Doxorubicin Activity Is Modulated by Traditional Herbal Extracts in a 2D and 3D Multicellular Sphere Model of Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
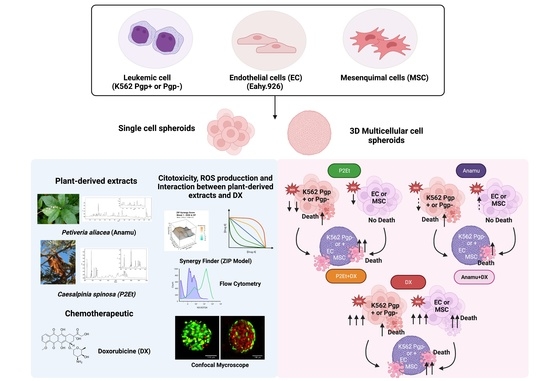
2.2. Culture Conditions of Leukemic Cells and Endothelial Cells
2.3. Isolation and Cultivation of MSC from Human Bone Marrow
2.4. In Vitro Cytotoxicity Assays
2.5. Determination of Intracellular Levels of ROS in Cell Populations Treated with Plant Extracts P2Et and Anamú-SC
2.6. Evaluation of the Synergistic, Additive or Antagonistic Effect of Plant Extracts with Doxorubicin in Leukemia Cells
2.7. Generation of Cell Spheroids
2.8. Cytotoxicity of Plant Extracts P2Et and Anamú-SC SC on 3D Cultures (Multicellular and Single Cell-Type Spheroids)
2.9. Statistical Analysis
3. Results
3.1. P2Et and Anamú-SC Extracts Have a Selective Cytotoxic Effect on Leukemic Cells Compared to Doxorubicin on 2D and 3D Culture
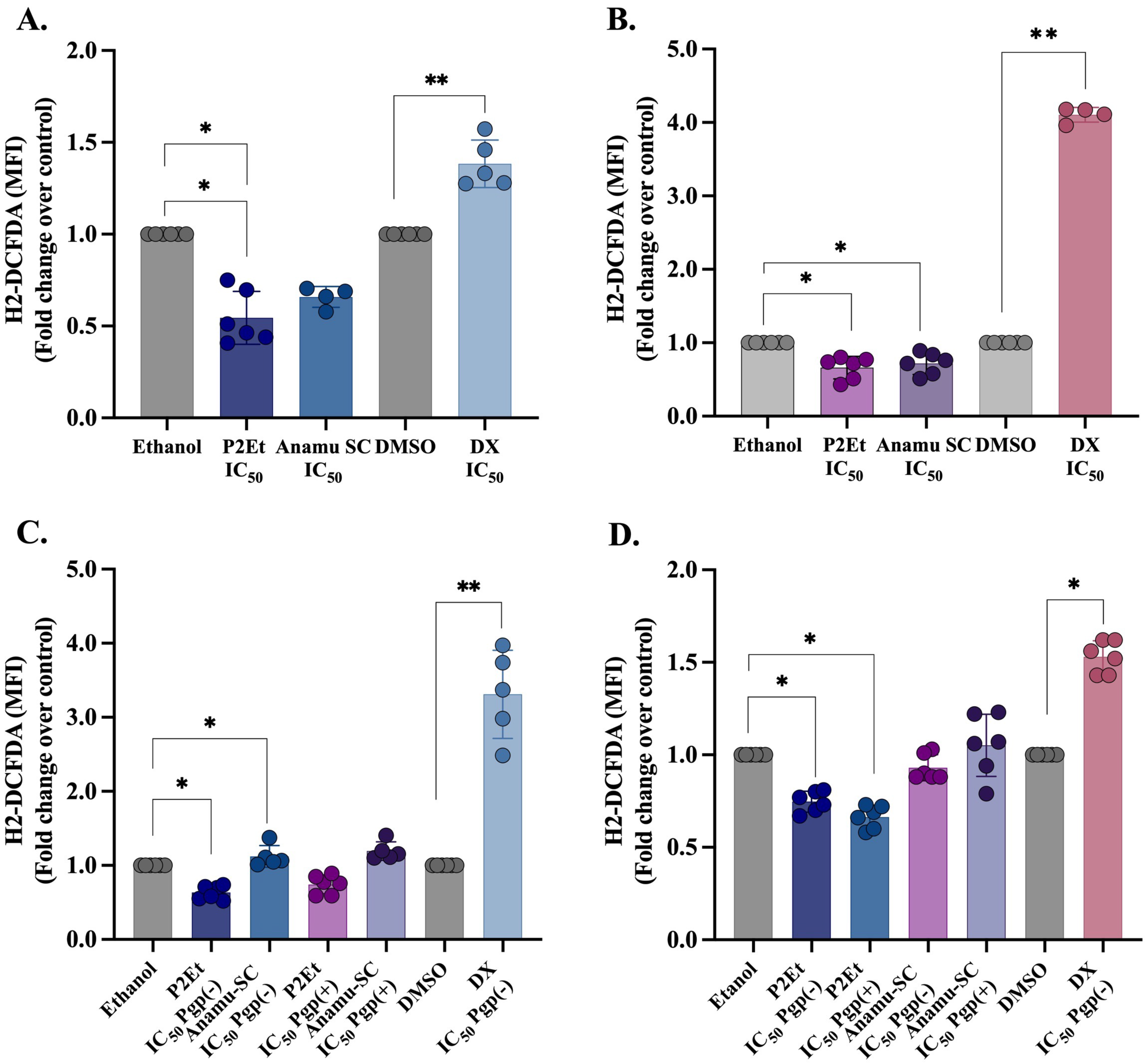
3.2. Cytotoxicity Induced by P2Et and Anamu-SC Extracts Is Not Related to a Pro-Oxidant Effect
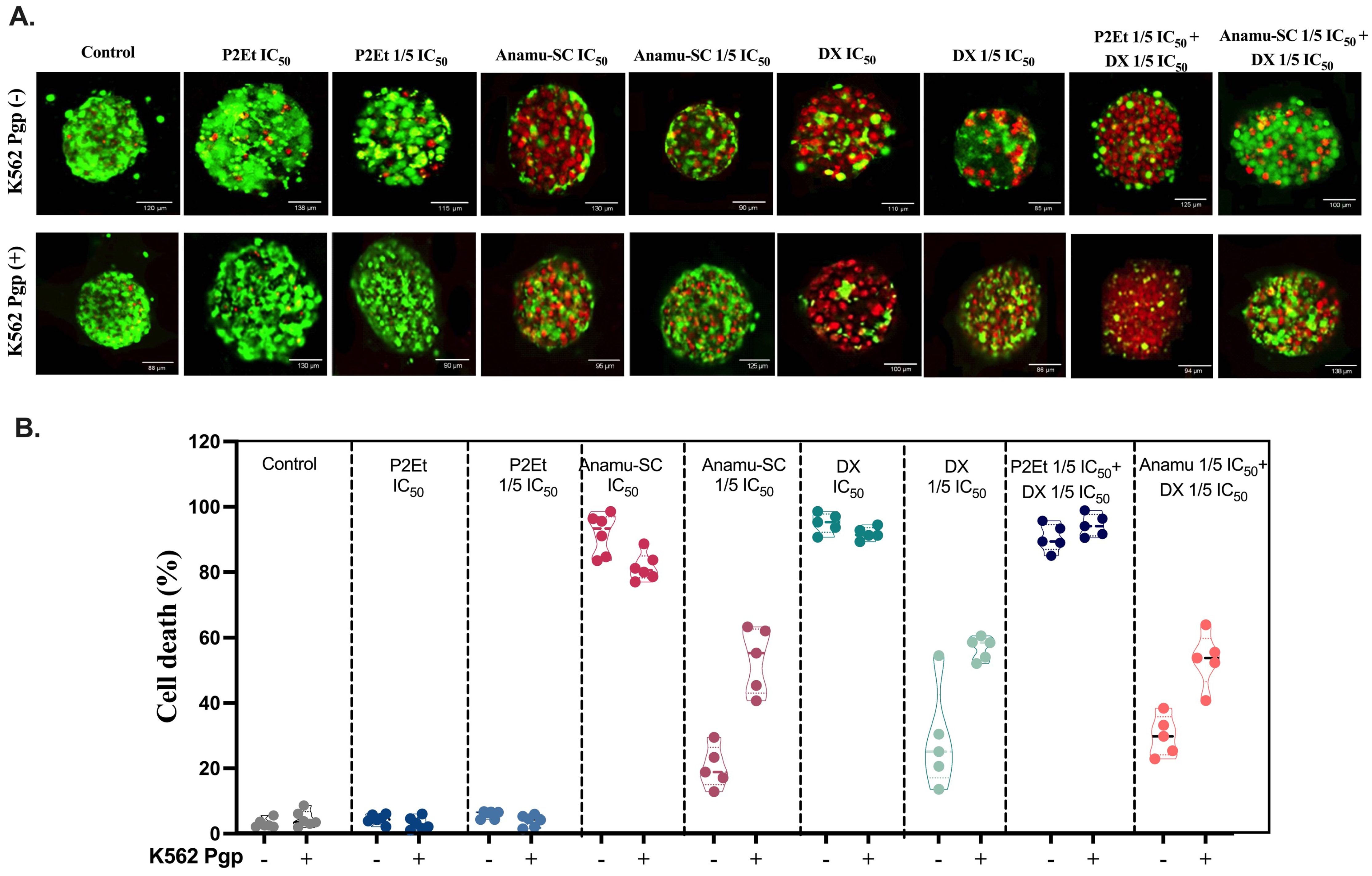
3.3. Natural Extracts’ Interaction with DX May Depend on Chemical Complexity and Pgp Expression
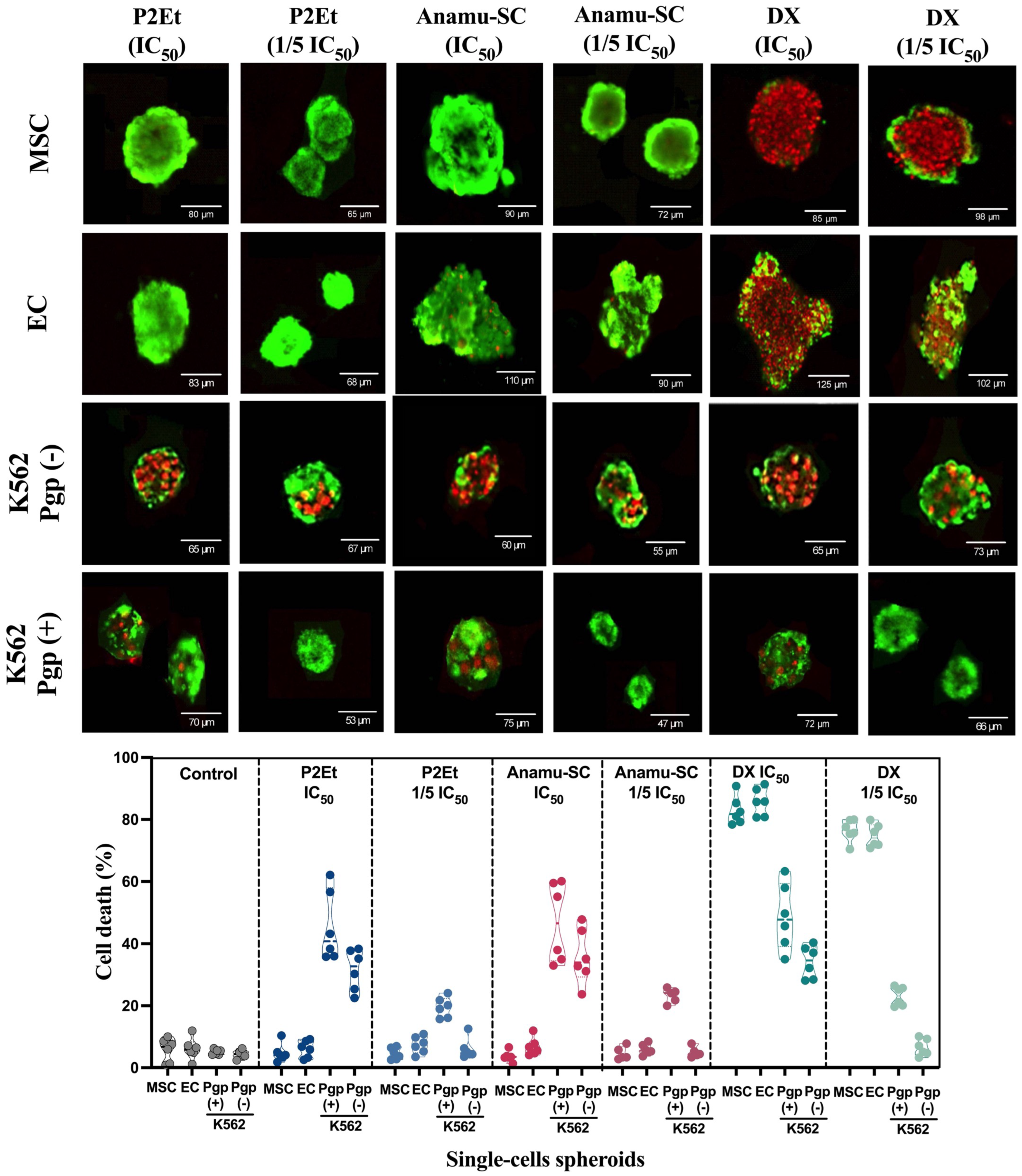
3.4. Cytotoxic Effect of P2Et and Anamú-SC Extracts Is Modified in Multicellular Spheroid, Favoring Selectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Röllig, C.; Bornhäuser, M.; Thiede, C.; Taube, F.; Kramer, M.; Mohr, B.; Aulitzky, W.; Bodenstein, H.; Tischler, H.-J.; Stuhlmann, R.; et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: Evaluation of the proposed reporting system. J. Clin. Oncol. 2011, 29, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Irving, J.A.E.; Enshaei, A.; Parker, C.A.; Sutton, R.; Kuiper, R.P.; Erhorn, A.; Minto, L.; Venn, N.C.; Law, T.; Yu, J.; et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood 2016, 128, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K. Etiology of Acute Leukemia: A Review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Galán-Díez, M.; Cuesta-Domínguez, Á.; Kousteni, S. The Bone Marrow Microenvironment in Health and Myeloid Malignancy. Cold Spring Harb. Perspect. Med. 2018, 8, a031328. [Google Scholar] [CrossRef]
- Boutin, L.; Arnautou, P.; Trignol, A.; Ségot, A.; Farge, T.; Desterke, C.; Soave, S.; Clay, D.; Raffoux, E.; Sarry, J.-E.; et al. Mesenchymal stromal cells confer chemoresistance to myeloid leukemia blasts through Side Population functionality and ABC transporter activation. Haematologica 2020, 105, 987–9998. [Google Scholar] [CrossRef]
- Forte, D.; García-Fernández, M.; Sánchez-Aguilera, A.; Stavropoulou, V.; Fielding, C.; Martín-Pérez, D.; López, J.A.; Costa, A.S.H.; Tronci, L.; Nikitopoulou, E.; et al. Bone Marrow Mesenchymal Stem Cells Support Acute Myeloid Leukemia Bioenergetics and Enhance Antioxidant Defense and Escape from Chemotherapy. Cell Metab. 2020, 32, 829–843.e9. [Google Scholar] [CrossRef]
- Pezeshkian, B.; Donnelly, C.; Tamburo, K.; Geddes, T.; Madlambayan, G.J. Leukemia Mediated Endothelial Cell Activation Modulates Leukemia Cell Susceptibility to Chemotherapy through a Positive Feedback Loop Mechanism. PLoS ONE 2013, 8, e60823. [Google Scholar] [CrossRef]
- Jacamo, R.; Chen, Y.; Wang, Z.; Ma, W.; Zhang, M.; Spaeth, E.L.; Wang, Y.; Battula, V.L.; Mak, P.Y.; Schallmoser, K.; et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-κB mediates chemoresistance. Blood 2014, 123, 2691–2702. [Google Scholar] [CrossRef]
- Takam Kamga, P.; Bassi, G.; Cassaro, A.; Midolo, M.; Di Trapani, M.; Gatti, A.; Carusone, R.; Resci, F.; Perbellini, O.; Gottardi, M.; et al. Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget 2016, 7, 21713–21727. [Google Scholar] [CrossRef]
- Purroy, N.; Abrisqueta, P.; Carabia, J.; Carpio, C.; Palacio, C.; Bosch, F.; Crespo, M. Co-culture of primary CLL cells with bone marrow mesenchymal cells, CD40 ligand and CpG ODN promotes proliferation of chemoresistant CLL cells phenotypically comparable to those proliferating in vivo. Oncotarget 2015, 6, 7632–7643. [Google Scholar] [CrossRef]
- Burt, R.; Dey, A.; Aref, S.; Aguiar, M.; Akarca, A.; Bailey, K.; Day, W.; Hooper, S.; Kirkwood, A.; Kirschner, K.; et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 2019, 134, 1415–1429. [Google Scholar] [CrossRef]
- Li, C.; Cheung, M.K.H.; Han, S.; Zhang, Z.; Chen, L.; Chen, J.; Zeng, H.; Qiu, J. Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci. Rep. 2019, 39, BSR20182417. [Google Scholar] [CrossRef]
- Passaro, D.; Di Tullio, A.; Abarrategi, A.; Rouault-Pierre, K.; Foster, K.; Ariza-McNaughton, L.; Montaner, B.; Chakravarty, P.; Bhaw, L.; Diana, G.; et al. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell 2017, 32, 324–341.e6. [Google Scholar] [CrossRef]
- Wilde, L.; Roche, M.; Domingo-Vidal, M.; Tanson, K.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin. Oncol. 2017, 44, 198–203. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Hou, D.; Huang, Q.; Zhan, W.; Chen, C.; Liu, J.; You, R.; Xie, J.; Chen, P.; et al. Exosomes derived from acute myeloid leukemia cells promote chemoresistance by enhancing glycolysis-mediated vascular remodeling. J. Cell. Physiol. 2019, 234, 10602–10614. [Google Scholar] [CrossRef]
- Rashidi, A.; Uy, G.L. Targeting the microenvironment in acute myeloid leukemia. Curr. Hematol. Malig. Rep. 2015, 10, 126–131. [Google Scholar] [CrossRef]
- Kumar, A.; Anand, T.; Bhattacharyya, J.; Sharma, A.; Jaganathan, B.G. K562 chronic myeloid leukemia cells modify osteogenic differentiation and gene expression of bone marrow stromal cells. J. Cell Commun. Signal. 2018, 12, 441–450. [Google Scholar] [CrossRef]
- Azadniv, M.; Myers, J.R.; McMurray, H.R.; Guo, N.; Rock, P.; Coppage, M.L.; Ashton, J.; Becker, M.W.; Calvi, L.M.; Liesveld, J.L. Bone marrow mesenchymal stromal cells from acute myelogenous leukemia patients demonstrate adipogenic differentiation propensity with implications for leukemia cell support. Leukemia 2020, 34, 391–403. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Q.; Cang, H.; Wang, Z.; Hu, X.; Pan, R.; Yang, Y.; Chen, Y. Acute Myeloid Leukemia Cells Educate Mesenchymal Stromal Cells toward an Adipogenic Differentiation Propensity with Leukemia Promotion Capabilities. Adv. Sci. 2022, 9, 2105811. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef]
- Krevvata, M.; Silva, B.C.; Manavalan, J.S.; Galan-Diez, M.; Kode, A.; Matthews, B.G.; Park, D.; Zhang, C.A.; Galili, N.; Nickolas, T.L.; et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 2014, 124, 2834–2846. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, F.; Cumbo, C.; Anelli, L.; Zagaria, A.; Specchia, G.; Musto, P.; Albano, F. Can the New and Old Drugs Exert an Immunomodulatory Effect in Acute Myeloid Leukemia? Cancers 2021, 13, 4121. [Google Scholar] [CrossRef] [PubMed]
- Egen, J.G.; Ouyang, W.; Wu, L.C. Human Anti-tumor Immunity: Insights from Immunotherapy Clinical Trials. Immunity 2020, 52, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-T.; Yang, C.-C.; Shyur, L.-F. Phytomedicine—Modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol. Res. 2016, 114, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yin, S.; Lu, J.; Zhou, S.; Shao, Y.; Bao, X.; Wang, T.; Qiu, Y.; Yu, H. Tumor microenvironment: A prospective target of natural alkaloids for cancer treatment. Cancer Cell Int. 2021, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines 2019, 6, 6. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, M.; Park, H.; Jeong, M.I.; Jung, W.; Kim, B. Natural Products and Acute Myeloid Leukemia: A Review Highlighting Mechanisms of Action. Nutrients 2019, 11, 1010. [Google Scholar] [CrossRef]
- Cheon, C. Synergistic effects of herbal medicines and anticancer drugs: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e27918. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, J.; Zhao, C.; Lu, S.; Qiao, J.; Han, M. The combinatory effects of natural products and chemotherapy drugs and their mechanisms in breast cancer treatment. Phytochem. Rev. 2020, 19, 1179–1197. [Google Scholar] [CrossRef]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef]
- Hernández, J.F.; Urueña, C.P.; Cifuentes, M.C.; Sandoval, T.A.; Pombo, L.M.; Castañeda, D.; Asea, A.; Fiorentino, S. A Petiveria alliacea standardized fraction induces breast adenocarcinoma cell death by modulating glycolytic metabolism. J. Ethnopharmacol. 2014, 153, 641–649. [Google Scholar] [CrossRef]
- Hernández, J.F.; Urueña, C.P.; Sandoval, T.A.; Cifuentes, M.C.; Formentini, L.; Cuezva, J.M.; Fiorentino, S. A cytotoxic Petiveria alliacea dry extract induces ATP depletion and decreases β-F1-ATPase expression in breast cancer cells and promotes survival in tumor-bearing mice. Rev. Bras. Farmacogn. 2017, 27, 306–314. [Google Scholar] [CrossRef]
- Sandoval, T.A.; Urueña, C.P.; Llano, M.; Gómez-Cadena, A.; Hernández, J.F.; Sequeda, L.G.; Loaiza, A.E.; Barreto, A.; Li, S.; Fiorentino, S. Standardized Extract from Caesalpinia spinosa is Cytotoxic Over Cancer Stem Cells and Enhance Anticancer Activity of Doxorubicin. Am. J. Chin. Med. 2016, 44, 1693–1717. [Google Scholar] [CrossRef]
- Ballesteros-Ramírez, R.; Aldana, E.; Herrera, M.V.; Urueña, C.; Rojas, L.Y.; Echeverri, L.F.; Costa, G.M.; Quijano, S.; Fiorentino, S. Preferential Activity of Petiveria alliacea Extract on Primary Myeloid Leukemic Blast. Evid.-Based Complement. Altern. Med. 2020, 2020, 4736206. [Google Scholar] [CrossRef]
- Castañeda, D.M.; Pombo, L.M.; Urueña, C.P.; Hernandez, J.F.; Fiorentino, S. A gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line. BMC Complement. Altern. Med. 2012, 12, 38. [Google Scholar] [CrossRef]
- Cifuentes, M.C.; Castañeda, D.M.; Urueña, C.P.; Fiorentino, S. A fraction from Petiveria alliacea induces apoptosis via a mitochondria-dependent pathway and regulates HSP70 expression. Univ. Sci. 2009, 14, 125–134. [Google Scholar] [CrossRef]
- Phan, N.L.-C.; Pham, K.D.; Nguyen, M.T.-T.; Phan, N.K.; Truong, K.D.; Van Pham, P. Anti-tumor activity of plant extracts against human breast cancer cells are different in monolayer and three-dimensional cell culture screening models: A comparison on 34 extracts. Biomed. Res. Ther. 2020, 7, 3667–3677. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Urueña, C.; Sandoval, T.A.; Lasso, P.; Tawil, M.; Barreto, A.; Torregrosa, L.; Fiorentino, S. Evaluation of chemotherapy and P2Et extract combination in ex-vivo derived tumor mammospheres from breast cancer patients. Sci. Rep. 2020, 10, 19639. [Google Scholar] [CrossRef]
- Prieto, K.; Lozano, M.P.; Urueña, C.; Alméciga-Díaz, C.J.; Fiorentino, S.; Barreto, A. The delay in cell death caused by the induction of autophagy by P2Et extract is essential for the generation of immunogenic signals in melanoma cells. Apoptosis 2020, 25, 875–888. [Google Scholar] [CrossRef]
- Prieto, K.; Cao, Y.; Mohamed, E.; Trillo-Tinoco, J.; Sierra, R.A.; Urueña, C.; Sandoval, T.A.; Fiorentino, S.; Rodriguez, P.C.; Barreto, A. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. 2019, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cadena, A.; Urueña, C.; Prieto, K.; Martinez-Usatorre, A.; Donda, A.; Barreto, A.; Romero, P.; Fiorentino, S. Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell Death Dis. 2016, 7, e2243. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Duran, E.; Mejia-Cruz, C.C.; Jaramillo-Garcia, L.F.; Leal-Garcia, E.; Barreto-Prieto, A.; Rodriguez-Pardo, V.M. 3D Multicellular Spheroid for the Study of Human Hematopoietic Stem Cells: Synergistic Effect Between Oxygen Levels, Mesenchymal Stromal Cells and Endothelial Cells. J. Blood Med. 2021, 12, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Cruz, C.C.; Barreto-Durán, E.; Pardo-Pérez, M.A.; Jimenez, M.C.; Rincón, J.; Vanegas, K.; Rodríguez, J.L.; Jaramillo-Garcia, L.F.; Ulloa, J.C.; Díaz, R.M.; et al. Generation of Organotypic Multicellular Spheres by Magnetic Levitation: Model for the Study of Human Hematopoietic Stem Cells Microenvironment. Int. J. Stem Cells 2019, 12, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Urueña, C.; Ballesteros-Ramírez, R.; Gomez-Cadena, A.; Barreto, A.; Prieto, K.; Quijano, S.; Aschner, P.; Martínez, C.; Zapata-Cardona, M.I.; El-Ahanidi, H.; et al. Randomized double-blind clinical study in patients with COVID-19 to evaluate the safety and efficacy of a phytomedicine (P2Et). Front. Med. 2022, 9, 991873. [Google Scholar] [CrossRef]
- Duran, M.I.; Ballesteros-Ramírez, R.; Tellez, A.; Torregrosa, L.; Olejua, P.A.; Galvis, S.; Urueña, C.; Fiorentino, S. Safety Evaluation in Healthy Colombian Volunteers of P2Et Extract Obtained from Caesalpinia spinosa: Design 3+3 Phase I Clinical Trial. Evid.-Based Complement. Altern. Med. 2022, 2022, 7943001. [Google Scholar] [CrossRef]
- Barreto-Durán, E.; Mejía-Cruz, C.C.; Leal-García, E.; Pérez-Núñez, R.; Rodríguez-Pardo, V.M. Impact of donor characteristics on the quality of bone marrow as a source of mesenchymal stromal cells. Am. J. Stem Cells 2018, 7, 114–120. [Google Scholar]
- Rodríguez-Pardo, V.M.; Aristizabal, J.A.; Jaimes, D.; Quijano, S.M.; de los Reyes, I.; Herrera, M.V.; Solano, J.; Vernot, J.P. Mesenchymal stem cells promote leukaemic cells aberrant phenotype from B-cell acute lymphoblastic leukaemia. Hematol./Oncol. Stem Cell Ther. 2013, 6, 89–100. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine 2012, 19, 1288–1297. [Google Scholar] [CrossRef]
- Urueña, C.; Cifuentes, C.; Castañeda, D.; Arango, A.; Kaur, P.; Asea, A.; Fiorentino, S. Petiveria alliacea extracts uses multiple mechanisms to inhibit growth of human and mouse tumoral cells. BMC Complement. Altern. Med. 2008, 8, 60. [Google Scholar] [CrossRef]
- Urueña, C.; Mancipe, J.; Hernandez, J.; Castañeda, D.; Pombo, L.; Gomez, A.; Asea, A.; Fiorentino, S. Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model. BMC Complement. Altern. Med. 2013, 13, 74. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef]
- Seebacher, N.; Lane, D.J.; Richardson, D.R.; Jansson, P.J. Turning the gun on cancer: Utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance. Free. Radic. Biol. Med. 2016, 96, 432–445. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; Cross, N.A.; Jordan-Mahy, N. Polyphenols act synergistically with doxorubicin and etoposide in leukaemia cell lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postep. Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, W.; Wu, J.; Zhu, Y. The synergistic reversal effect of multidrug resistance by quercetin and hyperthermia in doxorubicin-resistant human myelogenous leukemia cells. Int. J. Hyperth. 2008, 24, 151–159. [Google Scholar] [CrossRef]
- Wauchope, S.; Roy, M.A.; Irvine, W.; Morrison, I.; Brantley, E.; Gossell-Williams, M.; Timme-Laragy, A.R.; Delgoda, R. Dibenzyl trisulfide binds to and competitively inhibits the cytochrome P450 1A1 active site without impacting the expression of the aryl hydrocarbon receptor. Toxicol. Appl. Pharmacol. 2021, 419, 115502. [Google Scholar] [CrossRef]
- Murray, J.; Picking, D.; Lamm, A.; McKenzie, J.; Hartley, S.; Watson, C.; Williams, L.; Lowe, H.; Delgoda, R. Significant inhibitory impact of dibenzyl trisulfide and extracts of Petiveria alliacea on the activities of major drug-metabolizing enzymes in vitro: An assessment of the potential for medicinal plant-drug interactions. Fitoterapia 2016, 111, 138–146. [Google Scholar] [CrossRef]
- Caru, M.; Corbin, D.; Périé, D.; Lemay, V.; Delfrate, J.; Drouin, S.; Bertout, L.; Krajinovic, M.; Laverdière, C.; Andelfinger, G.; et al. Doxorubicin treatments induce significant changes on the cardiac autonomic nervous system in childhood acute lymphoblastic leukemia long-term survivors. Clin. Res. Cardiol. 2019, 108, 1000–1008. [Google Scholar] [CrossRef]
- Efferth, T.; Saeed, M.E.M.; Kadioglu, O.; Seo, E.-J.; Shirooie, S.; Mbaveng, A.T.; Nabavi, S.M.; Kuete, V. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol. Adv. 2020, 38, 107342. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Nichol, D.; Kinose, F.; Abazeed, M.E.; Marusyk, A.; Haura, E.B.; Scott, J.G. Collateral sensitivity networks reveal evolutionary instability and novel treatment strategies in ALK mutated non-small cell lung cancer. Sci. Rep. 2017, 7, 1232. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Saeed, M.E.M.; Mirghani, E.; Alim, A.; Yassin, Z.; Saeed, E.; Khalid, H.E.; Daak, S. Integration of phytochemicals and phytotherapy into cancer precision medicine. Oncotarget 2017, 8, 50284–50304. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Augustine, D.; Rao, R.S.; Sowmya, S.V.; Haragannavar, V.C.; Nambiar, S.; Prasad, K.; Awan, K.H.; Patil, S. Naturally Available Extracts Inhibiting Cancer Progression: A Systematic Review. J. Evid.-Based Complement. Altern. Med. 2017, 22, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Kim, S.-A.; Lee, E.K.; Kuh, H.-J. Co-culture of 3D tumor spheroids with fibroblasts as a model for epithelial–mesenchymal transition in vitro. Exp. Cell Res. 2015, 335, 187–196. [Google Scholar] [CrossRef]
- Fonseca-Benitez, A.; Morantes Medina, S.J.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Perdomo, S.J. Passiflora mollissima Seed Extract Induced Antiproliferative and Cytotoxic Effects on CAL 27 Spheroids. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 4602413. [Google Scholar] [CrossRef]
- Rubert, J.; Gatto, P.; Pancher, M.; Sidarovich, V.; Curti, C.; Mena, P.; Del Rio, D.; Quattrone, A.; Mattivi, F. A Screening of Native (Poly)phenols and Gut-Related Metabolites on 3D HCT116 Spheroids Reveals Gut Health Benefits of a Flavan-3-ol Metabolite. Mol. Nutr. Food Res. 2022, 66, e2101043. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S.K. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci. Rep. 2018, 8, 15787. [Google Scholar] [CrossRef]
| Cell Line | Doxorubicin (DX) (μM) | P2Et (μg/mL) | Anamu-SC (μg/mL) |
|---|---|---|---|
| MSC | 1.53 ± 0.128 | 388.81 ± 9.46 | >500 ± 10.1 |
| EC | 0.34 ± 0.101 | 175.0 ± 4.33 | 105.5 ± 11.8 |
| K562 Pgp (−) | 0.56 ± 0.018 | 81.28 ± 12.2 | 56.3 ± 8.37 |
| K562 Pgp (+) | >10 ± 9.53 | 114.9 ± 5.88 | 158 ± 2.53 |
| Cell Line | Combination | ZIP Score | Interpretation |
|---|---|---|---|
| K562 Pgp(−) | P2Et + DX | −8.94 ± 0.51 | Addition |
| Anamu-SC + DX | −14.88 ± 1.46 | Antagonism | |
| NAC + DX | −14.69 ± 0.69 | Antagonism | |
| K562 Pgp (+) | P2Et + DX | 0.21 ± 0.83 | Addition |
| Anamu-SC + DX | −5.72 ± 1.90 | Addition | |
| NAC + DX | −17.43 ± 1.45 | Antagonism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corzo Prada, L.; Urueña, C.; Leal-García, E.; Barreto, A.; Ballesteros-Ramírez, R.; Rodríguez-Pardo, V.; Fiorentino, S. Doxorubicin Activity Is Modulated by Traditional Herbal Extracts in a 2D and 3D Multicellular Sphere Model of Leukemia. Pharmaceutics 2023, 15, 1690. https://doi.org/10.3390/pharmaceutics15061690
Corzo Prada L, Urueña C, Leal-García E, Barreto A, Ballesteros-Ramírez R, Rodríguez-Pardo V, Fiorentino S. Doxorubicin Activity Is Modulated by Traditional Herbal Extracts in a 2D and 3D Multicellular Sphere Model of Leukemia. Pharmaceutics. 2023; 15(6):1690. https://doi.org/10.3390/pharmaceutics15061690
Chicago/Turabian StyleCorzo Prada, Laura, Claudia Urueña, Efraín Leal-García, Alfonso Barreto, Ricardo Ballesteros-Ramírez, Viviana Rodríguez-Pardo, and Susana Fiorentino. 2023. "Doxorubicin Activity Is Modulated by Traditional Herbal Extracts in a 2D and 3D Multicellular Sphere Model of Leukemia" Pharmaceutics 15, no. 6: 1690. https://doi.org/10.3390/pharmaceutics15061690
APA StyleCorzo Prada, L., Urueña, C., Leal-García, E., Barreto, A., Ballesteros-Ramírez, R., Rodríguez-Pardo, V., & Fiorentino, S. (2023). Doxorubicin Activity Is Modulated by Traditional Herbal Extracts in a 2D and 3D Multicellular Sphere Model of Leukemia. Pharmaceutics, 15(6), 1690. https://doi.org/10.3390/pharmaceutics15061690