Systemic Delivery of Magnetogene Nanoparticle Vector for Gene Expression in Hypoxic Tumors
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Plasmids
2.3. In Vitro Studies
2.4. Magnetic Nanoparticles (MNPs) Synthesis and Characterization
2.5. Magnetogene Synthesis and Characterization
2.6. In Vivo Studies
2.7. Statistical Analysis
3. Results
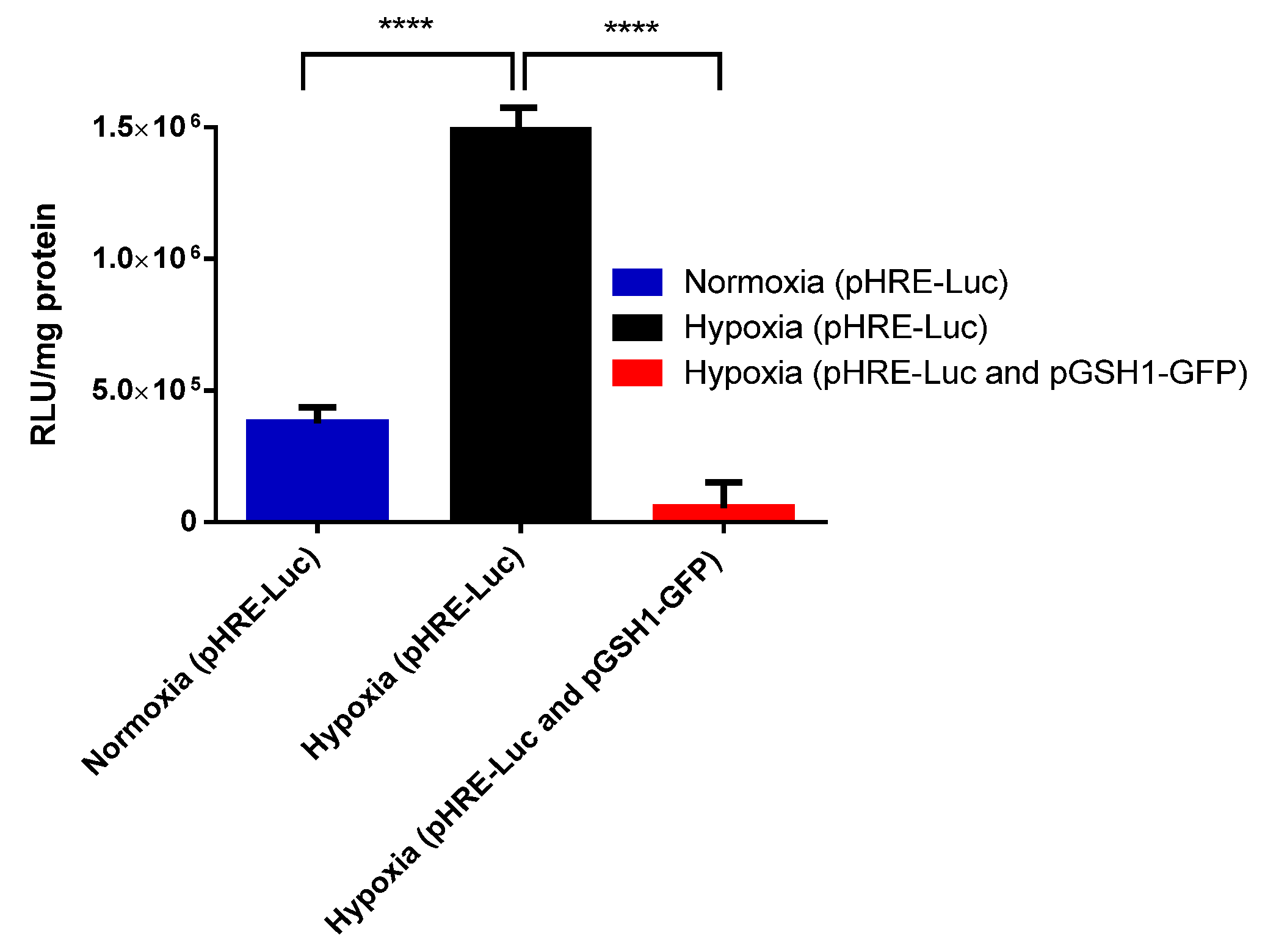
3.1. Selective Hypoxic Expression of pHRE-Luc Vector in B16F10 Cells
3.2. Magnetogene Synthesis and Characterization
3.3. Measurements of Magnetogene Levels in Mice Tissue
4. Discussion
5. Conclusions
- ○
- The major component of the Magnetogene nanoparticle vector (pHRE-Luc) had increased gene expression under hypoxia conditions; this was demonstrated in B16F10 cells.
- ○
- Magnetogene vector nanoparticles with a size of ~60 nm were synthesized.
- ○
- The Magnetogene nanoparticle vector can be targeted and concentrated in tumor cells by applying a magnetic field, since a higher concentration of this vector was obtained in the tumor tissue of mice after intravenous inoculation into the tail of the mice.
- ○
- The Magnetogene nanoparticle vector has a higher selective gene expression in hypoxic tumor cells than in healthy cells and tissues.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Montaño, S.; Bravo, D.; Méndez, O.; Alarcón, E.; Ibáñez, M. Strategies for targeting gene therapy in cancer cells with tumor-specific promoters. Front. Oncol. 2020, 10, 605380. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.F.; Ni, J.Y.; Sun, H.L.; Chen, Y.T.; Wu, Y.D. Effects of hypoxia inducible factor 1 α silencing on the proliferation of CBRH-7919 hepatoma cells. World J. Gastroenterol. 2013, 19, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 7, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, P.; Pan, W.; Singh, S.R.; Wei, Y. Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer Lett. 2015, 356, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Monti, E.; Gariboldi, M.B. HIF-1 as a target for cancer chemotherapy, chemosensitization and chemoprevention. Curr. Mol. Pharmacol. 2011, 4, 62–77. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Arvelo, F.; Cotte, C. Hipoxia en la malignidad del cáncer revisión. Investig. Clin. 2009, 50, 529–546. [Google Scholar]
- Wenger, R.; Stiehl, D.; Camenish, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE. 2005, 306, re12. [Google Scholar] [CrossRef]
- Sun, L.; Hao, Y.; Nie, X.; Zhang, X.; Yang, G.; Wang, Q. Construction of PR39 recombinant AAV under control of the HRE promoter and the effect of recombinant AAV on gene therapy of ischemic heart disease. Exp. Ther. Med. 2012, 4, 811–814. [Google Scholar] [CrossRef]
- Greco, O.; Marples, B.; Dachs, G.; Williams, K.; Patterson Scott, S. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Ther. 2002, 9, 1403–1411. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Kim, J.; Kim, K.; Lee, J.; Jang, S.; Kim, D. A novel chimeric promoter that is highly responsive to hypoxia and metals. Gene Ther. 2006, 13, 857–868. [Google Scholar] [CrossRef]
- Ruan, H.; Hu, L.; Lamborn, K.; Kan, W.; Deen, D. A hypoxia regulated adeno-associated virus vector for cancer specific gene therapy. Nature 2001, 3, 255–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paolicchi, E.; Gemignani, F.; Krstic-Demonacos, M.; Dedhar, S.; Mutti, L.; Landi, S. Targeting hypoxic response for cancer therapy. Oncotarget 2016, 7, 13464–13478. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.; Unni, M.; Rinaldi, C. Magnetic characterization of iron oxide nanoparticles for biomedical applications. Methods Mol. Biol. 2017, 1570, 47–71. [Google Scholar]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape, size and structure controlled synthesis and biocompatibility of iron oxide nanoparticle for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.; Albiss, B.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S.; Fakhari, Z.; Berijani, S. Synthesis and characterization of Fe3O4 magnetic nanoparticles coated with carboxymethyl chitosan grafted sodium methacrylate. J. Nanostruct. 2014, 4, 55–63. [Google Scholar]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An update on potential biomedical and pharmaceutical applications. Marine Drugs. 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Mody, V.; Cox, A.; Shah, S.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Alvizo, C.; Peña, A.; Terrazas, L.; Luna, I.; Uscanga, A.; Sampayo, A.; Tamez, R.; Rodríguez, C.; Alcocer, J. Synergic effect between TRAIL gene and curcumin in magnetic chitosan nanoparticles on cancer cells apoptosis enhanced by laser photoactivation. J. Nanopart. Res. 2022, 24, 165. [Google Scholar] [CrossRef]
- Dobson, J. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Ahmad, T.; Rhee, I.; Chang, Y.; Jin, S.U.; Hong, S. Carbon-coated iron oxide nanoparticles as contrast agents in magnetic resonance imaging. Nanoscale Res. Lett. 2012, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remunan, C.; Vila, J.; Alonso, M. Novel hydrophilic chitosan-plyethylene oxide nanoparticles as protein carriers. J. Appl. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Boticario, C.; Cascales, M. Hipoxia y cáncer. An. R. Acad. Nac. Farm. 2010, 76, 379–408. [Google Scholar]
- Zhou, W.; Dosey, T.; Biechele, T.; Moon, R.; Horwitz, M.; Baker, H. Assessment of hypoxia inducible factor levels in cancer cell lines upon hypoxic induction using a novel reporter construct. PLoS ONE 2011, 6, e27460. [Google Scholar] [CrossRef]
- Shibata, T.; Giaccia, A.; Brown, J. Development of a hypoxia-responsive vector for tumor specific gene therapy. Gene Ther. 2000, 7, 493–498. [Google Scholar] [CrossRef]
- Salatin, S.; Yari, K.A. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef]
- Altankov, G.; Richau, K.; Groth, T. The role of surface zeta potential and substratum chemistry for regulation of dermal fibroblasts interaction. Mater. Wiss. Werkst. Tech. 2003, 34, 1120–1128. [Google Scholar] [CrossRef]
- Terrazas, L.; Luna, I.; Alvizo, C.; Cavazos, A.; Rodríguez, C.; Tamez, R.; Alcocer, J. Microbots Gene Delivery System Based on Bifidobacteria in a Tumor Model. Appl. Sci. 2021, 11, 5544. [Google Scholar] [CrossRef]
- Soheyla, H.; Foruhe, Z. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Prosen, L.; Hudoklin, S.; Cemazar, M.; Stimac, M.; Tratar, U.L.; Ota, M.; Scancar, J.; Romih, R.; Sersa, G. Magnetic field contributes to the cellular uptake for effective therapy with magnetofection using plasmid DNA encoding against Mcam in B16F10 melanoma in vivo. Nanomedicine 2016, 11, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Denny, W. Hypoxia activated prodrugs in cancer therapy: Progress to clinic. Future Oncol. 2010, 6, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Alvizo, C.; Luna, I.; Vilches, N.; Rodríguez, C.; Alcocer, J. Systemic delivery and activation of the TRAIL gene in lungs, with magnetic nanoparticles of chitosan controlled by an external magnetic field. Int. J. Nanomed. 2016, 11, 6449–6458. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrazas-Armendáriz, L.D.; Alvizo-Báez, C.A.; Luna-Cruz, I.E.; Hernández-González, B.A.; Uscanga-Palomeque, A.C.; Ruiz-Robles, M.A.; Pérez Tijerina, E.G.; Rodríguez-Padilla, C.; Tamez-Guerra, R.; Alcocer-González, J.M. Systemic Delivery of Magnetogene Nanoparticle Vector for Gene Expression in Hypoxic Tumors. Pharmaceutics 2023, 15, 2232. https://doi.org/10.3390/pharmaceutics15092232
Terrazas-Armendáriz LD, Alvizo-Báez CA, Luna-Cruz IE, Hernández-González BA, Uscanga-Palomeque AC, Ruiz-Robles MA, Pérez Tijerina EG, Rodríguez-Padilla C, Tamez-Guerra R, Alcocer-González JM. Systemic Delivery of Magnetogene Nanoparticle Vector for Gene Expression in Hypoxic Tumors. Pharmaceutics. 2023; 15(9):2232. https://doi.org/10.3390/pharmaceutics15092232
Chicago/Turabian StyleTerrazas-Armendáriz, Luis Daniel, Cynthia Aracely Alvizo-Báez, Itza Eloisa Luna-Cruz, Becky Annette Hernández-González, Ashanti Concepción Uscanga-Palomeque, Mitchel Abraham Ruiz-Robles, Eduardo Gerardo Pérez Tijerina, Cristina Rodríguez-Padilla, Reyes Tamez-Guerra, and Juan Manuel Alcocer-González. 2023. "Systemic Delivery of Magnetogene Nanoparticle Vector for Gene Expression in Hypoxic Tumors" Pharmaceutics 15, no. 9: 2232. https://doi.org/10.3390/pharmaceutics15092232
APA StyleTerrazas-Armendáriz, L. D., Alvizo-Báez, C. A., Luna-Cruz, I. E., Hernández-González, B. A., Uscanga-Palomeque, A. C., Ruiz-Robles, M. A., Pérez Tijerina, E. G., Rodríguez-Padilla, C., Tamez-Guerra, R., & Alcocer-González, J. M. (2023). Systemic Delivery of Magnetogene Nanoparticle Vector for Gene Expression in Hypoxic Tumors. Pharmaceutics, 15(9), 2232. https://doi.org/10.3390/pharmaceutics15092232






