Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management
Abstract
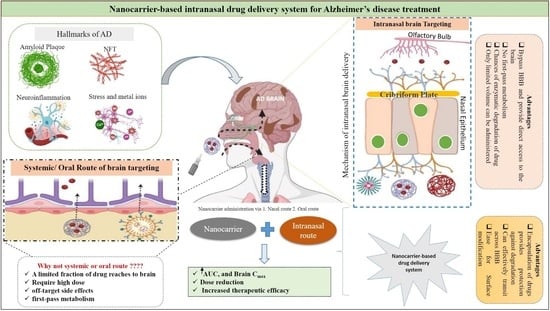
:1. Introduction
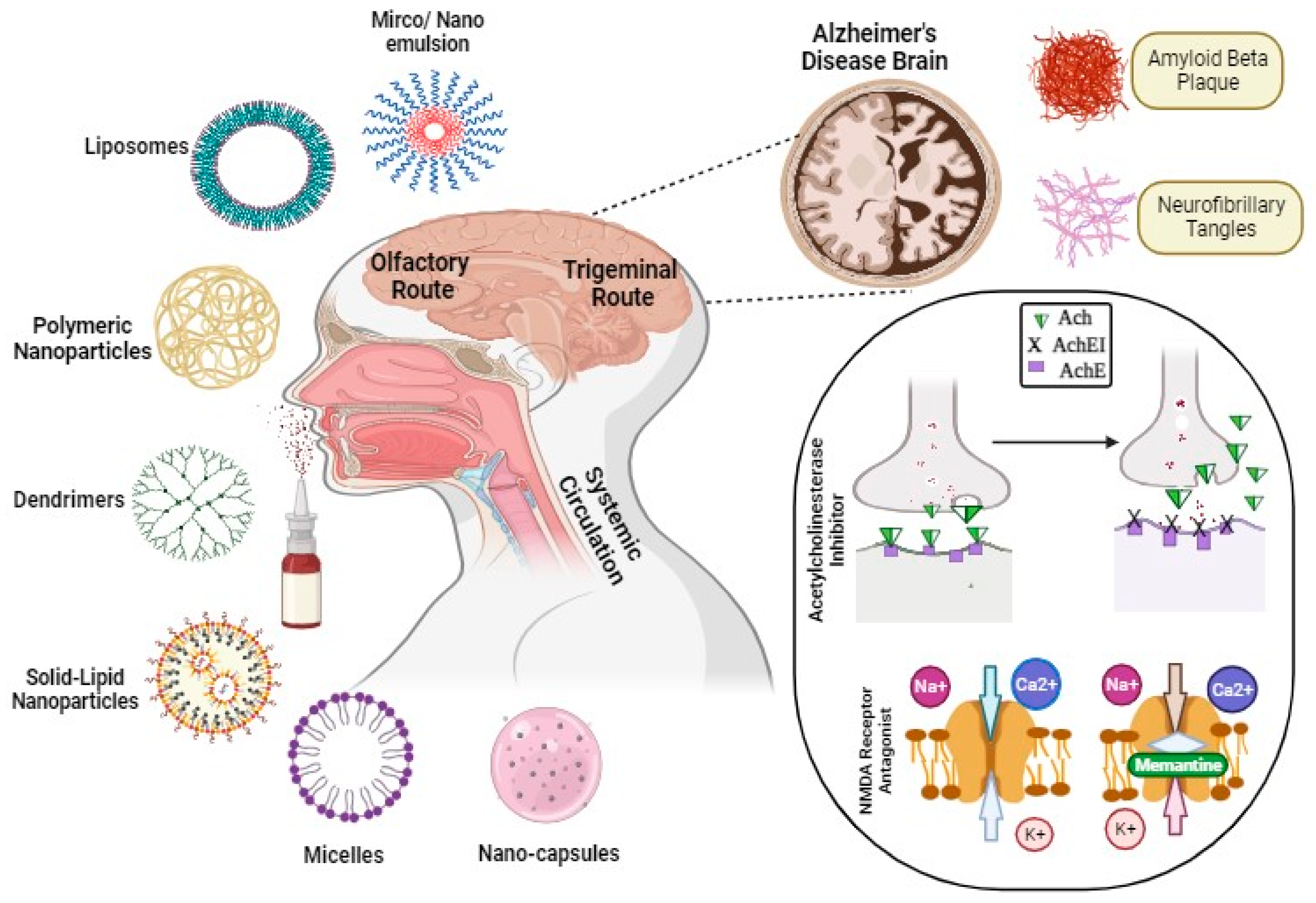
2. Exploring Nanocarriers for Alzheimer’s Disease Therapy
2.1. Polymeric Nanoparticles
2.2. Lipid-Based Nanocarriers
2.3. Metal Nanoparticles
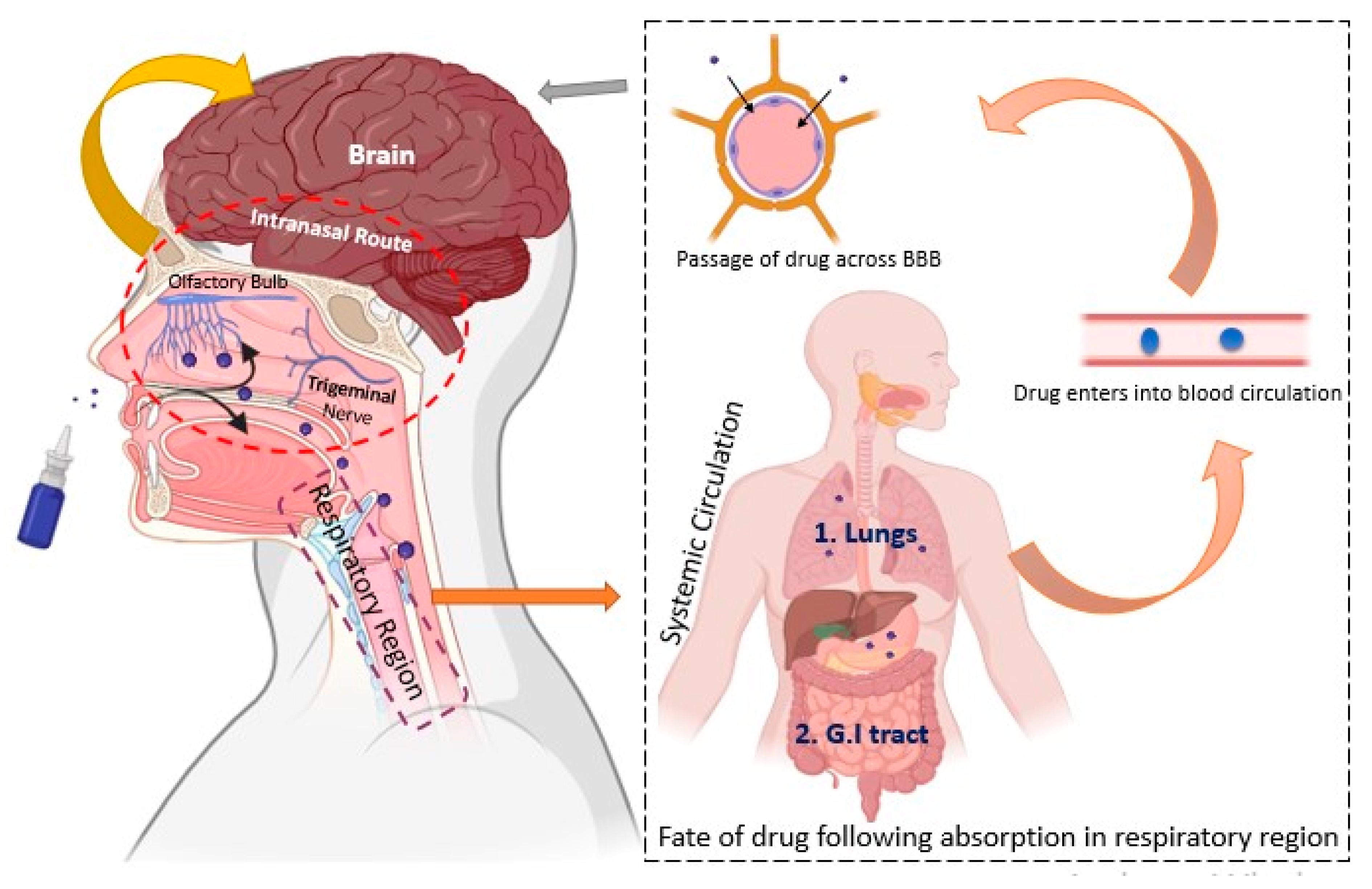
3. Transport Mechanisms of Intranasal Route
4. Intra-Nasal Nanoparticulate System for Alzheimer’s Disease Treatment
4.1. Nanoparticle-Based System
4.2. Lipid Nanocarriers
4.3. Nanoemulsions and Microemulsions
4.4. Miscellaneous Nanocarriers
4.5. InSitu Gelling System
5. Toxicity and Safety Aspects of Nanoparticulate Delivery
6. Regulatory Aspects/Challenges of Intranasal Nanocarrier Drug Delivery
7. Conclusions
8. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AD | Alzheimer’s disease |
| CNS | Central nervous system |
| BBB | Blood–brain barrier |
| APOE | Apolipoprotein E |
| PSEN | Presenilin |
| APP | Amyloid precursor protein |
| NMDA | N-methyl-D-aspartate receptor |
| NFT | Neurofibrillary tangles |
| CS-NP | Chitosan nanoparticles |
| ChAT | Choline acetyltransferase |
| PIP | Piperine |
| PAMAM | Poly (amidoamine) |
| PVA | Polyvinyl alcohol |
| PLGA | poly lactic co glycolic acid |
| HPMC | Hydroxypropyl methylcellulose |
| i.n | Intranasal |
| i.v | Intravenous |
| NP | Nanoparticles |
| SLN | Solid lipid nanoparticles |
| NLC | Nanostructured lipid carrier |
| Lf-TMC-NP | Lactoferrin conjugated N-methylated chitosan nanoparticles |
| RSLN | Risperidone solid lipid nanoparticles |
| PDI | Polydispersibility Index |
| EE | Entrapment efficiency |
| BuChE | Butyrylcholinesterases |
| AChEI | Acetylcholinesterase inhibitor |
| ROS | Reactive oxygen species |
| ARIA | Amyloid-related imaging abnormality |
| FDC | Fixed dose combination |
| OLE | Open label extension |
| PET | Photon emission topography |
| PEG | Polytheylene glycol |
| AMT | Adsorption mediated transcytosis |
| Tg | Glass transition temperature |
| PLGA | Polylactide-co-glycolide |
| LNP | Lipid nanoparticles |
| MOF | Metal organic framework |
| MDA | Malonyldialdehyde |
| IVIVC | In vitro in vivo correlation |
| RHT | Rivastigmine |
| TNF | Tumour necrosis factor |
| ELISA | Enzyme-linked immunosorbent assay |
| TRAIL | TNF-related apoptosis-inducing ligand |
| mi-RNA | Micro ribonucleic acid |
| PLA | Polylactic acid |
| AuNP | Gold nanoparticles |
| SPION | Super paramagnetic iron oxide nanoparticles |
| HAS | Hydroxy-α-sanshool |
| GH | Galantamine hydroxide |
| OVAL | Ovalalbumin |
| AUC | Area under the curve |
| SNF | Simulated nasal fluid |
| ACSF | Artificial cerebrospinal fluid |
| PBS | Phosphate buffer saline |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing ability of plasma |
| NE | Nanoemulsion |
| GQR | G alpha subunits |
| DTE | Drug transport efficiency |
| DTP | Drug targeting potential |
| MPP | 1-Methyl-4-phenylpyridinium |
| QD | Quantum dots |
| WGA | Wheat germ agglutinin |
| ApoE4 | Apolipoprotein E4 |
| GQD | Graphene quantum dots |
| FTIR | Fourier transform infrared spectroscopy |
| HPLC | High-performance liquid chromatography |
| Cmax | Maximum concentration |
| EMA | European Medicines Agency |
| NBCD | Non-biological complex drugs |
| IND | Investigational new drug |
| Aβ42 | 42-amino acidβ amyloid |
References
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Vinicius, M.; De Mello, C.; Vieira, L.; de Souza, L.C.; Gomes, K.; Carvalho, M. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6507104/ (accessed on 16 April 2021).
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.U.; Levey, A.I. Alzheimer’sdisease: A clinical perspective and future nonhuman primate research opportunities. Proc. Natl. Acad. Sci. USA 2019, 116, 26224–26229. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Avila, J.; Lucas, J.J.; Pérez, M.; Hernández, F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef]
- Pérez, M.J.; Jara, C.; Quintanilla, R.A. Contribution of Tau pathology to mitochondrial impairment in neurodegeneration. Front. Neurosci. 2018, 12, 441. [Google Scholar] [CrossRef]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2020, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular Pathogenesis of Alzheimer’s Disease: An Update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Tzoneva, R. Oxidative Stress and Aging as Risk Factors for Alzheimer’s Disease and Parkinson’s Disease: The Role of the Antioxidant Melatonin. Int. J. Mol. Sci. 2023, 24, 3022. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2019, 49, 102294. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integrationof the aluminum and amyloid cascadehypotheses. Int. J. Alzheimers Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef]
- Chukwu, L.C.; Ekenjoku, J.A.; Ohadoma, S.C.; Olisa, C.L.; Okam, P.C.; Okany, C.C.; Ramalam, M.A.; Innocent, O.C. Advances in the pathogenesis of Alzheimer’s disease: A reevaluation of the Amyloid cascade hypothesis. World J. Adv. Res. Rev. 2023, 17, 882–904. [Google Scholar] [CrossRef]
- Sutinen, E.M.; Pirttilä, T.; Anderson, G.; Salminen, A.; Ojala, J.O. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J. Neuroinflamm. 2012, 9, 199. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Briyal, S.; Ranjan, A.K.; Gulati, A. Oxidative stress: A target to treat Alzheimer’s disease and stroke. Neurochem. Int. 2023, 165, 105509. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Bao, X.; Wang, R. Clinical PET imaging of microglial activation: Implications for microglial therapeutics in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Zu, H.B. Microglial polarization: Novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology 2020, 28, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Tondo, G.; Iaccarino, L.; Caminiti, S.P.; Presotto, L.; Santangelo, R.; Iannaccone, S.; Magnani, G.; Perani, D. The combined effects of microglia activation and brain glucose hypometabolism in early-onset Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-M.; Miao, D.; Cao, X.-P.; Tan, L.; Tan, L. Innate immune activation in Alzheimer’s disease. Ann. Transl. Med. 2018, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arjona, M.d.M.; Grondona, J.M.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglial Morphometric Parameters Correlate with the Expression Level of IL-1β, and Allow Identifying Different Activated Morphotypes. Front. Cell. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef]
- Chakraborty, B.; Mukerjee, N.; Maitra, S.; Zehravi, M.; Mukherjee, D.; Ghosh, A.; Massoud, E.E.S.; Rahman, M.H. Therapeutic Potential of Different Natural Products for the Treatment of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 6873874. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Tayeb, H.O.; Yang, H.D.; Price, B.H.; Tarazi, F.I. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol. Ther. 2012, 134, 8–25. [Google Scholar] [CrossRef]
- Dominik, G.; Glinz, D.; Gloy, V.L.; Monsch, A.U.; Kressig, R.W.; Patel, C.; McCord, K.A.; Ademy, Z.; Tomonaga, Y.; Schwenkglenks, M.; et al. Acetylcholinesterase inhibitors combined with memantine for moderate to severe Alzheimer’s disease: A meta-analysis. Swiss Med. Wkly. 2019, 149, w20093. [Google Scholar] [CrossRef]
- Kuns, B.; Rosani, A.; Varghese, D.; Kuns, B.; Rosani, A.; Varghese, D. Memantine; StatPearls Publishing: St. Petersburg, FA, USA, 2022. [Google Scholar]
- Rosini, M.; Simoni, E.; Caporaso, R.; Minarini, A.A. Multitarget strategies in Alzheimer’s disease: Benefits and challenges on the road to therapeutics. Future Med. Chem. 2016, 8, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Grossberg, G.T. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des. Devel. Ther. 2016, 10, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, Y.; Ren, J. Basic information about memantine and its treatment of Alzheimer’s disease and other clinical applications. Ibrain 2023, 9, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.C.; Silva, L.F.A.L.; Pais, M.V.; Forlenza, O.V. Anti-amyloid agents for treating incipient Alzheimer’s disease: A new hope? Braz. J. Psychiatry 2022, 44, 368–369. [Google Scholar] [CrossRef]
- Bespalov, A.; Courade, J.P.; Khiroug, L.; Terstappen, G.C.; Wang, Y. A call for better understanding of target engagement in Tau antibody development. Drug Discov. Today 2022, 27, 103338. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Parmar, M. Aducanumab; StatPearls Publishing: St. Petersburg, FA, USA, 2023. [Google Scholar]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Orzeł-Sajdłowska, A. Aducanumab—Hope or Disappointment for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4367. [Google Scholar] [CrossRef]
- Haeberlein, S.B.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Rahman, A.; Hossen, M.A.; Chowdhury, M.F.I.; Bari, S.; Tamanna, N.; Sultana, S.S.; Haque, S.N.; Al Masud, A.; Saif-Ur-Rahman, K.M. Aducanumab for the treatment of Alzheimer’s disease: A systematic review. Psychogeriatrics 2023, 23, 512–522. [Google Scholar] [CrossRef]
- Management, P. Letter to Editor Accelerated Approval of Highly Expensive Disease-modifying Agents: Lessons Learned from the Aducanumab Approval. J. Pharmacoecon. Pharm. Manag. 2022, 8, 2–5. [Google Scholar]
- Brockmann, R.; Nixon, J.; Love, B.L.; Yunusa, I. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer’s disease: Patient outcomes, healthcare costs, and drug development. Lancet Reg. Health-Am. 2023, 20, 100467. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.; Frank, C. Challenges with new treatments for Alzheimer disease: Accelerated approval of aducanumab in the United States raises questions. Can. Fam. Physician 2023, 69, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.B. Accelerated Approval of Aducanumab: Where Do We Stand Now? Ann. Pharmacother. 2021, 56, 736–739. [Google Scholar] [CrossRef]
- Qin, Q.; Tang, Y. Lecanemab: The game changer in the ongoing fight to treat Alzheimer’s disease? Human Brain 2022, 2, 1–4. [Google Scholar] [CrossRef]
- Hardy, J.; Mummery, C. An anti-amyloid therapy works for Alzheimer’s disease: Why has it taken so long and what is next? Brain 2023, 146, 1240–1242. [Google Scholar] [CrossRef]
- McDade, E.; Cummings, J.L.; Dhadda, S.; Swanson, C.J.; Reyderman, L.; Kanekiyo, M.; Koyama, A.; Irizarry, M.; Kramer, L.D.; Bateman, R.J. Lecanemab in patients with early Alzheimer’s disease: Detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimer’s Res. Ther. 2022, 14, 191. [Google Scholar] [CrossRef]
- Honig, L.S.; Barakos, J.; Dhadda, S.; Kanekiyo, M.; Reyderman, L.; Irizarry, M.; Kramer, L.D.; Swanson, C.J.; Sabbagh, M. ARIA in Alzheimer’s disease background. Alzheimer’s Dement. 2023, 9, e12377. [Google Scholar] [CrossRef]
- Gautam, A.S.; Pandey, S.K.; Lasure, V.; Dubey, S. Monoclonal antibodies for the management of central nervous system diseases: Clinical success and future strategies. Expert Opin. Biol. Ther. 2023, 23, 603–618. [Google Scholar] [CrossRef]
- Revheim, M.; Carlsen, P.F.H.; Costa, T.; Alavi, A.; Kepp, K.P.; Sensi, S.L.; Perry, G.; Robakis, N.K.; Barrio, J.R.; Vissel, B. Passive Alzheimer’s immunotherapy: A promising or uncertain option? Ageing Res. Rev. 2023, 90, 101996. [Google Scholar]
- Lois, F.; Lavand, P.; Leonard, D.; Remue, C.; Bellemans, V.; First, A.K. Background Connect with Wiley. Photodermatol. Photoimmunol. Photomed. 2019, 29, 4–6. [Google Scholar]
- Bateman, R.J.; Cummings, J.; Schobel, S.; Salloway, S.; Vellas, B.; Boada, M.; Black, S.E.; Blennow, K.; Fontoura, P.; Klein, G.; et al. An anti-amyloid monoclonal antibody with potential disease-modifying effects in early Alzheimer’s disease. Alzheimer’s Res. Ther. 2022, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- Valiukas, Z.; Ephraim, R.; Tangalakis, K.; Davidson, M.; Apostolopoulos, V.; Feehan, J. Immunotherapies for Alzheimer’s Disease—A Review. Vaccines 2022, 10, 1527. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. I Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef]
- Hoque, M.; Samanta, A.; Sahajada, S.; Alam, M.; Zughaibi, T.A. Neuroscience & Biobehavioral Reviews Nanomedicine-based immunotherapy for Alzheimer’s disease. Neurosci. Biobehav. Rev. 2023, 144, 104973. [Google Scholar]
- Ramanan, V.K.; Day, G.S. Molecular Neurodegeneration Anti-amyloid therapies for Alzheimer disease: Finally, good news for patients. Mol. Neurodegener. 2023, 18, 42. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Elmaraezy, A.; Aglan, A.; Salama, R.; Fouda, S.; Fouda, R.; AlSafadi, A.M. Bapineuzumab for mild to moderate Alzheimer’s disease: A meta-analysis of randomized controlled trials. BMC Neurol. 2017, 17, 66. [Google Scholar] [CrossRef]
- Godyń, J.; Jończyk, J.; Panek, D.; Malawska, B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep. 2016, 68, 127–138. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Bittner, T.; Sink, K.M.; Mackey, H.; Rabe, C.; Honig, L.S.; Cassetta, E.; Woodward, M.; Boada, M.; Van Dyck, C.H.; et al. Evaluating the Safety and Efficacy of Crenezumab vs Placebo in Adults with Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials. JAMA Neurol. 2022, 79, 1113–1121. [Google Scholar] [CrossRef]
- Landen, J.W.; Andreasen, N.; Cronenberger, C.L.; Schwartz, P.F.; Hanson, A.B.; Östlund, H.; Sattler, C.A.; Binneman, B.; Bednar, M.M. Ponezumab in mild-to-moderate Alzheimer’s disease: Randomized phase II PET-PIB study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 393–401. [Google Scholar] [CrossRef]
- Teng, E.; Manser, P.T.; Pickthorn, K.; Brunstein, F.; Blendstrup, M.; Bohorquez, S.S.; Wildsmith, K.R.; Toth, B.; Dolton, M.; Ramakrishnan, V.; et al. Safety and Efficacy of Semorinemab in Individuals with Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.; Boxer, A.L.; Golbe, L.I.; Höglinger, G.U.; Morris, H.R.; Litvan, I.; Lang, A.E.; Corvol, J.-C.; Aiba, I.; Grundman, M.; et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: A phase 2, randomized, placebo-controlled trial. Nat. Med. 2021, 27, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Florian, H.; Wang, D.; Arnold, S.E.; Boada, M.; Guo, Q.; Jin, Z.; Zheng, H.; Fisseha, N.; Kalluri, H.V.; Rendenbach-Mueller, B.; et al. Tilavonemab in early Alzheimer’s disease: Results from a phase 2, randomized, double-blind study. Brain 2023, 146, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Unnisa, A.; Greig, N.; Kamal, M. Nanotechnology-based gene therapy as a credible tool in the treatment of Alzheimer’s disease. Neural Regen. Res. 2023, 18, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Mathew, G.E.; Uddin, M.S.; Kim, H.; Mathew, B. Advancements in nanotherapeutics for Alzheimer’s disease: Current perspectives. J. Pharm. Pharmacol. 2019, 71, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Medicinal, F.; Hassan, N.A.; Alshamari, A.K.; Hassan, A.A.; Elharrif, M.G. Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnologyg. Molecules 2022, 27, 4839. [Google Scholar]
- Ming, M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef]
- Ferreira, M.D.; Duarte, J.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Nanosystems for Brain Targeting of Antipsychotic Drugs: An Update on the Most Promising Nanocarriers for Increased Bioavailability and Therapeutic Efficacy. Pharmaceutics 2023, 15, 678. [Google Scholar] [CrossRef]
- Tiwari, V.; Tiwari, A.; Sharma, A.; Kumar, M.; Kaushik, D.; Sagadevan, S. An optimistic approach to nanotechnology in Alzheimer’s disease management: An overview. J. Drug Deliv. Sci. Technol. 2023, 86, 104722. [Google Scholar] [CrossRef]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules 2020, 25, 5294. [Google Scholar] [CrossRef]
- Karthivashan, G.; Ganesan, P.; Park, S.Y.; Kim, J.S.; Choi, D.K. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.D.; De Carvalho, S.G.; Duarte, J.L.; Luiz, M.T.; Paes Dutra, J.A.; De Paula, G.A.; Chorilli, M.; Conde, J. A receptor-mediated landscape of druggable and targeted nanomaterials for gliomas. Mater. Today Bio 2023, 20, 100671. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dang, S. Nanocarrier-Based Drug Delivery to Brain: Interventions of Surface Modification. Curr. Neuropharmacol. 2022, 21, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; Pereira, M.D.C. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2020, 10, 380–402. [Google Scholar] [CrossRef]
- Puranik, N.; Yadav, D.; Song, M. Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. Int. J. Mol. Sci. 2023, 24, 14044. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Pingale, P.; Dhapte-Pawar, V. Nasal delivery of neurotherapeutics via nanocarriers: Facets, aspects, and prospects. Front. Pharmacol. 2022, 13, 979682. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated progress of nanocarrier-based intranasal drug delivery systems for treatment of brain diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–468. [Google Scholar] [CrossRef]
- Kou, D.; Gao, Y.; Li, C.; Zhou, D.; Lu, K.; Wang, N.; Zhang, R.; Yang, Z.; Zhou, Y.; Chen, L.; et al. Intranasal Pathway for Nanoparticles to Enter the Central Nervous System. Nano Lett. 2023, 23, 5381–5390. [Google Scholar] [CrossRef]
- Chu, J.; Zhang, W.; Liu, Y.; Gong, B.; Ji, W.; Yin, T.; Gao, C.; Liangwen, D.; Hao, M.; Chen, C.; et al. Biomaterials-based anti-inflammatory treatment strategies for Alzheimer’s disease. Neural Regen. Res. 2024, 19, 100–115. [Google Scholar] [CrossRef]
- Saucier-Sawyer, J.K.; Deng, Y.; Seo, Y.-E.; Cheng, C.J.; Zhang, J.; Quijano, E.; Saltzman, W.M. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J. Drug Target. 2015, 23, 736–749. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Colson, Y.L.; Grinstaff, M.W. Biologically responsive polymeric nanoparticles for drug delivery. Adv. Mater. 2012, 24, 3878–3886. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, G.; Tosi, G.; et al. PLGA-PEG-Ang–2 nanoparticles for blood–brain barrier crossing: Proof-of-concept study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tong, Y.; Bai, L.; Ye, L.; Zhong, L.; Duan, X.; Zhu, Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018, 107, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Desfoux, A.; Martinez, J.; Amblard, M.; Mehdi, A. Bottom-up strategies for the synthesis of peptide-based polymers. Prog. Polym. Sci. 2021, 115, 101377. [Google Scholar] [CrossRef]
- La Barbera, L.; Mauri, E.; D’Amelio, M.; Gori, M. Functionalization strategies of polymeric nanoparticles for drug delivery in Alzheimer’s disease: Current trends and future perspectives. Front. Neurosci. 2022, 16, 939855. [Google Scholar] [CrossRef]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the blood-brain barrier: Functionalised chitosan nanocarriers. Pharmaceutics 2020, 12, 1013. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, K.; Huang, Y.; Pang, Z. Brain drug delivery by adsorption-mediated transcytosis. In Brain Targeted Drug Delivery Systems: A Focus on Nanotechnology and Nanoparticulates; Academic Press: Cambridge, MA, USA, 2018; pp. 159–183. [Google Scholar] [CrossRef]
- Cano, A.; Sánchez-López, E.; Ettcheto, M.; López-Machado, A.; Espina, M.; Souto, E.B.; Galindo, R.; Camins, A.; García, M.L.; Turowski, P. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine 2020, 15, 1239–1261. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Mittal, G.; Carswell, H.; Brett, R.; Currie, S.; Kumar, M.N.V.R. Development and evaluation of polymer nanoparticles for oral delivery of estradiol to rat brain in a model of Alzheimer’s pathology. J. Control. Release 2011, 150, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Baysal, I.; Ucar, G.; Gultekinoglu, M.; Ulubayram, K.; Yabanoglu-Ciftci, S. Donepezil loaded PLGA-b-PEG nanoparticles: Their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J. Neural Transm. 2017, 124, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Bhavna, B.; Shadab; Ali, M.; Baboota, S.; Sahni, J.K.; Bhatnagar, A.; Ali, J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2014, 40, 278–287. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Muthu, M.S.; Vinothapooshan, G. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of rivastigmine to treat Alzheimer’s disease. Ther. Deliv. 2011, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Jagaran, K.; Singh, M. Lipid Nanoparticles: Promising Treatment Approach for Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 9361. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.C.; Velho, S.; Amaral, M.H. Lipid Nanoparticles Functionalized with Antibodies for Anticancer Drug Therapy. Pharmaceutics 2023, 15, 216. [Google Scholar] [CrossRef]
- Gugleva, V.; Andonova, V. Drug delivery to the brain—Lipid nanoparticles-based approach. Pharmacia 2023, 70, 113–120. [Google Scholar] [CrossRef]
- Yan, D.; Qu, X.; Chen, M.; Wang, J.; Li, X.; Zhang, Z.; Liu, Y.; Kong, L.; Yu, Y.; Ju, R.; et al. Functionalized curcumin/ginsenoside Rb1 dual-loaded liposomes: Targeting the blood-brain barrier and improving pathological features associated in APP/PS-1 mice. J. Drug Deliv. Sci. Technol. 2023, 86, 104633. [Google Scholar] [CrossRef]
- Sokolik, V.V.; Berchenko, O.G. The cumulative effect of the combined action of miR-101 and curcumin in a liposome on a model of Alzheimer’s disease in mononuclear cells. Front. Cell. Neurosci. 2023, 17, 1169980. [Google Scholar] [CrossRef]
- Andrade, S.; Pereira, M.C.; Loureiro, J.A. Caffeic acid loaded into engineered lipid nanoparticles for Alzheimer’s disease therapy. Colloids Surf. B Biointerfaces 2023, 225, 113270. [Google Scholar] [CrossRef] [PubMed]
- Shivananjegowda, M.G.; Hani, U.; Osmani, R.A.M.; Alamri, A.H.; Ghazwani, M.; Alhamhoom, Y.; Rahamathulla, M.; Paranthaman, S.; Gowda, D.V.; Siddiqua, A. Development and Evaluation of Solid Lipid Nanoparticles for the Clearance of Aβ in Alzheimer’s Disease. Pharmaceutics 2023, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Dara, T.; Vatanara, A.; Sharifzadeh, M.; Khani, S.; Vakilinezhad, M.A.; Vakhshiteh, F.; Meybodi, M.N.; Malvajerd, S.S.; Hassani, S.; Mosaddegh, M.H. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol. Learn. Mem. 2019, 166, 107082. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.; Kunde, S.S.; Auti, S.T.; Kulkarni, Y.A.; Wairkar, S. Berberine loaded nanostructured lipid carrier for Alzheimer’s disease: Design, statistical optimization and enhanced in vivo performance. Life Sci. 2021, 285, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Abu Bakar, M.F.; Yida, Z.; Abdullah, M.A.; Basri, H. Thymoquinone-rich fraction nanoemulsion (TQRFNE) decreases Aβ40 and Aβ42 levels by modulating APP processing, up-regulating IDE and LRP1, and down-regulating BACE1 and RAGE in response to high fat/cholesterol diet-induced rats. Biomed. Pharmacother. 2017, 95, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Gyanani, V.; Goswami, R. Key Design Features of Lipid Nanoparticles and Electrostatic Charge-Based Lipid Nanoparticle Targeting. Pharmaceutics 2023, 15, 1184. [Google Scholar] [CrossRef]
- Hernandez, C.; Shukla, S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1190–1198. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.-L.; Jan, J.-S.; Tang, R.-D.; Wang, J.-Y.; Taliyan, R.; Yang, C.-H. Solid lipid nanoparticles (Slns): An advanced drug delivery system targeting brain through bbb. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Mosallaei, N.; Jaafari, M.R.; Hanafi-Bojd, M.Y.; Golmohammadzadeh, S.; Malaekeh-Nikouei, B. Docetaxel-loaded solid lipid nanoparticles: Preparation, characterization, in vitro, and in vivo evaluations. J. Pharm. Sci. 2013, 102, 1994–2004. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Souto, E.B.; Singh, K.K. Advances in brain drug targeting and delivery: Limitations and challenges of solid lipid nanoparticles. Expert Opin. Drug Deliv. 2013, 10, 889–905. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Abbas, H.; Zewail, M.; Noureldin, M.H.; Ali, M.M.; Shamaa, M.M.; Khattab, M.A. Neuroprotective Effect of Artichoke-Based Nanoformulation in Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceuticals 2022, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.; Baskaran, M.; Humtsoe, L.C.; Vadivelan, R.; Justin, A. Enhanced brain targeting efficacy of Olanzapine through solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Patr, A.B.; Prata, M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar]
- Nasar, S.; Afzal, O.; Altamimi, A.S.A.; Ather, H.; Sultana, S.; Almalki, W.H.; Bharti, P.; Sahoo, A.; Dwivedi, K.; Khan, G.; et al. Nanomedicine in the Management of Alzheimer’s Disease: State-of-the-Art Biomedicines. Biomedicines 2023, 11, 1752. [Google Scholar] [CrossRef]
- Garg, J.; Pathania, K.; Sah, S.P.; Pawar, S.V. Nanostructured lipid carriers: A promising drug carrier for targeting brain tumours. Future J. Pharm. Sci. 2022, 8, 25. [Google Scholar] [CrossRef]
- Mendes, I.T.; Carvalho, F.C.; Bonfilio, R.; Pereira, G.R. Colloids and Surfaces B: Biointerfaces Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf. B Biointerfaces 2019, 177, 274–281. [Google Scholar] [CrossRef]
- Chauhan, M.K. Optimization and characterization of rivastigmine nanolipid carrier loaded transdermal patches for the treatment of dementia. Chem. Phys. Lipids 2019, 224, 104794. [Google Scholar] [CrossRef]
- Hamano, N.; Li, S.; Chougule, M.; Shoyele, S.A.; Alexander, A. Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin. Drug Deliv. 2018, 15, 589–617. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Nirale, P.; Paul, A.; Yadav, K.S. Nanoemulsions for targeting the neurodegenerative diseases: Alzheimer’s, Parkinson’s and Prion’s. Life Sci. 2020, 245, 117394. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.; Khan, S.; Gaba, B.; Alam, T. Optimization of rivastigmine nanoemulsion for enhanced brain delivery: In-vivo and toxicity evaluation. J. Mol. Liq. 2018, 255, 384–396. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Ishida, K.; de Araujo, G.L.B.; de Castro Spadari, C.; de Souza, A.; Löbenberg, R.; Henostroza, M.A.B.; Folchini, B.R.; Peroni, C.M.; Peters, M.C.C.; et al. Rational design of oral flubendazole-loaded nanoemulsion for brain delivery in cryptococcosis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127631. [Google Scholar] [CrossRef]
- Atinderpal, K.; Kuldeep, N.; Sukriti, S.; Amit, T.; Shweta, D. Memantine nanoemulsion: A new approach to treat Alzheimer’s disease. J. Microencapsul. 2020, 37, 355–365. [Google Scholar] [CrossRef]
- Line, S.; Guyon, L.; Maurel, M.; Verdié, P.; Davis, A.; Corvaisier, S.; Lisowski, V.; Dallemagne, P.; Groo, A.-C.; Malzert-Fréon, A. Active Targeted Nanoemulsions for Repurposing of Tegaserod in Alzheimer’s Disease Treatment. Pharmaceutics 2021, 13, 1626. [Google Scholar]
- Valmiki, V.C.; Gangadhara, A. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Sharma, B.; Pervushin, K. Magnetic nanoparticles as in vivo tracers for Alzheimer’s disease. Magnetochemistry 2020, 6, 13. [Google Scholar] [CrossRef]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, N.; Yang, X.; Ling, G.; Zhang, P. The roles of gold nanoparticles in the detection of amyloid-β peptide for Alzheimer’s disease. Colloid Interface Sci. Commun. 2022, 46, 100579. [Google Scholar] [CrossRef]
- Barrier, B.; Wong, K.H.; Riaz, M.K.; Xie, Y.; Zhang, X.; Liu, Q. Review of Current Strategies for Delivering Alzheimer’s Disease Drugs across the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 381. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Lee, H.; Nam, J. How Do the Size, Charge and Shape of Nanoparticles Affect Amyloid β Aggregation on Brain Lipid Bilayer? Sci. Rep. 2016, 6, 19548. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T.; Ullah, F. Biosynthesized Metal Nanoparticles as Potential Alzheimer’s Disease Therapeutics; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Carro, C.E.; Pilozzi, A.R.; Huang, X. Nanoneurotoxicity and Potential Nanotheranostics for Alzheimer’s Disease. EC Pharmacol. Toxicol. 2019, 7, 1–7. [Google Scholar] [PubMed]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef]
- Tajahmadi, S.; Molavi, H.; Ahmadijokani, F.; Shamloo, A.; Shojaei, A.; Sharifzadeh, M.; Rezakazemi, M.; Fatehizadeh, A.; Aminabhavi, T.M.; Arjmand, M. Metal-organic frameworks: A promising option for the diagnosis and treatment of Alzheimer’s disease. J. Control. Release 2023, 353, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Elmonem, H.A.A.; Morsi, R.M.; Mansour, D.S.; El-Sayed, E.S.R. Myco-fabricated ZnO nanoparticles ameliorate neurotoxicity in mice model of Alzheimer’s disease via acetylcholinesterase inhibition and oxidative stress reduction. BioMetals 2023, 36, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Jia, Z.; Yuan, X.; Liu, J. Electrostatic assembly of gold nanoparticle and metal-organic framework nanoparticles attenuates amyloid β aggregate-mediated neurotoxicity. J. Mater. Chem. B 2023, 11, 4453–4463. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Ahmad, A.; Chinnathambi, S. Protein-Capped Metal Nanoparticles Inhibit Tau Aggregation in Alzheimer’s Disease. ACS Omega 2019, 4, 12833–12840. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Z.; Gao, D.; Luo, G.; Ma, T.; Wang, Y.; Lu, L.; Gao, X. Stepwise Coordination-Driven Metal-Phenolic Nanoparticle as a Neuroprotection Enhancer for Alzheimer’s Disease Therapy. ACS Appl. Mater. Interfaces 2022, 15, 524–540. [Google Scholar] [CrossRef]
- Tang, R.; Yuan, X.; Jia, Z.; Yang, F.; Ye, G.; Liu, J. Ruthenium Dioxide Nanoparticles Treat Alzheimer’s Disease by Inhibiting Oxidative Stress and Alleviating Neuroinflammation. ACS Appl. Nano Mater. 2023, 6, 11661–11678. [Google Scholar] [CrossRef]
- Ling, T.S.; Chandrasegaran, S.; Xuan, L.Z.; Suan, T.L.; Elaine, E.; Visva Nathan, D.; Chai, Y.H.; Gunasekaran, B.; Salvamani, S. Review Article the Potential Benefits of Nanotechnology in Treating Alzheimer’s Disease. BioMed Res. Int. 2021, 2021, 5550938. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Iannitelli, A.; Laserra, S.; Sozio, P. Drug delivery strategies for Alzheimer’s disease treatment. Expert Opin. Drug Deliv. 2011, 8, 581–603. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood–brain barrier. Ital. J. Pediatr. 2018, 44, 127–133. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Access to the CNS: Strategies to overcome the BBB. Int. J. Pharm. 2023, 636, 122759. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Lobo, J.M.S.; Silva, A.C. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood—Brain barrier (BBB) to manage neurological disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef] [PubMed]
- Gyimesi, G. Transporter-Mediated Drug Delivery. Molecules 2023, 28, 1151. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, X.; Hu, K. Efflux Pump Inhibition to Enhance Brain Targeting Delivery; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Botti, G.; Dalpiaz, A.; Pavan, B. Targeting systems to the brain obtained by merging prodrugs, nanoparticles, and nasal administration. Pharmaceutics 2021, 13, 1144. [Google Scholar] [CrossRef]
- Saxena, S.; Bhardwaj, S.; Aggarwal, A. Brain Targeted Drug Delivery System: A Review. Res. Anal. J. 2023, 6, 16–29. [Google Scholar] [CrossRef]
- Pardridge, W.M. Brain Delivery of Nanomedicines: Trojan Horse Liposomes for Plasmid DNA Gene Therapy of the Brain. Front. Med. Technol. 2020, 2, 602236. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, S.L.; Shalgunov, V.; Herth, M.M. Transport of nanomedicines across the blood-brain barrier: Challenges and opportunities for imaging and therapy. Biomater. Adv. 2022, 141, 213125. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.; Schindowski, K.; Sonvico, F. Editorial: Intranasal Drug Delivery: Challenges and Opportunities. Front. Pharmacol. 2022, 13, 868986. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Kumar, N.N.; Nehra, G.; Stenslik, M.J.; Bradley, L.H.; Thorne, R.G. Intranasal Drug Delivery to the Brain; Springer: Berlin/Heidelberg, Germany, 2022; Volume 33. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, P.; Biswal, S.S.; Kumar, N.; Jha, C.B.; Singh, G.; Kaur, C.; Wadhwa, S.; Kumar, R. Drug delivery through nose: A noninvasive technique for brain targeting. J. Rep. Pharm. Sci. 2020, 9, 168–175. [Google Scholar] [CrossRef]
- Patel, D.; Thakkar, H. Formulation considerations for improving intranasal delivery of CNS acting therapeutics. Ther. Deliv. 2022, 13, 371–381. [Google Scholar] [CrossRef]
- Kumar, H.; Mishra, G.; Sharma, A.K.; Gothwal, A.; Kesharwani, P.; Gupta, U. Intranasal Drug Delivery: A Non-Invasive Approach for the Better Delivery of Neurotherapeutics. Pharm. Nanotechnol. 2017, 5, 203–214. [Google Scholar] [CrossRef]
- Bahadur, S.; Jha, M.K. Emerging nanoformulations for drug targeting to brain through intranasal delivery: A comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 78, 103932. [Google Scholar] [CrossRef]
- Marcello, E.; Chiono, V. Biomaterials-Enhanced Intranasal Delivery of Drugs as a Direct Route for Brain Targeting. Int. J. Mol. Sci. 2023, 24, 3390. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Govender, M.; Indermun, S.; Kumar, P.; Choonara, Y.E. Potential Targeting Sites to the Brain Through Nasal Passage. In Nasal Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2023; pp. 83–99. [Google Scholar] [CrossRef]
- Misra, S.K.; Pathak, K. Nose-to-Brain Targeting via Nanoemulsion: Significance and Evidence. Colloids Interfaces 2023, 7, 23. [Google Scholar] [CrossRef]
- Rai, G.; Gauba, P.; Dang, S. Recent advances in nanotechnology for Intra-nasal drug delivery and clinical applications. J. Drug Deliv. Sci. Technol. 2023, 86, 104726. [Google Scholar] [CrossRef]
- Journal, A.I.; Selvaraj, K.; Gowthamarajan, K.; Venkata, V. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Borkar, S.P.; Raizaday, A. Different Strategies for Nose-to-Brain Delivery of Small Molecules. In Nasal Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2023; pp. 361–379. [Google Scholar] [CrossRef]
- Alabsi, W.; Eedara, B.B.; Encinas-Basurto, D.; Polt, R.; Mansour, H.M. Nose-to-Brain Delivery of Therapeutic Peptides as Nasal Aerosols. Pharmaceutics 2022, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.K.S.; Keshari, R.K.; Rai, A.K. Advances in nasal trans-mucosal drug delivery. J. Appl. Pharm. Sci. 2011, 1, 21–28. [Google Scholar]
- Hemalatha, B.; Kalpana, M.; Rekha, B.S.; Varalakshmi, A.; Padmalatha, K. An Overview on Nasal Drug Delivery System. Asian J. Pharm. Res. 2022, 12, 249–258. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Kotha, A.K.; Ghosh, S.; Komanduri, N.; Wang, R.; Bhowmick, S.; Chougule, M.B. Approaches in barriers, modifications, route of administrations, and formulations of therapeutic agents for brain delivery. In Novel Drug Delivery Technologies: Innovative Strategies for Drug Re-Positioning; Springer: Berlin/Heidelberg, Germany, 2020; pp. 383–401. [Google Scholar] [CrossRef]
- Duan, X.; Mao, S. New strategies to improve the intranasal absorption of insulin. Drug Discov. Today 2010, 15, 416–427. [Google Scholar] [CrossRef]
- De Ponti, R.; Lardini, E. Use of chemical enhancers for nasal drug delivery. Drug Dev. Ind. Pharm. 1991, 17, 1419–1436. [Google Scholar] [CrossRef]
- Chavanpatil, M.D.; Vavia, P.R. The influence of absorption enhancers on nasal absorption of acyclovir. Eur. J. Pharm. Biopharm. 2004, 57, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.S.; Illum, L. Absorption Enhancers for Nasal Drug Delivery. Clin. Pharmacokinet. 2003, 42, 1107–1128. [Google Scholar] [CrossRef] [PubMed]
- Baldassi, D.; Ambike, S.; Feuerherd, M.; Cheng, C.-C.; Peeler, D.J.; Feldmann, D.P.; Porras-Gonzalez, D.L.; Wei, X.; Keller, L.-A.; Kneidinger, N.; et al. Inhibition of SARS-CoV-2 replication in the lung with siRNA/VIPER polyplexes. J. Control. Release 2022, 345, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Nazem, A.; Mansoori, G.A. Nanotechnology Solutions for Alzheimer’s Disease: Advances in Research Tools, Diagnostic Methods and Therapeutic Agents. J. Alzheimer’s Dis. 2020, 13, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Ahmadi, S.; Afshari, R.; Khalaji, S.; Rabiee, M.; Bagherzadeh, M.; Fatahi, Y.; Dinarvand, R.; Tahriri, M.; Tayebi, L.; et al. Polymeric Nanoparticles for Nasal Drug Delivery to the Brain: Relevance to Alzheimer’s Disease. Adv. Ther. 2020, 4, 2000076. [Google Scholar] [CrossRef]
- Brambilla, D.; Droumaguet, B.L.; Nicolas, J.; Hashemi, S.H.; Wu, L.-P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy, and safety issues. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 521–540. [Google Scholar] [CrossRef]
- Petschauer, J.S.; Madden, A.J.; Kirschbrown, W.P.; Song, G.; Zamboni, W.C. The effects of nanoparticle drug loading on the pharmacokinetics of anticancer agents. Nanomedicine 2015, 10, 447–463. [Google Scholar] [CrossRef]
- Modi, G.; Pillay, V.; Choonara, Y.E. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann. N. Y. Acad. Sci. 2010, 1184, 154–172. [Google Scholar] [CrossRef]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. International Journal of Biological Macromolecules Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef]
- Wilson, B.; Nasralla, B.; Alobaid, M.; Mukundan, K.; Leno, J. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2020, 61, 102176. [Google Scholar] [CrossRef]
- Kandil, L.S.; Farid, R.M.; Elgamal, S.S.; Hanafy, A.S. Intranasal galantamine/chitosan complex nanoparticles elicit neuroprotection potentials in rat brains via antioxidant effect. Drug Dev. Ind. Pharm. 2021, 47, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C. In Vitro and In Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly (lactic-co-glycolic acid) Nanoparticles and Curcumin/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer ’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef] [PubMed]
- Pawar, D.; Mangal, S.; Goswami, R.; Jaganathan, K.S. European Journal of Pharmaceutics and Biopharmaceutics Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: Effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur. J. Pharm. Biopharm. 2013, 85, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Fazil, M.; Haque, S.; Kumar, M.; Baboota, S. European Journal of Pharmaceutical Sciences Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Musumeci, T.; Di Benedetto, G.; Carbone, C.; Bonaccorso, A.; Amato, G.; Faro, M.J.O.; Burgaletto, C.; Puglisi, G.; Bernardini, R.; Cantarella, G. Intranasal Administration of a TRAIL Neutralizing Monoclonal Antibody Adsorbed in PLGA Nanoparticles and NLC Nanosystems: An In Vivo Study on a Mouse Model of Alzheimer’s Disease. Biomedicines 2022, 10, 985. [Google Scholar] [CrossRef]
- Su, Y.; Sun, B.; Gao, X.; Dong, X.; Fu, L.; Zhang, Y.; Li, Z.; Wang, Y.; Jiang, H.; Han, B. Intranasal Delivery of Targeted Nanoparticles Loaded With miR-132 to Brain for the Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 1165. [Google Scholar] [CrossRef]
- Nanaki, S.G.; Spyrou, K.; Bekiari, C.; Veneti, P.; Baroud, T.N.; Karouta, N.; Grivas, I.; Papadopoulos, G.C.; Gournis, D.; Bikiaris, D.N. Hierarchical porous Carbon—PLLA and PLGA hybrid nanoparticles for intranasal delivery of galantamine for Alzheimer’s disease therapy. Pharmaceutics 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Shamarekh, K.S.; Gad, H.A.; Soliman, M.E.; Sammour, O.A. Development and evaluation of protamine-coated PLGA nanoparticles for nose-to-brain delivery of tacrine: In-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101724. [Google Scholar] [CrossRef]
- Meng, Q.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Araya, E.; Olmedo, I.; Bastus, N.G.; Guerrero, S.; Puntes, V.F.; Giralt, E.; Kogan, M.J. Gold nanoparticles and microwave irradiation inhibit beta-amyloid amyloidogenesis. Nanoscale Res. Lett. 2008, 3, 435–443. [Google Scholar] [CrossRef]
- Nazem, A.; Mansoori, G.A. Nanotechnology for Alzheimer’s disease detection and treatment. Insciences J. 2011, 1, 169–193. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Brain targeting of resveratrol through intranasal lipid vesicles labelled with gold nanoparticles: In vivo evaluation and bioaccumulation investigation using computed tomography and histopathological examination. J. Drug Target. 2019, 27, 1127–1134. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, W.F.; Sun, H.B. Multifunctional superparamagnetic iron oxide nanoparticles: Design, synthesis and biomedical photonic applications. Nanoscale 2013, 5, 7664–7684. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Quinlan-Pluck, F.; Monopoli, M.P.; Sheibani, S.; Vali, H.; Dawson, K.A.; Lynch, I. Influence of the physiochemical properties of superparamagnetic iron oxide nanoparticles on amyloid β protein fibrillation in solution. ACS Chem. Neurosci. 2013, 4, 475–485. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta—Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Csóka, I. Progress and perspectives of brain-targeting lipid-based nanosystems via the nasal route in Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2020, 148, 38–53. [Google Scholar] [CrossRef]
- Patel, S.; Chavhan, S.; Soni, H.; Babbar, A.K.; Mathur, R.; Mishra, A.K.; Sawant, K. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J. Drug Target. 2011, 19, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Bhatt, S.; Kumar, M.; Verma, R.; Taneja, Y.; Kaushal, N.; Tiwari, A.; Tiwari, V.; Alexiou, A.; Albogami, S.; et al. QbD-based rivastigmine tartrate-loaded solid lipid nanoparticles for enhanced intranasal delivery to the brain for Alzheimer’s therapeutics. Front. Aging Neurosci. 2022, 14, 960246. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Sara, U.V.S.; Chauhan, I.; Gaur, P.K.; Singh, A.P.; Puri, D. Ameeduzzafar, Solid lipid nanoparticles for nose to brain delivery of donepezil: Formulation, optimization by Box–Behnken design, in vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1838–1851. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Khan, R.A.; Ahmed, B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J. Drug Target. 2013, 21, 300–311. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, T.; Jain, A.; Kaur, H.; Katare, O.P.; Singh, B. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: A preclinical evidence. Colloids Surf. B Biointerfaces 2021, 205, 111838. [Google Scholar] [CrossRef]
- Anand, A.; Arya, M.; Kaithwas, G.; Singh, G.; Saraf, S.A. Sucrose stearate as a biosurfactant for development of rivastigmine containing nanostructured lipid carriers and assessment of its activity against dementia in C. elegans model. J. Drug Deliv. Sci. Technol. 2019, 49, 219–226. [Google Scholar] [CrossRef]
- Wavikar, P.; Pai, R.; Vavia, P. Nose to Brain Delivery of Rivastigmine by In Situ Gelling Cationic Nanostructured Lipid Carriers: Enhanced Brain Distribution and Pharmacodynamics. J. Pharm. Sci. 2017, 106, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Jojo, G.M.; Kuppusamy, G.; De, A.; Narayan, V.V.S. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef]
- Rompicherla, S.K.L.; Arumugam, K.; Bojja, S.L.; Kumar, N.; Rao, C.M. Pharmacokinetic and pharmacodynamic evaluation of nasal liposome and nanoparticle based rivastigmine formulations in acute and chronic models of Alzheimer’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1737–1755. [Google Scholar] [CrossRef]
- Sokolik, V.; Berchenko, O.G.; Shulga, S. Comparative Analysis of Nasal Therapy with Soluble and Liposomal Forms of Curcumin on Rats with Alzheimer’s Disease Model. J. Alzheimer’s Dis. Park. 2017, 7, 2161–0460. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Zhao, N.; Hao, B.; Wang, X.; Kong, P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ. Toxicol. Pharmacol. 2012, 34, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.M.; Vyas, T.K.; Campbell, R.B.; Amiji, M.M.; Waszczak, B.L. Brain delivery of proteins by the intranasal route of administration: A comparison of cationic liposomes versus aqueous solution formulations. J. Pharm. Sci. 2010, 99, 1745–1761. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shao, X.; Zhang, C.; Tan, Y.; Liu, Q.; Wan, X.; Zhang, Q.; Xu, S.; Jiang, X. Intranasal H102 Peptide-Loaded Liposomes for Brain Delivery to Treat Alzheimer’s Disease. Pharm. Res. 2015, 32, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Zhang, Y.Q.; Wang, Z.Z.; Wu, K.; Lou, J.N.; Qi, X.R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef] [PubMed]
- El-Helaly, S.N.; Elbary, A.A.; Kassem, M.A.; El-Nabarawi, M.A. Electrosteric stealth rivastigmine loaded liposomes for brain targeting: Preparation, characterization, ex vivo, bio-distribution and in vivo pharmacokinetic studies. Drug Deliv. 2017, 24, 692–700. [Google Scholar] [CrossRef]
- Arumugam, K.; Subramanian, G.S.; Mallayasamy, S.R.; Averineni, R.K.; Reddy, M.S.; Udupa, N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008, 58, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Mourtas, S.; Lazar, A.N.; Markoutsa, E.; Duyckaerts, C.; Sophia, G. Multifunctional nanoliposomes with curcumin–lipid derivative and brain targeting functionality with potential applications for Alzheimer disease. Eur. J. Med. Chem. 2014, 80, 175–183. [Google Scholar] [CrossRef]
- Fonseca-santos, B. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.W.; Helmy, M.W.; ElGamal, S.S. Pharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: Future potential contribution in Alzheimer’s disease management Pharmacological, toxicological and neuronal localization asse. Drug Deliv. 2016, 7544, 3111–3122. [Google Scholar] [CrossRef]
- Wang, X.; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef]
- Ke, W.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Li, J.; Kuang, Y.; Ye, L.; Lou, J.; Jiang, C. Biomaterials Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 2009, 30, 6976–6985. [Google Scholar] [CrossRef] [PubMed]
- Pal, I.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Rassu, G.; Soddu, E.; Posadino, A.M.; Pintus, G.; Sarmento, B.; Giuchedi, P.; Gavini, E. Colloids and Surfaces B: Biointerfaces Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf. B Biointerfaces 2017, 152, 296–301. [Google Scholar] [CrossRef]
- Vaz, G.R.; Hädrich, G.; Bidone, J.; Rodrigues, J.L.; Falkembach, M.C.; Putaux, J.-L.; Hort, M.A.; Monserrat, J.M.; Varela Junior, A.S.; Teixeira, H.F.; et al. Development of Nasal Lipid Nanocarriers Containing Curcumin for Brain Targeting. J. Alzheimer’s Dis. 2017, 59, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Chen, Z.; et al. Biomaterials Lactoferrin-modi fi ed PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Bana, L.; Minniti, S.; Salvati, E.; Sesana, S.; Zambelli, V.; Cagnotto, A.; Orlando, A.; Cazzaniga, E.; Zwart, R.; Scheper, W.; et al. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect A β aggregation features and cross the blood—Brain-barrier: Implications for therapy of Alzheimer disease. Nanomed. Nanotechnol. Biol. Med. 2013, 10, 1583–1590. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, M.; Wang, X.-R.; Fu, J.; Han, M.; Shen, Y.-Q.; Xia, Z.; Gao, J.-Q. Transferrin-modified liposome promotes α-Mangostin to penetrate the blood-brain barrier. Nanomed. Nanotechnol. Biol. Med. 2015, 12, 421–430. [Google Scholar] [CrossRef]
- Salvati, E.; Sesana, S.; Sancini, G. Liposomes functionalized to overcome the blood—Brain barrier and to target amyloid-β peptide: The chemical design affects the permeability across an in vitro model. Int. J. Nanomed. 2013, 8, 1749–1758. [Google Scholar]
- Papadia, K.; Giannou, A.D.; Markoutsa, E.; Bigot, C.; Vanhoute, G.; Mourtas, S.; Van der Linded, A.; Stathopoulos, G.T.; Antimisiaris, S.G. European Journal of Pharmaceutical Sciences Multifunctional LUV liposomes decorated for BBB and amyloid targeting—B. In vivo brain targeting potential in wild-type and APP/PS1 mice. Eur. J. Pharm. Sci. 2017, 102, 180–187. [Google Scholar] [CrossRef]
- Qiang, F.; Shin, H.J.; Lee, B.J.; Han, H.K. Enhanced systemic exposure of fexofenadine via the intranasal administration of chitosan-coated liposome. Int. J. Pharm. 2012, 430, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, S.; Giunchedi, P. Nanoemulsions for ‘nose-to-brain’ drug delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.L.; Jindal, A.B.; Devarajan, P.V. Microemulsions and Nanoemulsions for Targeted Drug Delivery to the Brain. Curr. Nanosci. 2011, 7, 119–133. [Google Scholar] [CrossRef]
- Kaur, A.; Nigam, K.; Bhatnagar, I.; Sukhpal, H.; Awasthy, S.; Shankar, S.; Tyagi, A.; Dang, S. Treatment of Alzheimer’s diseases using donepezil nanoemulsion: An intranasal approach. Drug Deliv. Transl. Res. 2020, 10, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Wang, X.; Wang, J.; Hao, Q.; Hao, J. Osthole- Loaded Nanoemulsion Enhances Brain Target in the Treatment of Alzheimer’s Disease via Intranasal Administration. Oxidative Med. Cell. Longev. 2021, 2021, 8844455. [Google Scholar] [CrossRef] [PubMed]
- Vasdev, N.; Handa, M. Rosemary oil low energy nanoemulsion: Optimization, µrheology, in silico, in vitro, and ex vivo characterization. J. Biomater. Sci. Polym. Ed. 2022, 33, 1901–1923. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S. Coconut oil-based resveratrol nanoemulsion: Optimization using response surface methodology, stability assessment and pharmacokinetic evaluation. Food Chem. 2021, 357, 129721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, C.; Zhai, W.; Zhuang, N.; Han, T.; Ding, Z. The Optimization Design of Lactoferrin Loaded HupA Nanoemulsion for Targeted Drug Transport Via Intranasal Route. Int. J. Nanomed. 2019, 14, 9217–9234. [Google Scholar] [CrossRef]
- Wen, M.M.; Ismail, N.I.K.; Nasra, M.M.A.; El-Kamel, A.H. Repurposing ibuprofen-loaded microemulsion for the management of Alzheimer’s disease: Evidence of potential intranasal brain targeting. Drug Deliv. 2021, 28, 1188–1203. [Google Scholar] [CrossRef]
- Zussy, C.; John, R.; Urgin, T.; Otaegui, L.; Vigor, C.; Acar, N.; Canet, G.; Vitalis, M.; Morin, F.; Planel, E.; et al. Intranasal Administration of Nanovectorized Docosahexaenoic Acid (DHA) Improves Cognitive Function in Two Complementary Mouse Models of Alzheimer’s Disease. Antioxidants 2022, 11, 838. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, G.; Hu, R.; Chen, S.; Lu, W.; Gao, S.; Xia, H.; Wang, B.; Sun, C.; Nie, X.; et al. A Nasal Temperature and pH Dual-Responsive In Situ Gel Delivery System Based on Microemulsion of Huperzine A: Formulation, Evaluation, and In Vivo Pharmacokinetic Study. AAPS PharmSciTech 2019, 20, 301. [Google Scholar] [CrossRef] [PubMed]
- Khunt, D.; Shrivas, M.; Polaka, S.; Gondaliya, P.; Misra, M. Role of Omega-3 Fatty Acids and Butter Oil in Targeting Delivery of Donepezil Hydrochloride Microemulsion to Brain via the Intranasal Route: A Comparative Study. AAPS PharmSciTech 2020, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Formulation and In-vivo Pharmacokinetic Consideration of Intranasal Microemulsion and Mucoadhesive Microemulsion of Rivastigmine for Brain Targeting. Pharm. Res. 2017, 35, 8. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Prasad, R.; Misra, M. Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm. Sin. B 2014, 4, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Misra, A.; Babbar, A.K.; Mishra, A.K.; Mishra, P.; Pathak, K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008, 358, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014, 7544, 148–154. [Google Scholar] [CrossRef]
- Mizrahi, M.; Friedman-Levi, Y.; Larush, L.; Frid, K.; Binyamin, O.; Dori, D.; Fainstein, N.; Ovadia, H.; Ben-Hur, T.; Magdassi, S.; et al. Pomegranate seed oil nanoemulsions for the prevention and treatment of neurodegenerative diseases: The case of genetic CJD. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1353–1363. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Y.; Wang, Y.; Huang, M. Food Chemistry Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef]
- Yadav, S.; Gandham, S.K.; Panicucci, R.; Amiji, M.M. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 987–1002. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 1–16. [Google Scholar] [CrossRef]
- Dibaei, M.; Rouini, M.R.; Sheikholeslami, B.; Gholami, M.; Dinarvand, R. The effect of surface treatment on the brain delivery of curcumin nanosuspension: In vitro and in vivo studies. Int. J. Nanomed. 2019, 14, 5477–5490. [Google Scholar] [CrossRef] [PubMed]
- Bhavna; Shadab, M.; Ali, M.; Ali, R.; Bhatnagar, A.; Baboota, S.; Ali, J. Donepezil nanosuspension intended for nose to brain targeting: In vitro and in vivo safety evaluation. Int. J. Biol. Macromol. 2014, 67, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Junghanns, J.-U.A.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Singh, Y.; Meher, J.G.; Gupta, S.; Chourasia, M.K. Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery. J. Control. Release 2014, 183, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Benyue, L.; Yi, Z.; Tingting, C.; Chuangrong, C.; Wei, D.; Qi, W.; Tongkai, C. Intranasal delivery of paeoniflorin nanocrystals for brain targeting. Asian J. Pharm. Sci. 2020, 15, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Babylon, L.; Grewal, R.; Stahr, P.L.; Eckert, R.W.; Keck, C.M.; Eckert, G. P Hesperetin nanocrystals improve mitochondrial function in a cell model of early Alzheimer disease. Antioxidants 2021, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, S.; Pang, L.; Ou, G.; Zhu, L.; Ma, J.; Li, R.; Liu, Y.; Wang, L.; Wang, L.; et al. Effects of armodafinil nanocrystal nasal hydrogel on recovery of cognitive function in sleep-deprived rats. Int. J. Pharm. 2021, 597, 120343. [Google Scholar] [CrossRef]
- Fomicheva, A. Signal Enhancement in Antibody Microarrays Using Quantum Dots Nanocrystals: Application to Potential Alzheimer’s Disease Biomarker Screening. Anal. Chem. 2012, 84, 6821–6827. [Google Scholar] [CrossRef]
- Thakur, G.; Micic, M.; Yang, Y.; Li, W.; Movia, D.; Giordani, S.; Zhang, H.; Leblanc, R.M. Conjugated Quantum Dots Inhibit the Amyloid β (1–42) Fibrillation Process. Int. J. Alzheimer’s Dis. 2011, 2011, 502386. [Google Scholar] [CrossRef]
- Quan, L.; Wu, J.; Lane, L.A.; Wang, J.; Lu, Q.; Gu, Z.; Wang, Y. Enhanced Detection Specificity and Sensitivity of Alzheimer’s Disease Using Amyloid-beta Targeted Quantum Dots. Bioconjugate Chem. 2016, 27, 809–814. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Dai, W.; Dong, H.; Wen, Y.; Zhang, X. Graphene quantum dots for the inhibition of β amyloid aggregation. Nanoscale 2015, 7, 19060–19065. [Google Scholar] [CrossRef] [PubMed]
- Mars, A.; Hamami, M.; Bechnak, L.; Patra, D.; Raouafi, N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal. Chim. Acta 2018, 1036, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, N.; Nepovimova, E.; Korabecny, J.; Satnami, M.L.; Ghosh, K.K. Interaction of synthesized nitrogen enriched graphene quantum dots with novel anti-Alzheimer’s drugs: Spectroscopic insights. J. Biomol. Struct. Dyn. 2019, 38, 1822–1837. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Pi, J.; Long, Y.; Huang, N.; Cheng, Y.; Zheng, H. Quantum dots-based sandwich immunoassay for sensitive detection of Alzheimer’s disease-related Aβ1–42. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, D.; Chan, W.; Choi, M.M.F.; Li, H. Biomaterials Inhibition of beta 1–40 amy loid fibrillation with N-acetyl-L-cysteine capped quantum dots. Biomaterials 2010, 31, 91–98. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, D.; Luan, P.; Gu, B.; Feng, L.; Fan, S.; Liao, W.; Fang, W.; Yang, L.; Tao, E.; et al. Department of Neurology and Outpatient Department of Internal Medicine, Guangdong. Biomaterials 2016, 106, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanobiotechnology-based strategies for crossing the blood—Brain barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Al-azzawi, S.; Masheta, D.; Guildford, A.L.; Phillips, G. Dendrimeric Poly (Epsilon-Lysine) Delivery Systems for the Enhanced Permeability of Flurbiprofen across the Blood-Brain Barrier in Alzheimer’s Disease. Int. J. Mol. Sci. 2018, 19, 3224. [Google Scholar] [CrossRef]
- Al-azzawi, S.K. Improving Flurbiprofen Brain—Permeability and Targeting in Alzheimer’s Disease by Using a Novel Dendronised ApoE—Derived Peptide Carrier System. Ph.D. Thesis, University of Brighton, Brighton, UK, 2017. [Google Scholar]
- Agrawal, M.; Saraf, S.; Safar, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-responsive in situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef]
- Patil, R.P.; Pawara, D.D.; Gudewar, C.S.; Tekade, A.R. Nanostructured cubosomes in an in situ nasal gel system: An alternative approach for the controlled delivery of donepezil HCl to brain. J. Liposome Res. 2019, 29, 264–273. [Google Scholar] [CrossRef]
- Cunha, S.; Swedroska, M.; Bellahnid, Y.; Xu, Z.; Sousa Lobo, J.M.; Forbes, B.; Silva, A.C. Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: Characterisation, biocompatibility, and drug deposition studies. Int. J. Pharm. 2022, 620, 121720. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; Biagio, P.L.S.; Mule’, F.; Giacomazza, D.; Dispenze, C.; Carlo, M.D. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2017, 270, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Ditta, L.A.; Sabatino, M.A.; Militello, V.; Biagio, P.L.S.; Giacinto, M.L.D.; Cristaldi, L.; Nuzzo, D.; Dispenza, C.; Giacomazza, D.; et al. Biomaterials Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials 2016, 80, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Kalaiarasi, S.; Arjun, P.; Nandhagopal, S.; Brijitta, J.; Iniyan, A.M.; Vincent, S.G.P.; Kannan, R.R. ScienceDirect Development of biocompatible nanogel for sustained drug release by overcoming the blood brain barrier in zebrafish model. J. Appl. Biomed. 2016, 14, 157–169. [Google Scholar] [CrossRef]
- Azadi, A.; Hamidi, M.; Khoshayand, M.; Amini, M. Preparation and optimization of surface-treated methotrexate-loaded nanogels intended for brain delivery. Carbohydr. Polym. 2012, 90, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Boridy, S.; Takahashi, H.; Akiyoshi, K.; Maysinger, D. Biomaterials The binding of pullulan modified cholesteryl nanogels to A b oligomers and their suppression of cytotoxicity. Biomaterials 2009, 30, 5583–5591. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V.; Batrakova, E.V.; Kabanov, A.V. Nanogels for Oligonucleotide Delivery to the Brain. Bioconjugate Chem. 2003, 15, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Maurer-jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of Engineered Nanoparticles in the Environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J. C Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Accounts Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Jeng, H.A.; Swanson, J. Toxicity of Metal Oxide Nanoparticles in Mammalian Cells Toxicity of Metal Oxide Nanoparticles in Mammalian Cells. J. Environ. Sci. Health Part A 2006, 41, 2699–2711. [Google Scholar] [CrossRef]
- Badawy, A.M.E.L.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2010, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Gaur, N.; Sharma, N.; Dahiya, A.; Yadav, P.; Ojha, H. Toxicity and Regulatory Concerns for Nanoformulations in Medicine. In The ELSI Handbook of Nanotechnology: Risk, Safety, ELSI and Commercialization; ELSI: Ōta, Tokyo, 2020; pp. 333–357. [Google Scholar]
- Hejmady, S.; Singhvi, G.; Saha, R.N.; Dubey, S.K. Regulatory aspects in process development and scale-up of nanopharmaceuticals. Ther. Deliv. 2020, 11, 341–343. [Google Scholar] [CrossRef]
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Pérez de la Ossa, D.H.; Pita, R.; Vidal, J.M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine 2013, 8, 849–856. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Trows, S.; Wuchner, K.; Spycher, R.; Steckel, H. Analytical Challenges and Regulatory Requirements for Nasal Drug Products in Europe and the U.S. Pharmaceutics 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
| Name | Status | Outcome | Ref |
|---|---|---|---|
| Donanemab | Phase 3 | Donanemab showed maximum affinity towards Aβplaques, resulting in a deceleration of disease progression. Further, PET images revealed the absence of Aβ plaques in patients after12 months of treatment. | [58] |
| Bapineuzumab | Failed in Phase 3 | Had an anti-Alzheimer’s effect by targeting tau phosphorylation, thereby decreasing the tau concentration in CSF. However, bapineuzumab failed to show clinical efficacy and its clinical use was associated with a high risk of ARIA and TEAE. | [59] |
| Solanezumab | Terminated | Solanezumab acts by identifying and targeting soluble monomer Aβ except for fibrillary Aβ. However, the trial was terminated due to negligible benefits to mild AD patients and not meeting clinical endpoints. | [60] |
| Crenezumab | Completed | This antibody was well tolerated with no prominent side effects even when increasing the dosage. However, no commercial translation occurred as it failed to show clinical efficacy. | [61] |
| Ponezumab | Phase 2 | Treatment with ponezumab led to increased Aβ level in plasma. On completion of treatment, no alterations in CSF biomarkers, Aβ burden, and cognition were reported, which could be due to its low penetrability. | [62] |
| Semorinemab | Phase 2 | Semorinemab had a well-tolerated safety profile. The 73-week treatment did not reduce disease progression and no clinical outcome was reported. | [63] |
| Gosuranemab | Phase 2 | Considerable concentration of gosuranemab in serum and CSF was noted. Further, ~98% of unbound tau was reduced in CSF, yet no benefits were observed in a population at risk for PSP. | [64] |
| Tilavonemab | Phase 2 | Tilavonemab showed no effect on disease progression. Intriguingly, a reduced level of free tau in CSF (38.0–46.3%) was reported, which reached a plateau when the dosage was increased. | [65] |
| Drug | Polymer | Targeting | Route of Administration | Results | Ref |
|---|---|---|---|---|---|
| Estradiol | Polylactide-co-glycolide (PLGA) | Tween 80 (mimics LDL particles by adsorbing apolipoprotein and achieves targeting via LDL receptors) | Oral Route |
| [94] |
| Donepezil | PLGA-b-PEG | - | NA |
| [95] |
| Donepezil | Polylactide-co-glycolide (PLGA) | Tween-80 (internalize via LDL receptors) | Intravenous |
| [96] |
| Rivastigmine | Chitosan | Tween-80 (internalize through LDL receptors) | Intravenous |
| [97] |
| Drug | Carrier | Target | Route | Description | Ref |
|---|---|---|---|---|---|
| Curcumin/Ginsenoside Rb1 | Liposome | Mannose | i.v. |
| [102] |
| miR-101 and Curcumin | Liposome | - | NA |
| [103] |
| Caffeic Acid | Liposome | Transferrin | NA |
| [104] |
| Memantine HCl and Tramiprosate | SLN | - | oral |
| [105] |
| Erythropoietin | SLN | - | i.p. |
| [106] |
| Berberine | NLC | - | oral |
| [107] |
| Thymoquinone | NE | - | oral |
| [108] |
| Drug | Carrier | Target | Route of Administration | Description | Ref |
|---|---|---|---|---|---|
| - | Myco-fabricated ZnO nanoparticles | - | i.p. |
| [141] |
| PEG-MIL-101 (MOF) | AuNPs |
| [142] | ||
| - | Cadmium sulfide and Iron oxide nanoparticles | Protein capped | NA |
| [143] |
| Rhein and Polydopamine | Fe–Rh/Pda NPs | (KLVFFAED)/K8 peptide | i.v. |
| [144] |
| Ruthenium dioxide | Borneol | i.v. |
| [145] |
| Drug | Nanoparticle | Targeting Agent | Method of Preparation | Pharmacological Data | Ref |
|---|---|---|---|---|---|
| Tacrine | poly (n-butyl cyanoacrylate) | polysorbate 80 | Emulsion polymerization technique |
| [227] |
| Galantamine | Hydrobromide Chitosan complex NP (GH–chitosan NP) | - | Ionic interaction method |
| [228] |
| Estradiol | Chitosan NP | Ionic interaction method |
| [229] | |
| -Gene (DNA) | Polyamidoamine dendrimers-Polyethlene glycol (PAMAM-PEG-) NP | Angiopep | First, PEG PAMAM modification of angiopep was performed followed by complexation with DNA |
| [230] |
| Doxorubicin | Stealth(PEG2000) and non-stealth SLN | High-pressure homogenization |
| [231] | |
| RVG-9R -BACE1 siRNA A | Chitosan-coated and uncoated SLN | - | High-pressure homogenization |
| [232] |
| Curcumin | Lipid NP | - | Hot solvent diffusion method |
| [233] |
| Vasoactive intestinal peptide (VIP) | PEG-PLA NP | Wheat germ agglutinin | Double-emulsion solvent evaporation |
| [234] |
| Neuroprotective peptide | PEG-co-PCL NP | Lactoferrin | Emulsion solvent evaporation |
| [235] |
| Liposome Formulation | Problem to Encounter | Pharmacological Data | Ref |
|---|---|---|---|
| Bifunctionalized liposome mApoE-PA-LIP | Effective targeting of Aβ | The mApoE-PA-LIP showed temporal and dose-dependent inhibition of Aβ42 aggregates, while destabilization of preformed aggregates was found to be time- and lipid-dose-dependent. Also, five-fold increased radioactivity of brain/blood ratio was seen for mApoE-PA-LIP compared to PA-LIP. | [236] |
| Transferrin-modified alpha-M liposomes | Poor penetration | The alpha-M demonstrated an entrapment efficiency greater than 88% with improved bioavailability | [237] |
| Fluorescent liposomes functionalized with Antibody R17217 | Effective binding to Aβ | Functionalization improved cellular uptake and permeation. The functionalized liposomes also demonstrated higher EP (7.24 ± 0.39 ×10−6 cm/min) as compared to that of biotin/streptavidin-RI-A-LIP (4.97 ± 0.51 × 10−6 cm/min). | [238] |
| Multifunctionalized liposomes attached with two BBB-specific ligands and curcumin–lipid ligand | To locate and target formulation | In vivo study in mice demonstrated efficacy of liposomes to traverse across BBB. Addition of TREG–lipid curcumin derivative in liposome did not influence the functionality of ligands | [239] |
| Liposome coated with chitosan and encapsulated with fexofenadine | Effective brain targeting | Increased stability and retention time. Chitosan-coated liposomes showed enhanced bioavailability (34.7 ± 6.3%) as compared to non-liposomes (25.0 ± 8.0%) and uncoated liposomes (24.5 ± 7.5%). Sustained release was obtained for a period of 12 h | [240] |
| Drug and DDS | Pharmacological Evidence | Ref |
|---|---|---|
| Risperidone-loaded chitosan-based nanoemulsion | The mucoadhesive nanoemulsion was most effective with higher drug targeting efficiency (476 ± 2.14%) and rapid transport as compared to the drug solution | [254] |
| Saquinavir mesylate-loaded nanoemulsion | A higher concentration of drug (7290.46± 143.15 ng/g) was found at a faster rate with the NE with no toxicity and higher targeting efficiency (2919.261 ± 5.68%) | [255] |
| Pomegranate seed oil (PSO) nanoemulsion | PSO contains phytoconstituents such as polyunsaturated fatty acids and punicic acid, which reduced lipid oxidation and loss of neuronal functionality, suggesting the formulation to be neuro-protective and safe | [256] |
| Curcumin-based o/w nanoemulsion | Curcumin has low solubility and poor bioavailability. To improve its bioavailability, a curcumin-loaded NE was formulated. The prepared NE had a droplet size in the range of 618.6 nm to 79.5 nm. Anti-inflammatory action was shown using mouse ear inflammation model induced by TPA. The inhibition percentages observed were43% (in case of 618.6 nm droplets) and 85% (79.5 nm droplets), respectively. | [257] |
| anti-TNFα siRNA-encapsulated flaxseed nanoemulsion | SiRNA-loaded nanoemulsion showed 70 ± 10% encapsulation efficiency. Higher cellular uptake was observed at 15 min end point (10-fold greater) and after 2.5 h (25-fold greater), respectively. Nanoemulsion loaded with SiRNA showed improved brain targeting (two-fold greater) than SiRNA solution at the end point of 6 hr. Nanoemulsion-based delivery was found to be effective in gene knockdown and preventing neuroinflammation | [258] |
| Drug and Carrier | Investigation | Results | Ref |
|---|---|---|---|
| PEG-BTA quantum dots | Specificity and sensitivity of disease detection | Effective binding to amyloid beta peptide | [269] |
| Graphene QDs | Inhibitory effect on Aβ | Suppressed formation of fibrils. The inhibitory effect increased when surface negative charge decreased | [270] |
| Curcumin–graphene QD coated with Indium-TO electrode | For detection of ApoE4 | Reproducibility, repeatability, and high efficiency of curcumin platform for sensing even in a complex matrix | [271] |
| High-fluorescence NGQDs | To sense enzymatic action and efficacy | Decreased activity of AChE | [272] |
| Biotinylated N-Ab and streptavidinquantum dots | To detect Aβ | Successful for detecting Aβ in CSF | [273] |
| N-acetyl-L-cysteine-capped quantum dots | For inhibition of amyloid fibrillation | Inhibitory effect with an AUC that was 100 times increased | [274] |
| Grapheme quantum dots (GQDs) conjugated with peptide glycine–proline–glutamate | Neuroprotective effect | Inhibition of fibril with enhanced memory and reduced inflammation | [275] |
| Drug | Pharmacological Data | Ref |
|---|---|---|
| Poly (N-vinyl pyrrolidone) functionalized insulin nanogel | Receptor binding with protection from degradation and effective transport | [282] |
| E-beam-irradiation-based nanogel of poly(N-vinyl pyrrolidone) attached to insulin | Intranasal delivery was enhanced based on activated level of AKT with increased insulin delivery | [283] |
| Donepezil nanogel functionalized with Poly(N-isopropylacrylamide) (PNIPAM) | Biocompatible with sustained release pattern and enhanced entrapment efficiency of 87.5% | [284] |
| Methotrexate nanogels coated with polysorbate 80 | Effective brain targeting was achieved by coating with polysorbate 80 | [285] |
| Cholesterol-modified pullulan (CHP)- loaded hydrogel nanoparticles | Interacted with oligomeric Aβ and reduced its toxicity | [286] |
| Oligonucleotide-based nanogel | Less degradation with 15-fold enhanced biodistribution and two times less accumulation in the liver as compared to naked ODN | [287] |
| Nanomaterial | Pharmacological Data | Ref |
|---|---|---|
| Surface-modified gold NPs of various sizes | The concentration of gold atoms up to ~100µM does not cause any toxicity to leukemia cells. Cell viability studies demonstrated no cytotoxicity | [294] |
| Engineered gold NPs | NPs with a diameter of1–2 nm showed toxicity due to irreversible binding. No toxicity was observed in the case of NPs of the range of3–100 nm | [142] |
| Metal oxide NPs (TiO2, ZnO, FeSO4, Al2O3, and CrO) with a size range of 30–45 nm | FeSO4, Al2O3, and TiO2(concentration > 200 µg/mL) demonstrated no toxicity and at high doses, they showed LDH leakage. ZnO with a concentration range of 50–100µg/mL reduced mitochondrial function | [295] |
| Silver NPs | NPs showed toxicity via oxidative stress and a concentration of 5–50µg/mL reduced mitochondrial function along with enhanced LDH leakage | [293] |
| Silver NPs with surface charges | AgNPs exhibited toxicity depending upon their surface charge | [296] |
| Gold, silver, and platinum NPs | Exhibited toxicity via accumulation. Out of all three, the silver NPs were the most toxic whereas gold NPs were non-toxic | [297] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dighe, S.; Jog, S.; Momin, M.; Sawarkar, S.; Omri, A. Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics 2024, 16, 58. https://doi.org/10.3390/pharmaceutics16010058
Dighe S, Jog S, Momin M, Sawarkar S, Omri A. Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics. 2024; 16(1):58. https://doi.org/10.3390/pharmaceutics16010058
Chicago/Turabian StyleDighe, Sayali, Sunil Jog, Munira Momin, Sujata Sawarkar, and Abdelwahab Omri. 2024. "Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management" Pharmaceutics 16, no. 1: 58. https://doi.org/10.3390/pharmaceutics16010058
APA StyleDighe, S., Jog, S., Momin, M., Sawarkar, S., & Omri, A. (2024). Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics, 16(1), 58. https://doi.org/10.3390/pharmaceutics16010058