma Sci. 2020, 4, 013–020. [Google Scholar]
- Coates, J. Interpretation of infrared spectra: A practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Shurvell, H.F. Spectra-structure correlations in the mid and far infrared. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2002; pp. 1783–1816. [Google Scholar]
- Catauro, M.; Barrino, F.; Poggetto, G.D.; Crescente, G.; Piccolella, S.; Pacifico, S. New SiO2/Caffeic Acid Hybrid Materials: Synthesis, Spectroscopic Characterization, and Bioactivity. Materials 2020, 13, 394. [Google Scholar] [CrossRef] [Green Version]
- Al-Mussawi, Z.K.; Al-Hussani, I.M. Phytochemical study of calendula officinalis plant by used GC-MS and FTIR techniques. Plant Arch. 2019, 19, 845–851. [Google Scholar]
- El-Hashemy, M.A.; Sallam, A. The inhibitive action of Calendula officinalis flower heads extract for mild steel corrosion in 1 M HCl solution. J. Mater. Res. Technol. 2020, 9, 13509–13523. [Google Scholar] [CrossRef]
- Constantin, M.; Lupei, M.; Bucatariu, S.-M.; Pelin, I.M.; Doroftei, F.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. PVA/Chitosan Thin Films Containing Silver Nanoparticles and Ibuprofen for the Treatment of Periodontal Disease. Polymers 2022, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Asmarandei, I.; Filimon, A.; Fundueanu, G. Synthesis, characterization, and solution behavior of pullulan functionalized with tertiary amino groups. High Perform. Polym. 2015, 27, 625–636. [Google Scholar] [CrossRef]
- Morandim-Giannetti, A.A.; Silva, R.C.; Magalhaes, O., Jr.; Schor, P.; Bersanetti, P.A. Conditions for obtaining polyvinyl alcohol/trisodium trimetaphosphate hydrogels as vitreous humor substitute. J. Biomed. Mater. Res. Part B 2016, 104, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Thirumavalavan, K.; Manikkadan, T.R.; Dhanasekar, R. Pullulan production from coconut by-products by Aureobasidium pullulans. Afr. J. Biotechnol. 2009, 8, 254–258. [Google Scholar]
- Edikresnha, D.; Suciati, T.; Suprijadi; Khairurrijal, K. Freeze-thawed hydrogel loaded by Piper crocatum extract with in-vitro antibacterial and release tests. J. Mater. Res. Technol. 2021, 15, 17–36. [Google Scholar] [CrossRef]
- Bolgen, N.; Demir, D.; Serkan Yalcin, M.; Ozdemir, S. Development of Hypericum perforatum oil incorporated antimicrobial and antioxidant chitosan cryogel as a wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1581–1590. [Google Scholar] [CrossRef]
- Shang, K.; Tao, L.; Jiang, S.; Yan, J.; Hu, S.; Yang, G.; Ma, C.; Cheng, S.; Wang, X.; Yin, J. Highly flexible hydrogel dressing with efficient antibacterial, antioxidative, and wound healing performances. Biomater. Sci. 2022, 10, 1373–1383. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A review of the properties and applications of bioadhesive hydrogels. Polym. Chem. 2021, 12, 3721. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in Mucoadhesion and Mucoadhesive Polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Cirri, M.; Mennini, N.; Casella, G.; Maestrelli, F. Polymeric mucoadhesive tablets for topical or systemic buccal delivery of clonazepam: Effect of cyclodextrin complexation. Carbohydr. Polym. 2016, 152, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.P.; Fortes, A.C.; da Cruz Fonseca, S.G.; Breitkreutz, J.; Ferraz, H.G. Manufacture and characterization of mucoadhesive buccal films based on pectin and gellan gum containing triamcinolone acetonide. Int. J. Polym. Sci. 2018, 2018, 2403802. [Google Scholar] [CrossRef]
- Wollinger, A.; Perrin, E.; Chahboun, J.; Jeannot, V.; Touraud, D.; Kunz, W. Antioxidant activity of hydro distillation water residues from Rosmarinus officinalis L. leaves determined by DPPH assays. Comptes Rendus Chim. 2016, 19, 754–765. [Google Scholar] [CrossRef]
- Tahami, S.R.; Nemati, N.H.; Keshvari, H.; Khorasani, M.T. In vitro and in vivo evaluation of nanofibre mats containing Calendula officinalis extract as a wound dressing. J. Wound Care 2022, 31, 598–611. [Google Scholar] [CrossRef]
- Rathod, L.; Bhowmick, S.; Patel, P.; Sawant, K. Calendula flower extract loaded collagen film exhibits superior wound healing potential: Preparation, evaluation, in-vitro & in-vivo wound healing study. J. Drug Deliv. Sci. Technol. 2022, 72, 103363. [Google Scholar]
- Ishfaq, B.; Khan, I.U.; Khalid, S.H.; Asghar, S. Design and evaluation of sodium alginate-based hydrogel dressings containing Betula utilis extract for cutaneous wound healing. Front. Bioeng. Biotechnol. 2023, 11, 1042077. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Yang, C.; Liu, C.; Yu, G.; Guan, H. Preparation, characterization and antioxidant activities of polymannuronic acid phosphate, H-phosphonate and sulfate. Int. J. Biol. Macromol. 2013, 62, 281–286. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, J.Y.; Lee, Y.S. Development of polyvinyl alcohol and apple pomace biocomposite film with antioxidant properties for active food packaging application. J. Food Sci. Tecnol. 2016, 53, 1608–1619. [Google Scholar] [CrossRef] [Green Version]
- Nooeaid, P.; Chuysinuan, P.; Techasakul, S. Alginate/gelatine hydrogels: Characterisation and application of antioxidant release. Green Mater. 2017, 5, 153–164. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, Y.; Chen, Z.; Yu, Y.; Wang, H.; Lu, X.; Zhao, J.; Wang, S. Chitosan-based multifunctional hydrogel for sequential wound inflammation elimination, infection inhibition, and wound healing. Int. J. Biol. Macromol. 2023, 235, 123847. [Google Scholar] [CrossRef] [PubMed]
- Puca, V.; Marulli, R.Z.; Grande, R.; Vitale, I.; Niro, A.; Molinaro, G.; Prezioso, S.; Murano, R.; Di Giovani, P. Microbial species isolated from infected wounds and antimicrobial resistance analysis: Data emerging from a three-years retrospective study. Antibiotics 2021, 10, 1162. [Google Scholar] [CrossRef]
- Nassar, M.S.M.; Hazzah, W.A.; Bakr, W.M.K. Evaluation of antibiotic susceptibility test results: How guilty a laboratory could be? J. Egypt. Public Health Assoc. 2019, 94, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef]
- Langendonk, R.F.; Neill, D.R.; Fothergill, J.L. The Building Blocks of Antimicrobial Resistance in Pseudomonas aeruginosa: Implications for Current Resistance-Breaking Therapies. Front. Cell. Infect. Microbiol. 2021, 11, 665759. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 106. [Google Scholar]
- Masoko, P.; Eloff, J.N. The diversity of antifungal compounds of six south African Terminalia species (Combretaceae) determined by bioautography. Afr. J. Biotechnol. 2005, 4, 1425–1431. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods–A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmałek, T.; Kaproń, B.; Plech, T.; Szymanowska, D.; Karaźniewicz-Łada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel delivery system containing calendulae flos lyophilized extract with chitosan as a supporting strategy for wound healing applications. Pharmaceutics 2020, 12, 634. [Google Scholar] [CrossRef]
- Rogers, T.R.; Verweij, P.E.; Castanheira, M.; Dannaoui, E.; White, P.L.; Arendrup, M.C. Molecular mechanisms of acquired antifungal drug resistance in principal fungal pathogens and EUCAST guidance for their laboratory detection and clinical implications. J. Antimicrob. Chemother. 2022, 77, 2053–2073. [Google Scholar] [CrossRef] [PubMed]
- Cruceriu, D.; Diaconeasa, Z.; Socaci, S.; Socaciu, C.; Rakosy-Tican, E.; Balacescu, O. Biochemical profile, selective cytotoxicity and molecular effects of Calendula officinalis extracts on breast cancer cell lines. Not. Bot. Horti. Agrobo. 2020, 48, 24–39. [Google Scholar] [CrossRef] [Green Version]
- Matysik, G.; Wójciak-Kosior, M.; Paduch, R. The influence of Calendulae officinalis flos extracts on cell cultures, and the chromatographic analysis of extracts. J. Pharm. Biomed. Anal. 2005, 38, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A new extract of the plant calendula officinalis produces a dual in vitro effect: Cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer 2006, 6, 119. [Google Scholar] [CrossRef] [Green Version]
- Dinda, M.; Mazumdar, S.; Das, S.; Ganguly, D.; Dasgupta, U.B.; Dutta, A.; Jana, K.; Karmakar, P. The Water Fraction of Calendula officinalis Hydroethanol Extract Stimulates In Vitro and In Vivo Proliferation of Dermal Fibroblasts in Wound Healing. Phytother. Res. 2016, 30, 1696–1707. [Google Scholar] [CrossRef]
- Park, E.; Kim, S.; Moon, J. The Effects of Marigold (Tagetes L.) Extract and Calendula (Calendula officinalis L.) Extract on Collagen Growth and MMP-1 Expression in Human Dermal Fibroblasts. J. Korean Appl. Sci. Technol. 2017, 34, 769–777. [Google Scholar]
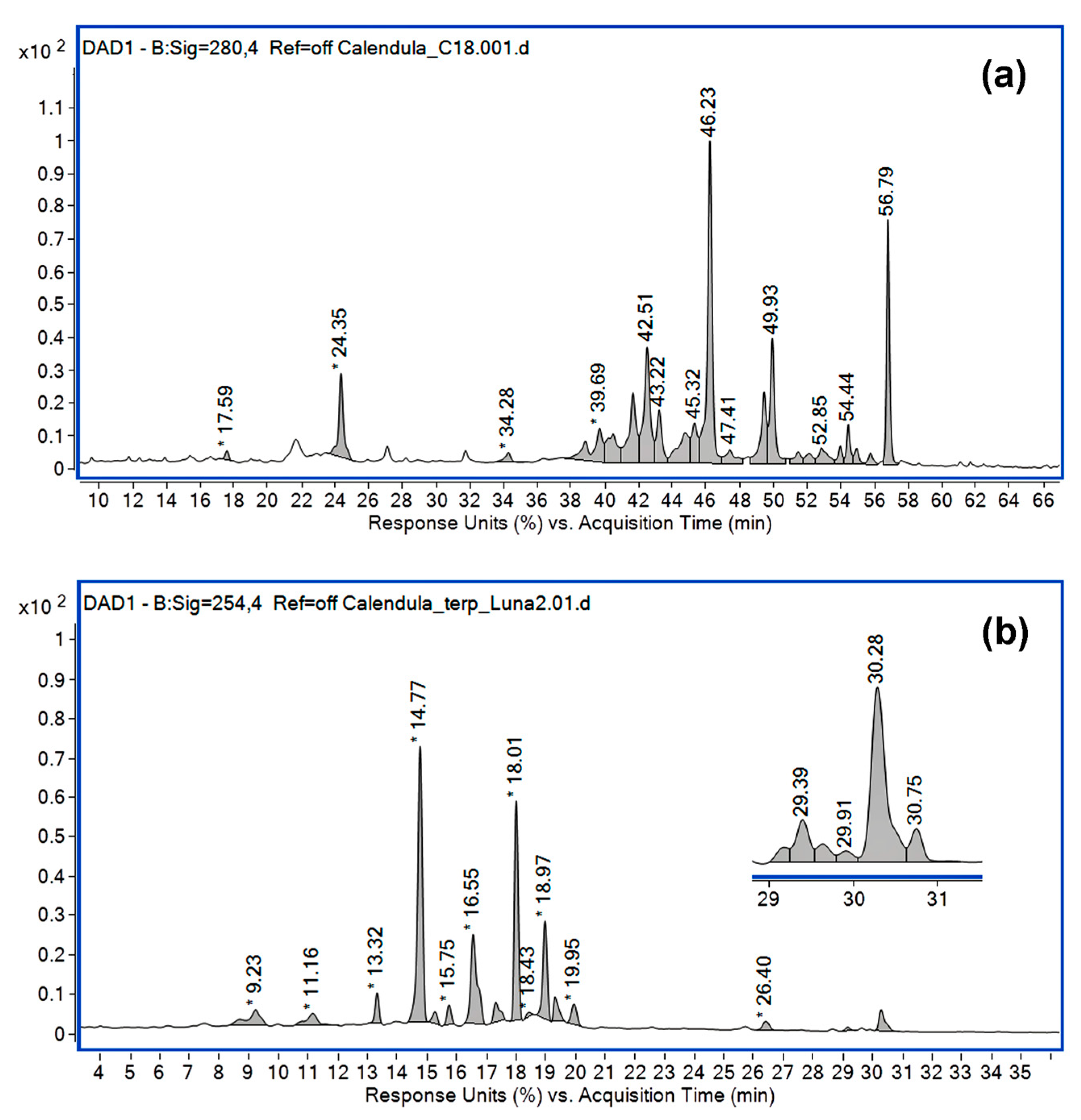








| No. | Identified Compound | RT (min) | Column/ Ionization Mode | Major ESI m/z Observed | Ref. |
|---|---|---|---|---|---|
| 1 | Chlorogenic acid | 9.23 24.34 | Phenomenex C18 Luna/ESI(−) Mediterranea Sea 18/ESI(+) | 707.22 [2M-H]− 353.11 [M-H]− 191.06 [QA-H]− 355.31 [M+H]+ 163.14 [M-QA]+ | [53,54,55] |
| 2 | Caffeic acid | 11.35 | Phenomenex C18 Luna/ESI(−) | 179.04 [M-H]− 135.05 [M-CO2]− | [59] |
| 3 | Quercitin-3-O-rhamnosylrutinoside | 13.32 | Phenomenex C18 Luna/ESI(−) | 1511.46 [2M-H]− 755.24 [M-H]− | [59,60] |
| 4 | Typhaneoside | 14.77 42.50 | Phenomenex C18 Luna/ESI(−) Mediterranea Sea 18/ESI(+) | 769.25 [M-H]− 771.162 [M+H]+ 625.35 [M-Rham+H]+ | [57,58] |
| 5 | Quercitin-3-O-rutinoside | 15.75 39.68 | Phenomenex C18 Luna/ESI(−) Mediterranea Sea 18/ESI(+) | 1219.34 [2M-H]− 609.18 [M-H]− 611.34 [M+H]+ 465.32 [M-Rham+H]+ | [59,60] |
| 6 | Isorhamnetin 3-O-rutinoside | 16.55 43.22 | Phenomenex C18 Luna/ESI(−) Mediterranea Sea 18/ESI(+) | 1247.37 [2M-H]− 623.20 [M-H]− 625.35 [M+H]+ 479.33 [M-Rham+H]+ | [59,60,62] |
| 7 | Quercetin-3-O-galactoside | 16.55 45.32 | Phenomenex C18 Luna/ESI(−) Mediterranea Sea 18/ESI(+) | 927.22 [2M-H]− 463.11 [M-H]− 301.12 [M-Glu-H]− 465.32 [M+H]+ | [60,61] |
| 8 | Quercitin-3-O-rutinoside isomer | 17.32 | Phenomenex C18 Luna/ESI(−) | 1219.34 [2M-H]− 609.17 [M-H]− | [59,60] |
| 9 | Caffeic acid 3-glucoside | 17.58 | Mediterranea Sea 18/ESI(+) | 343.54 [M+H]+ 181.15 [M-Glu+H]+ | [53,54,55] |
| 10 | Isorhamnetin rutinoside | 18.01 | Phenomenex C18 Luna/ESI(−) | 1247.37 [2M-H]− 623.20 [M-H]− | [60,62] |
| 11 | Dicaffeoylquinic acid | 18.43 | Phenomenex C18 Luna/ESI(−) | 515.14 [M-H]− | [61,62] |
| 12 | Isorhamnetin-3-O-glucoside | 18.97 49.93 | Phenomenex C18 Luna/ESI(−) Mediterranea Sea 18/ESI(+) | 955.25 [2M-H]− 477.12 [M-H]− 479.32 [M+H]+ | [56,59,60,62] |
| 13 | Hydroxycaffeic acid | 34.28 | Mediterranea Sea 18/ESI(+) | 197.26 [M+H]+ 179.13 [M-OH]+ | [53,54,55] |
| 14 | Dicaffeoylquinic acid | 18.43 | Phenomenex C18 Luna/ESI(−) | 515.14 [M-H]− 353.10 [CGA-H]− | [61,62] |
| 15 | Isorhamnetin 3,7-di-O-β-D-glucopyranoside | 46.22 | Mediterranea Sea 18/ESI(+) | 641.513 M+H]+ | [53,54,55] |
| 16 | Oleanolic acid glucuronide A | 26.40 | Phenomenex C18 Luna/ESI(−) | 1117.57 [M-H]− 793 [M-H-2Hex]− | [52,60,63] |
| 17 | Oleanolic acid glucuronide A isomer | 29.39 | Phenomenex C18 Luna/ESI(−) | 1117.56 [M-H]− 581.29 [M-H-2Hex-HexUA]− | [52,63,64] |
| 18 | Oleanolic acid glucuronide C | 30.28 | Phenomenex C18 Luna/ESI(−) | 955.50 [M-H]− 500.26 [M-OA]− | [52,63] |
| 19 | Oleanolic acid glucuronide D | 30.75 | Phenomenex C18 Luna/ESI(−) | 793.46 [M-H]− 569 [M-H-Hex-H2O-CO2]− | [52] |
| TPC (mg GAE/g) | TFC (mg QE/g) | TTPC (mg OAE/g) | DPPH, IC50 (μg/mL) | ||
|---|---|---|---|---|---|
| C. officinalis Extract | Ascorbic Acid | Quercetin | |||
| 15.03 ± 0.45 | 79.42 ± 2.92 | 53.58 ± 0.47 | 122.67 ± 4.5 | 3.1 ± 0.12 | 2.3 ± 0.14 |
| Sample Code | Initial Mixture | Final Composition | |||||
|---|---|---|---|---|---|---|---|
| P/PVA Ratio (%, w/w) | STMP/(P + PVA) Ratio (w/w) | NaOH/(P + PVA) Ratio (w/w) | GF * (%, w/w) | P (%, w/w) | PVA (%, w/w) | PO42− Content ** (meq./g) | |
| H #0 | 25/75 | 0.125/1 | 0.1/1 | 65.44 ± 3.9 | 36.08 ± 2.45 | 63.92 ± 2.45 | 0.74 ± 0.06 |
| Sample Code | C. officinalis Conc. (%, w/v) | Gravimetric Ratio Extract/H (w/w) | LC (mg C. officinalis/g H) | TPC (mg GAE/g H) | TTPC (mg OAE/g H) |
|---|---|---|---|---|---|
| H #1 | 5 | 1/1 | 211.4 ± 8.9 | 10.02 ± 0.73 | 9.98 ± 0.78 |
| H #2 | 10 | 334.4 ± 4.6 | 14.36 ± 1.09 | 12.18 ± 1.07 | |
| H #3 | 20 | 736.7 ± 2.1 | 24.33 ± 1.04 | 22.87 ± 1.11 |
| Sample Code | Young’s Modulus (KPa) | Compressive Strength (KPa) | Maximal Adhesive Force Fmax (N) |
|---|---|---|---|
| H #0 | 42.53 ± 5.22 | 61.67 ± 6.47 | 0.37 ± 0.04 |
| H #1 | 78.87 ± 3.40 b | 81.75 ± 3.44 a | 0.38 ± 0.04 |
| H #2 | 69.06 ± 1.13 b | 76.25 ± 2.97 | 0.43 ± 0.02 a |
| H #3 | 47.56 ± 4.80 | 66.33 ± 3.60 | 0.56 ± 0.03 |
| Mean Value and Standard Deviation of the Inhibition Zones (mm) * | |||||
|---|---|---|---|---|---|
| Species | C (+) | C (−) | H #1 | H #2 | H #3 |
| C. officinalis Extract Content (mg) | |||||
| 0 | 2.1 | 3.9 | 10.5 | ||
| S. aureus ATCC25923 | 22 | 5 | 11 ± 0.3 | 12 ± 0.25 | 13 ± 0.35 |
| Escherichia coli ATCC 25922 | 24 | 5 | 11.5 ± 0.1 | 11.6 ± 0.4 | 12 ± 0.5 |
| P. aeruginosa 9027 ATCC | 22 | 5 | 5 ± 0.1 | 5 ± 0.2 | 15 ± 0.1 |
| C. albicans ATCC 90028 | 16 | 5 | 6 ± 0.25 | 7 ± 0.3 | 8 ± 0.4 |
| Time (h) | Sample | Log Reduction/% Reduction | ||||
|---|---|---|---|---|---|---|
| Species | S. aureus | E. coli | P. aeruginosa | C. albicans | ||
| 24 | H #1 | 4.87/100 | 4.87/100 | 2.96/99.89 | 3.92/99.98 | |
| H #2 | 4.87/100 | 4.87/100 | 3.20/99.94 | 4.87/100 | ||
| H #3 | 4.87/100 | 4.87/100 | 4.87/100 | 4.87/100 | ||
| 72 | H #1 | 4.87/100 | 2.17/99.33 | 2.39/99.59 | 2.95/99.88 | |
| H #2 | 4.57/99.99 | 2.37/99.58 | 2.53/99.70 | 2.98/99.89 | ||
| H #3 | 4.57/99.99 | 4.57/99.99 | 4.57/99.99 | 4.87/100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelin, I.M.; Silion, M.; Popescu, I.; Rîmbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/Poly(vinyl alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications. Pharmaceutics 2023, 15, 1674. https://doi.org/10.3390/pharmaceutics15061674
Pelin IM, Silion M, Popescu I, Rîmbu CM, Fundueanu G, Constantin M. Pullulan/Poly(vinyl alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications. Pharmaceutics. 2023; 15(6):1674. https://doi.org/10.3390/pharmaceutics15061674
Chicago/Turabian StylePelin, Irina Mihaela, Mihaela Silion, Irina Popescu, Cristina Mihaela Rîmbu, Gheorghe Fundueanu, and Marieta Constantin. 2023. "Pullulan/Poly(vinyl alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications" Pharmaceutics 15, no. 6: 1674. https://doi.org/10.3390/pharmaceutics15061674
APA StylePelin, I. M., Silion, M., Popescu, I., Rîmbu, C. M., Fundueanu, G., & Constantin, M. (2023). Pullulan/Poly(vinyl alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications. Pharmaceutics, 15(6), 1674. https://doi.org/10.3390/pharmaceutics15061674







