Influence of ZnO Nanoparticles on the Properties of Ibuprofen-Loaded Alginate-Based Biocomposite Hydrogels with Potential Antimicrobial and Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
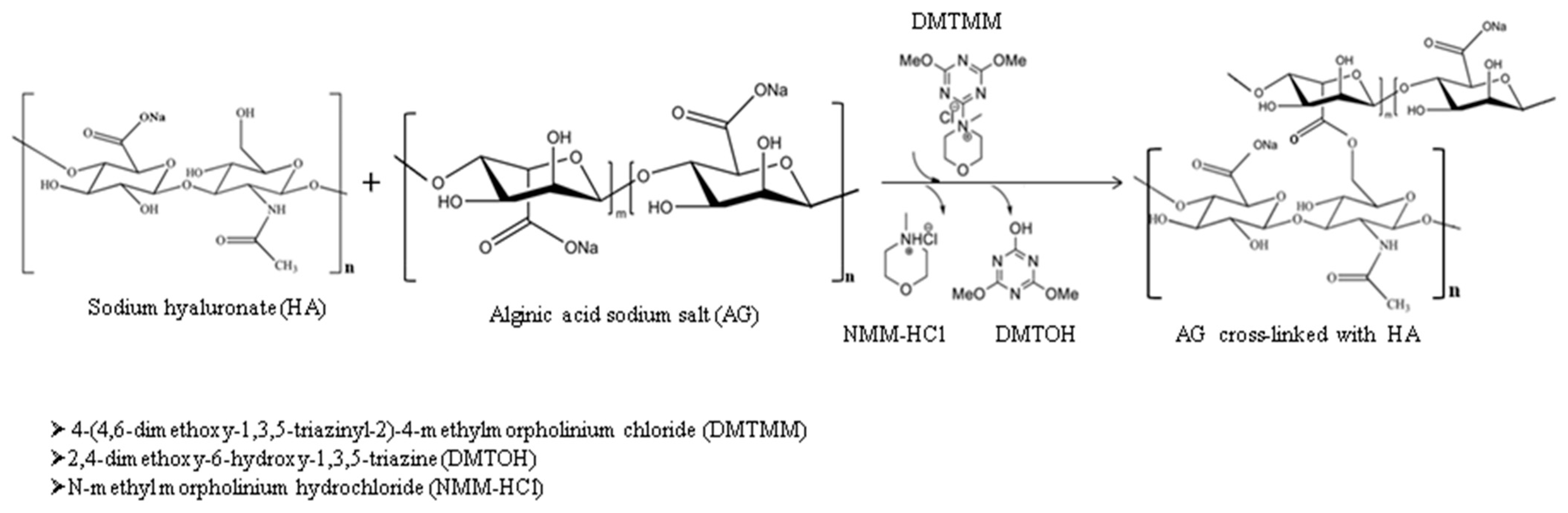
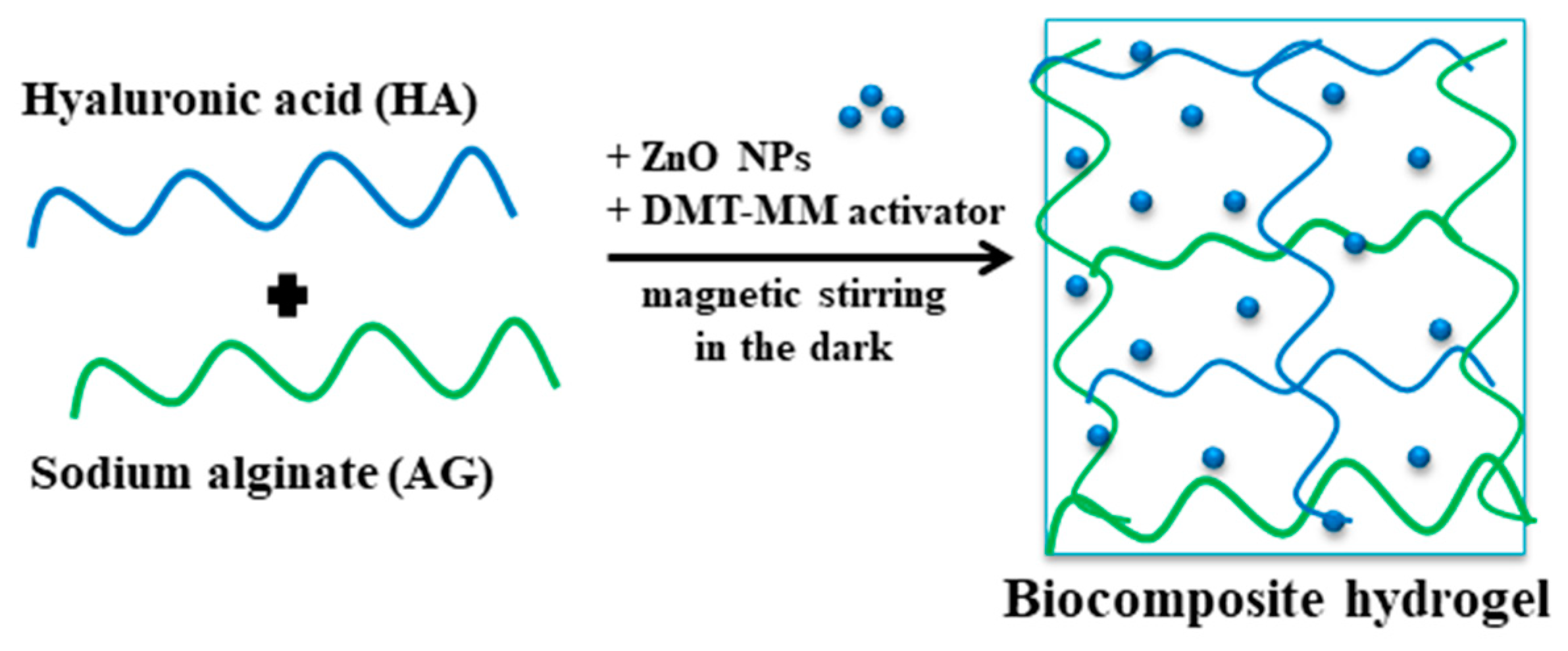
2.2. Biocomposite Hydrogel Preparation
2.3. Characterization
2.3.1. FTIR Spectroscopy
2.3.2. Hydrogel Microscopy
2.3.3. Swelling Behavior
2.3.4. Ibuprofen Release Test
2.3.5. Assessment of the In Vitro Cytotoxicity
2.3.6. Antimicrobial Activity of Biocomposite Hydrogels
3. Results and Discussion
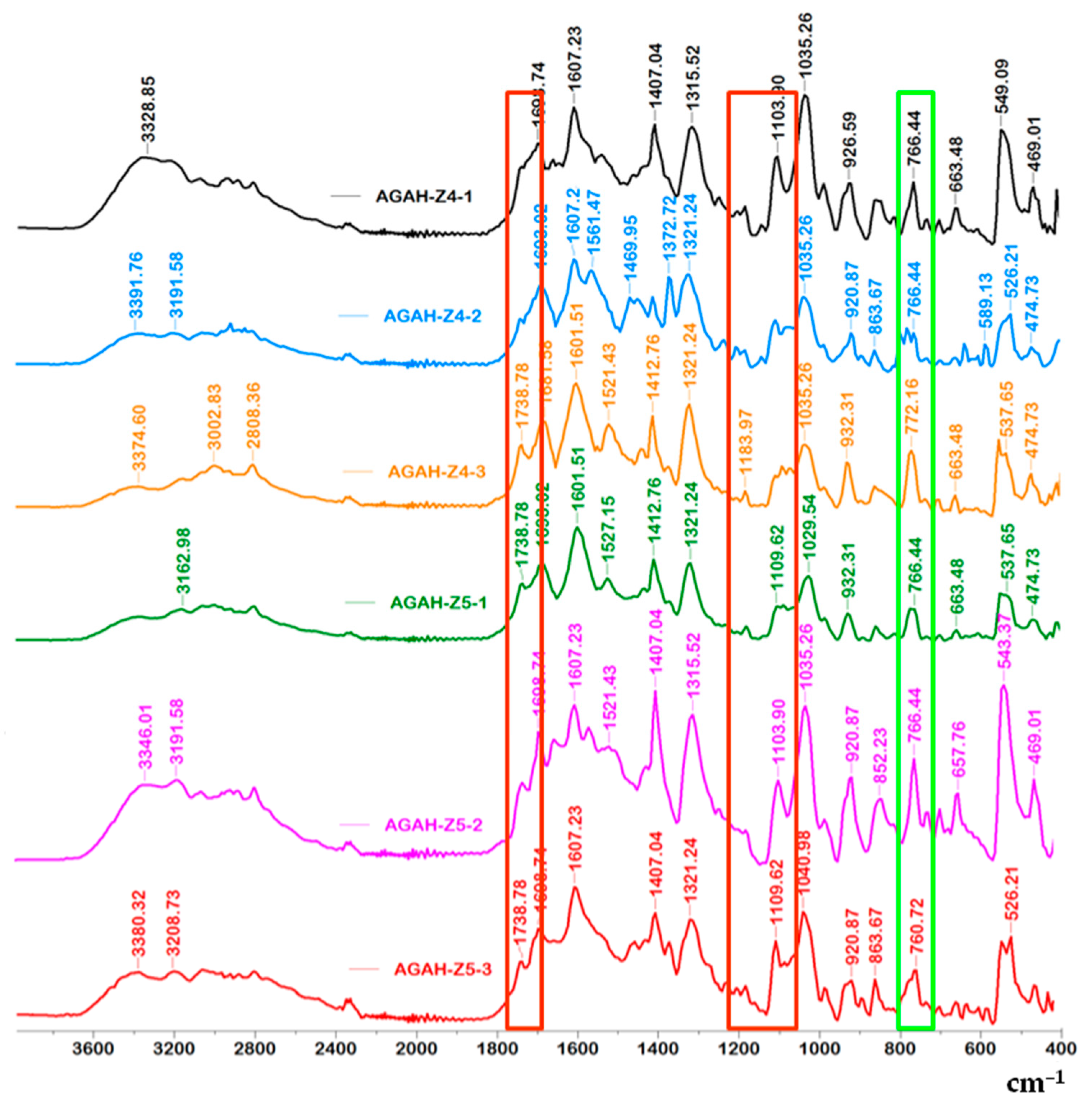
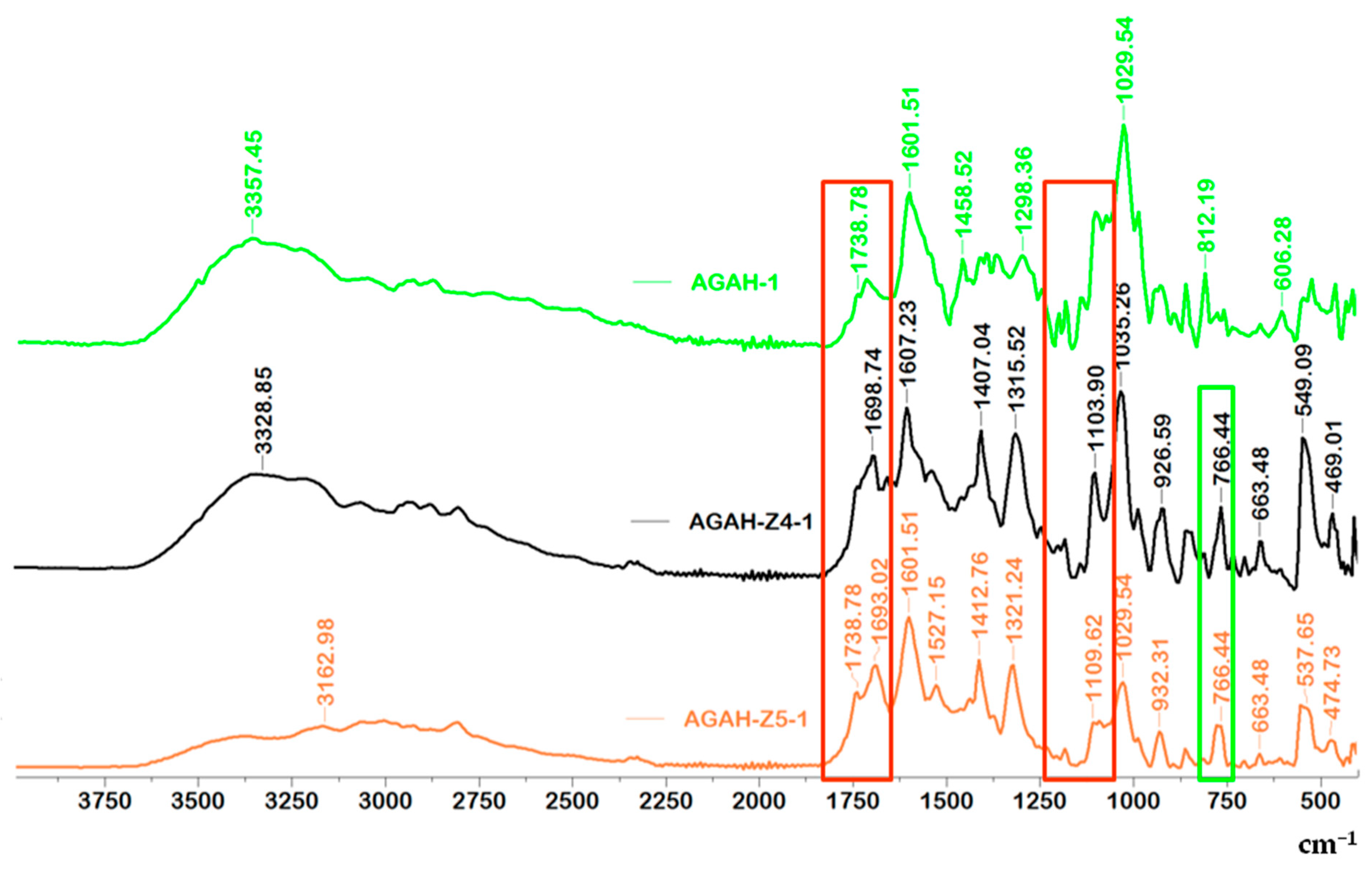
3.1. FTIR Spectroscopy
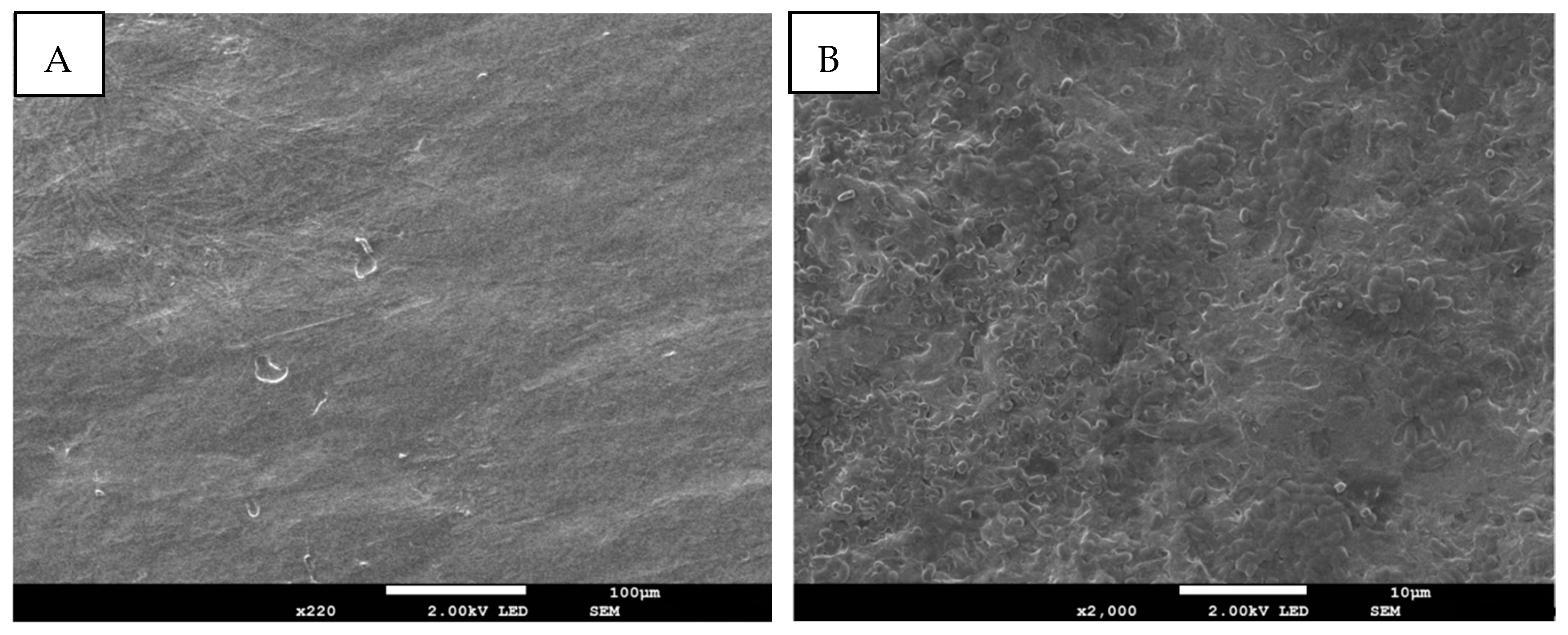
3.2. Morphological Characterization
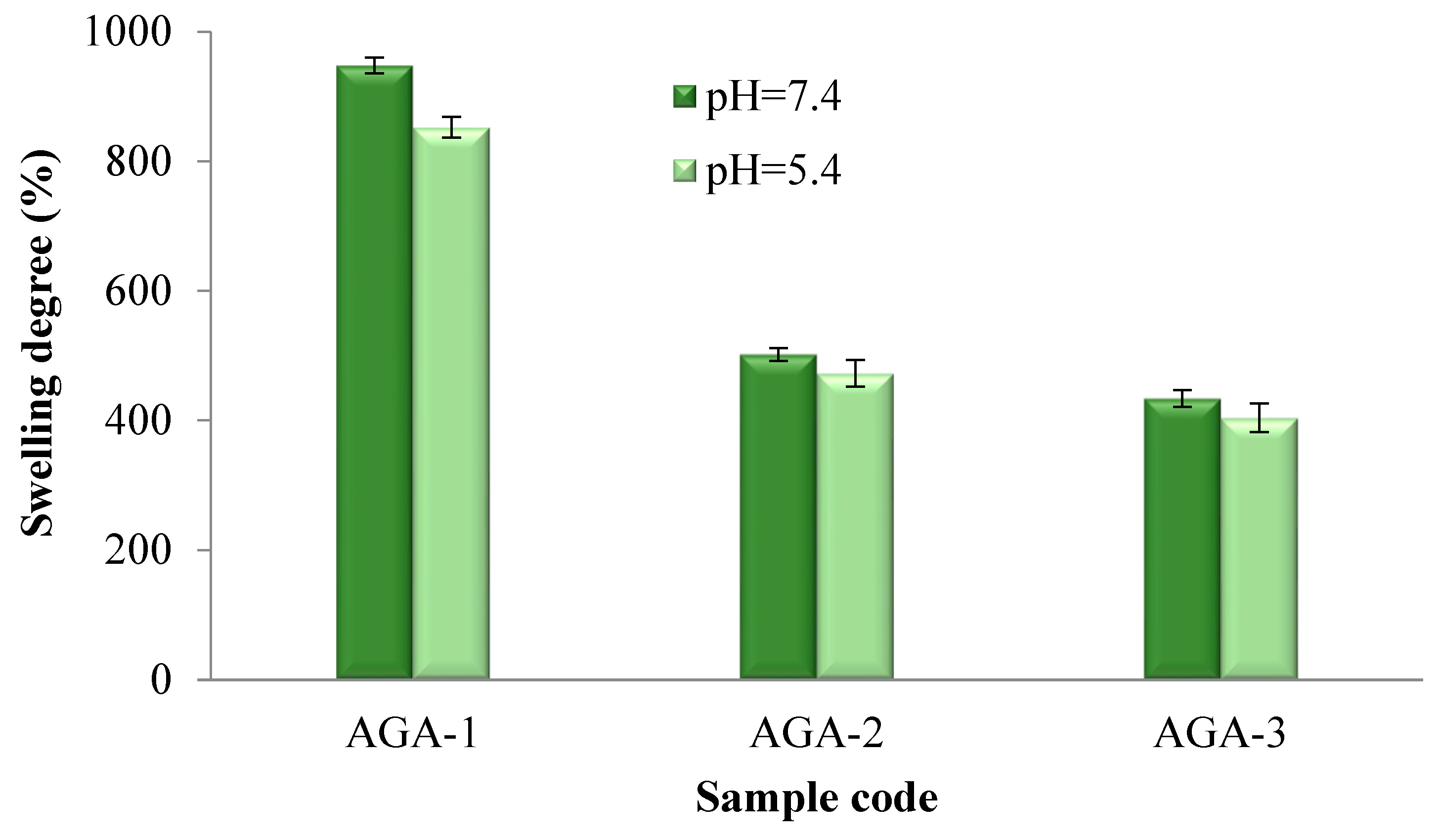
3.3. Hydrogel Swelling Capacity
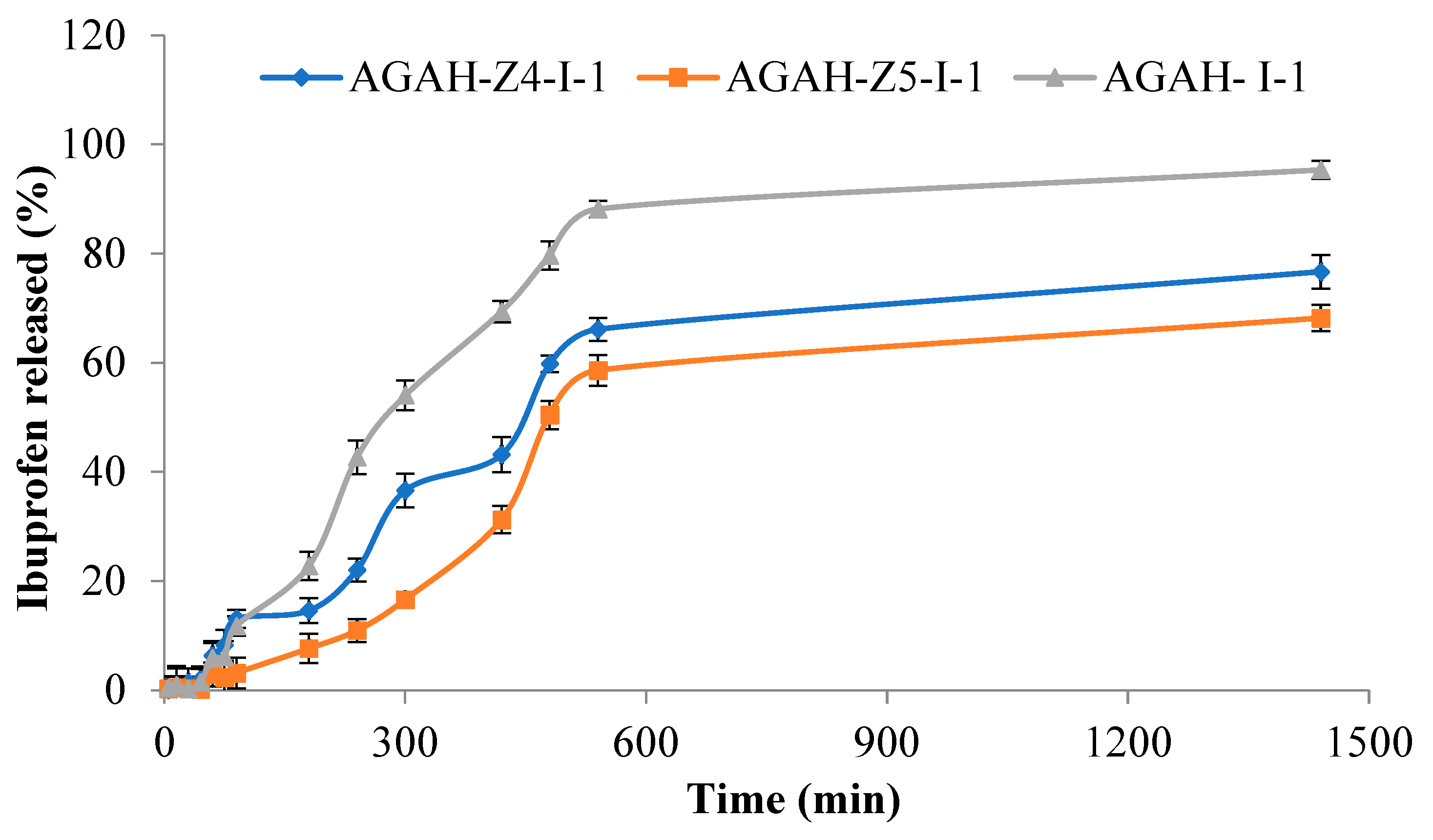
3.4. Ibuprofen Release Studies
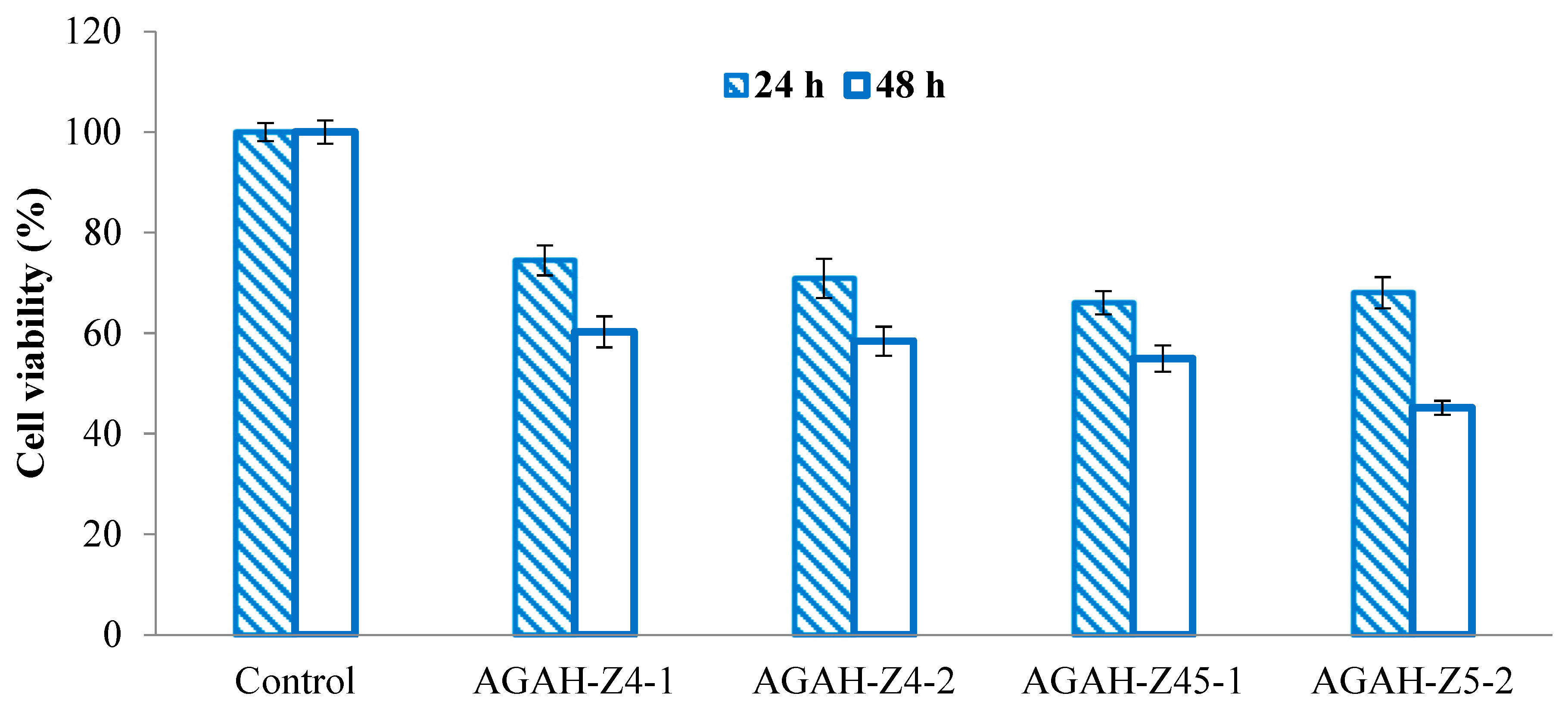
3.5. Cytotoxicity Assessment
3.6. Antimicrobial Activity of Biocomposite Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Zhong, M.; Wu, H.; Peng, Y.; Xia, H.; Tang, Q.; Huang, Q.; Wei, L.; Xiao, L.; Peng, C. Topical Application of Keratinocyte Growth Factor Conjugated Gold Nanoparticles Accelerate Wound Healing. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Stubbe, B.; Mignon, A.; Declercq, H.; Van Vlierberghe, S.; Dubruel, P. Development of Gelatin-Alginate Hydrogels for Burn Wound Treatment. Macromol. Biosci. 2019, 19, 1900123. [Google Scholar] [CrossRef]
- Shen, Y.-I.; Song, H.-H.G.; Papa, A.E.; Burke, J.A.; Volk, S.W.; Gerecht, S. Acellular Hydrogels for Regenerative Burn Wound Healing: Translation from a Porcine Model. J. Investig. Dermatol. 2015, 135, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of Systemic Complications and Current Management. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef]
- Madaghiele, M.; Sannino, A.; Ambrosio, L.; Demitri, C. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burn. Trauma 2014, 2, 153. [Google Scholar] [CrossRef]
- Saporito, F.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Boselli, C.; Icaro Cornaglia, A.; Ferrari, F. Essential oil-loaded lipid nanoparticles for wound healing. Int. J. Nanomed. 2017, 13, 175–186. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, Q.; Xin, P.; Yuan, Q.-J.; Wang, Y.; Wu, J. Polydopamine/Puerarin nanoparticles incorporated hybrid hydrogels for enhanced wound healing. Biomater. Sci. 2019, 7, 4230–4236. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Yudaev, P.; Butorova, I.; Chuev, V.; Posokhova, V.; Klyukin, B.; Chistyakov, E. Wound Gel with Antimicrobial Effects Based on Polyvinyl Alcohol and Functional Aryloxycyclotriphosphazene. Polymers 2023, 15, 2831. [Google Scholar] [CrossRef] [PubMed]
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800221. [Google Scholar] [CrossRef] [PubMed]
- Smagina, V.; Yudaev, P.; Kuskov, A.; Chistyakov, E. Polymeric Gel Systems Cytotoxicity and Drug Release as Key Features for their Effective Application in Various Fields of Addressed Pharmaceuticals Delivery. Pharmaceutics 2023, 15, 830. [Google Scholar] [CrossRef] [PubMed]
- Summa, M.; Russo, D.; Penna, I.; Margaroli, N.; Bayer, I.S.; Bandiera, T.; Athanassiou, A.; Bertorelli, R. A biocompatible sodium alginate/povidone iodine film enhances wound healing. Eur. J. Pharm. Biopharm. 2018, 122, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar] [CrossRef]
- Irache, J.M.; Esparza, I.; Gamazo, C.; Agüeros, M.; Espuelas, S. Nanomedicine: Novel approaches in human and veterinary therapeutics. Vet. Parasitol. 2011, 180, 47–71. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Abdelnabi, A.; Othman, M.S.; Bayoumi, R.E.; Abdelraouf, R.M. Effect of Different Mouthwashes on the Surface Microhardness and Color Stability of Dental Nanohybrid Resin Composite. Polymers 2023, 15, 815. [Google Scholar] [CrossRef]
- Singh, M.R.; Patel, S.; Singh, D. Natural polymer-based hydrogels as scaffolds for tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–260. [Google Scholar]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 1–22. [Google Scholar] [CrossRef]
- Daghdari, S.G.; Ahmadi, M.; Saei, H.D.; Tehrani, A.A. The effect of ZnO nanoparticles on bacterial load of experimental infectious wounds contaminated with Staphylococcus aureus in mice. Nanomed. J. 2017, 4, 232–236. [Google Scholar] [CrossRef]
- Sabbagh, F.; Khatir, N.M.; Kiarostami, K. Synthesis and Characterization of k-Carrageenan/PVA Nanocomposite Hydrogels in Combination with MgZnO Nanoparticles to Evaluate the Catechin Release. Polymers 2023, 15, 272. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology. 2009. Available online: https://asm.org/Protocols/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Pro (accessed on 1 June 2023).
- Singh, J.; Kaur, S.; Kaur, G.; Basu, S.; Rawat, M. Biogenic ZnO nanoparticles: A study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight. Green Process. Synth. 2019, 8, 272–280. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Popa, M.; Atanase, L.I.; Daraba, O.M.; Popescu, I.; Romila, L.E.; Ichim, D.L. Biocomposite Hydrogels for the Treatment of Bacterial Infections: Physicochemical Characterization and In Vitro Assessment. Pharmaceutics 2021, 13, 2079. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Gherasim, O.; Niculescu, A.-G.; Grumezescu, A.M.; Neacșu, I.A.; Chircov, C.; Vasile, B.Ș.; Oprea, O.C.; Andronescu, E.; Stan, M.S.; et al. A Microfluidic Approach for Synthesis of Silver Nanoparticles as a Potential Antimicrobial Agent in Alginate–Hyaluronic Acid-Based Wound Dressings. Int. J. Mol. Sci. 2023, 24, 11466. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.T. Anatase TiO2 nanocomposites for antimicrobial coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Dall Orto, V.C.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]




| Sample Code | AG/HA Ratio (mg/mg) | Moles DMT-MM/Moles COOH | ZnO NPs Compared to the Polymer Amount (%) | Ibuprofen (mg) |
|---|---|---|---|---|
| AGA-1 | 100/0 | 2/1 | - | - |
| AGA-2 | 100/0 | 3/1 | - | - |
| AGA-3 | 100/0 | 3.5/1 | - | - |
| AGAH-1 | 90/10 | 3/1 | - | - |
| AGAH-2 | 80/20 | 3/1 | - | - |
| AGAH-3 | 70/30 | 3/1 | - | - |
| AGAH-4 | 90/10 | 3.5/1 | - | - |
| AGAH-5 | 80/20 | 3.5/1 | - | - |
| AGAH-6 | 70/30 | 3.5/1 | - | - |
| AGAH-Z4-1 | 90/10 | 3/1 | 4 | - |
| AGAH-Z4-2 | 80/20 | 3/1 | 4 | - |
| AGAH-Z4-3 | 70/30 | 3/1 | 4 | - |
| AGAH-Z5-1 | 90/10 | 3/1 | 5 | - |
| AGAH-Z5-2 | 80/20 | 3/1 | 5 | - |
| AGAH-Z5-3 | 70/30 | 3/1 | 5 | - |
| AGAH- I-1 | 90/10 | 3/1 | - | 200 |
| AGAH-Z4-I-1 | 90/10 | 3/1 | 4 | 200 |
| AGAH-Z5-I-1 | 90/10 | 3/1 | 5 | 200 |
| Wavenumber (cm−1) | Functional Group | References |
|---|---|---|
| 766 | vibration band of ZnO | [25,26] |
| 812–932 | guluronic and mannuronic acids from AG | |
| 1035 | C-O-C functional group from AG | [27] |
| 1103–1183, 1738 | ester bonds (C=O and C–C(O)–C) | |
| 1521–1561 | amide I and amide II bonds from HA | |
| 1601, 1607 | asymmetric vibrations of the COO– | |
| 1407, 1412 | symmetric COO− vibration | |
| 1693, 1698 | C=O asymmetric stretching of the amide group | |
| 2808 | –CH vibration | |
| 3328–3391 | O-H groups |
| Inhibition Zone (mm) | |
|---|---|
| Samples | Gram-Positive Bacteria S. aureus (ATCC 25923) |
| AGAH-1 (Control) | no inhibition zone |
| AGAH-Z4-1 | 18 |
| AGAH-Z4-2 | 20 |
| AGAH-Z5-1 | 22 |
| AGAH-Z5-2 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rata, D.M.; Cadinoiu, A.N.; Daraba, O.M.; Gradinaru, L.M.; Atanase, L.I.; Ichim, D.L. Influence of ZnO Nanoparticles on the Properties of Ibuprofen-Loaded Alginate-Based Biocomposite Hydrogels with Potential Antimicrobial and Anti-Inflammatory Effects. Pharmaceutics 2023, 15, 2240. https://doi.org/10.3390/pharmaceutics15092240
Rata DM, Cadinoiu AN, Daraba OM, Gradinaru LM, Atanase LI, Ichim DL. Influence of ZnO Nanoparticles on the Properties of Ibuprofen-Loaded Alginate-Based Biocomposite Hydrogels with Potential Antimicrobial and Anti-Inflammatory Effects. Pharmaceutics. 2023; 15(9):2240. https://doi.org/10.3390/pharmaceutics15092240
Chicago/Turabian StyleRata, Delia Mihaela, Anca Niculina Cadinoiu, Oana Maria Daraba, Luiza Madalina Gradinaru, Leonard Ionut Atanase, and Daniela Luminita Ichim. 2023. "Influence of ZnO Nanoparticles on the Properties of Ibuprofen-Loaded Alginate-Based Biocomposite Hydrogels with Potential Antimicrobial and Anti-Inflammatory Effects" Pharmaceutics 15, no. 9: 2240. https://doi.org/10.3390/pharmaceutics15092240
APA StyleRata, D. M., Cadinoiu, A. N., Daraba, O. M., Gradinaru, L. M., Atanase, L. I., & Ichim, D. L. (2023). Influence of ZnO Nanoparticles on the Properties of Ibuprofen-Loaded Alginate-Based Biocomposite Hydrogels with Potential Antimicrobial and Anti-Inflammatory Effects. Pharmaceutics, 15(9), 2240. https://doi.org/10.3390/pharmaceutics15092240








