Microneedle-Assisted Transfersomes as a Transdermal Delivery System for Aspirin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Fabrication of Transfersomes and Drug Encapsulation
Characterisation of Transfersomes
2.2.2. Quantification of Aspirin-Encapsulated Transfersomes (TF–Asp)
2.2.3. Size, Polydispersity Index and Zeta Potential Characterisation
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR) of Aspirin and Transfersomes
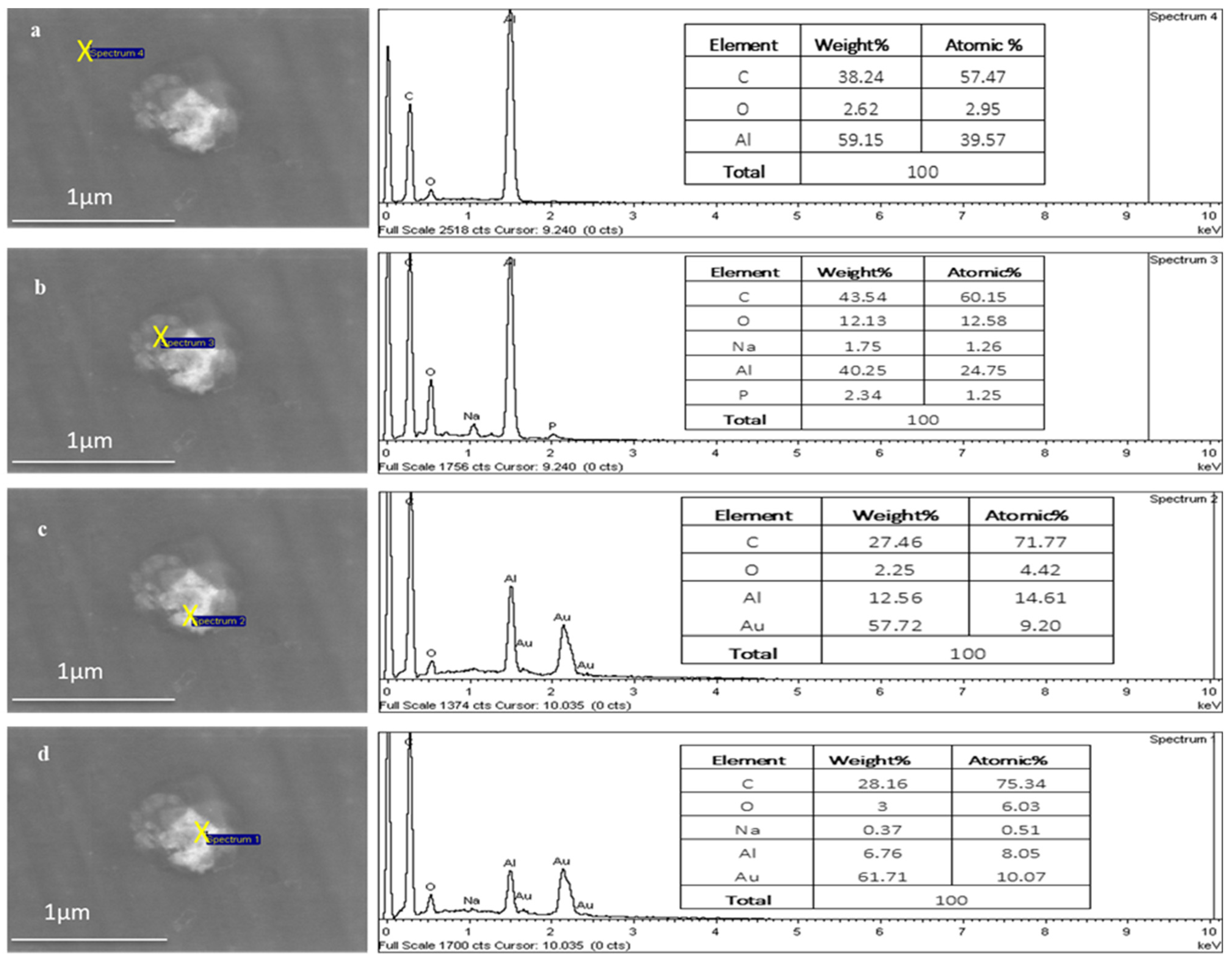
2.2.5. Energy-Dispersive X-ray Spectroscopy (EDX)
2.2.6. TEM Investigation of TF (TF and TF-Encapsulated Au-NPs)
Dissolution Studies of Encapsulated Aspirin
2.2.7. In Vitro Release of Aspirin Using Dialysis Sacks
2.2.8. Stability of TF–Asp, A Time-Dependant Release Study
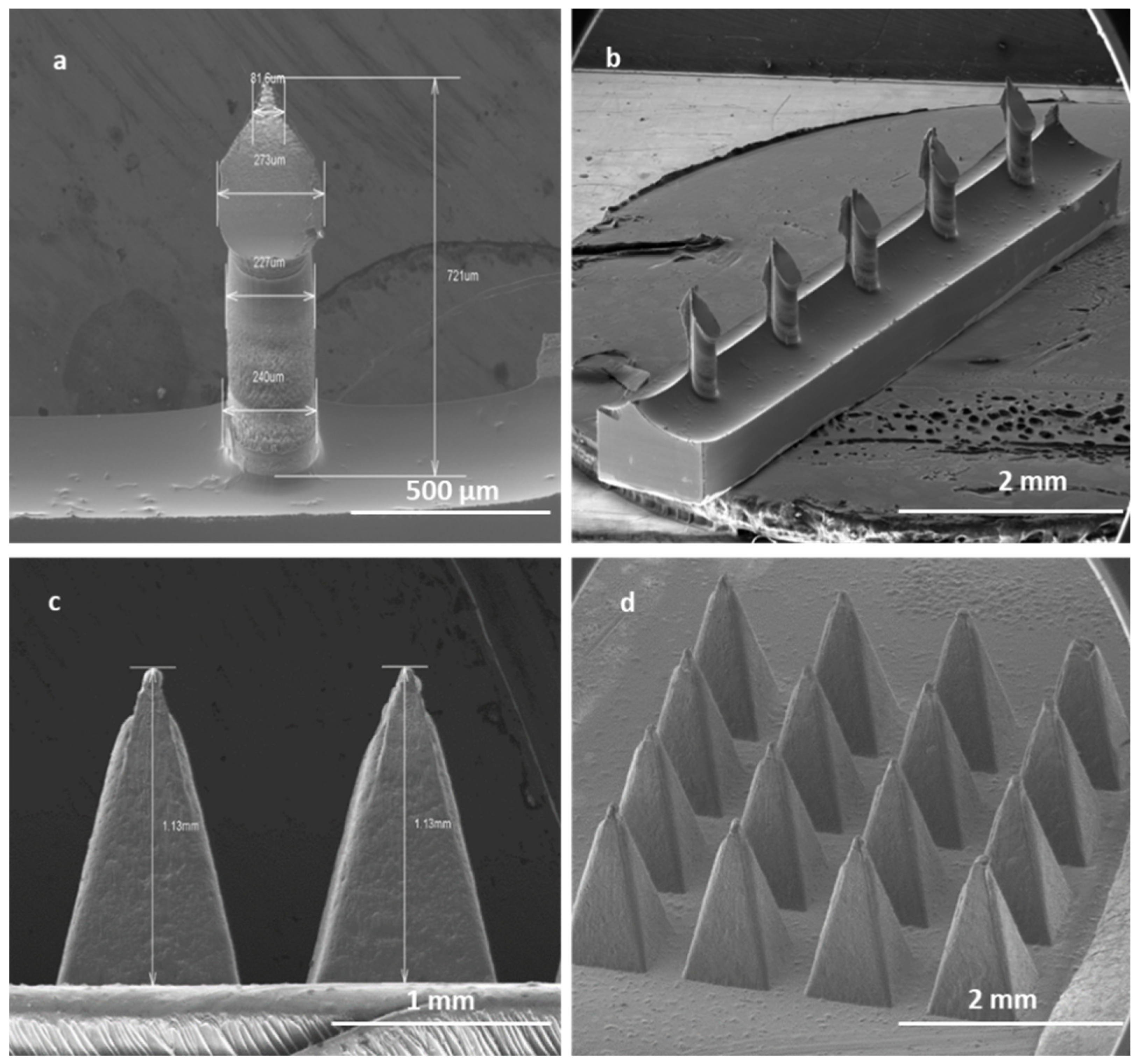
2.2.9. Microneedle Fabrication
Permeation Studies
2.2.10. Skin Sample Preparation
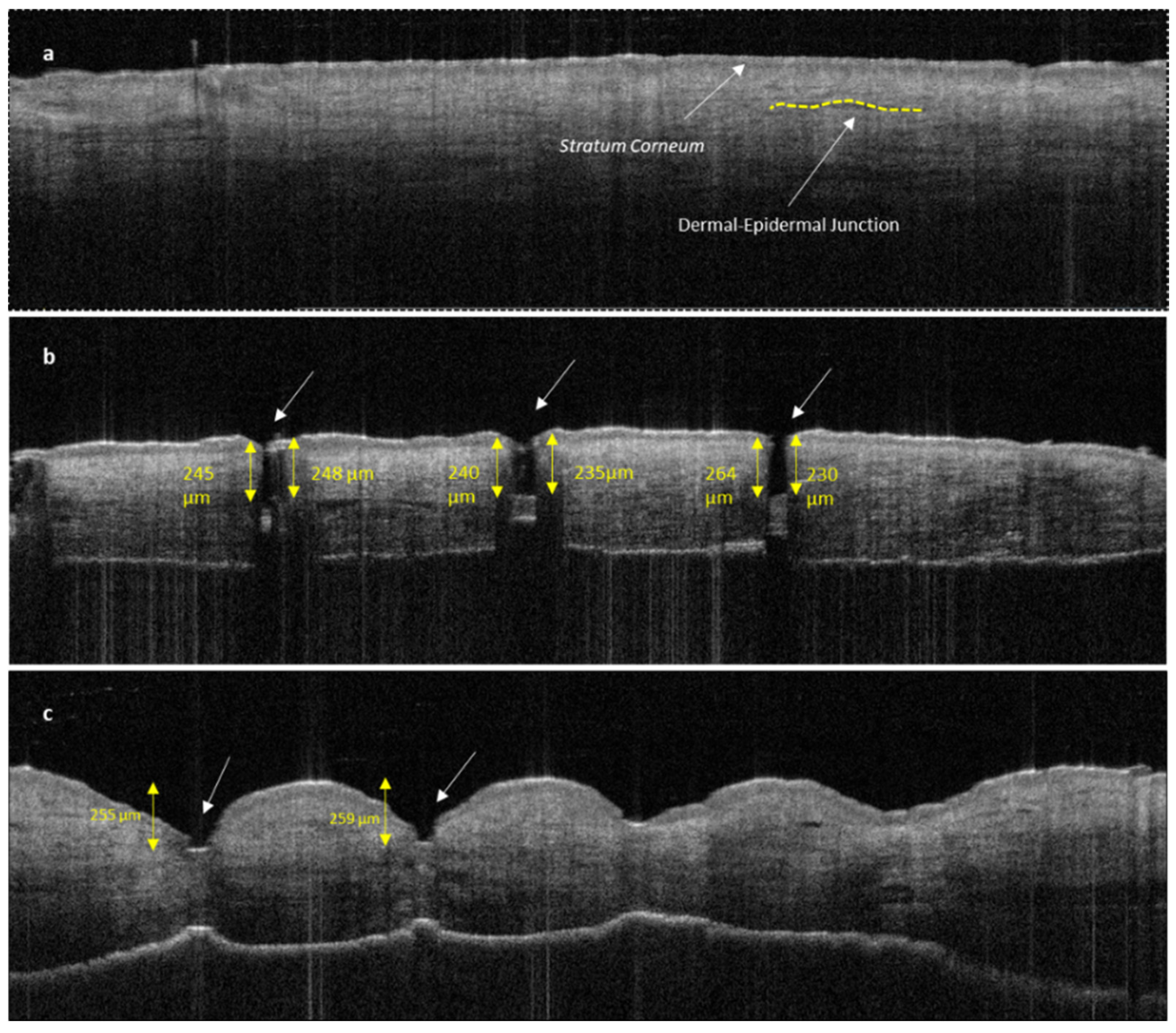
2.2.11. Optical Coherence Tomography (OCT)
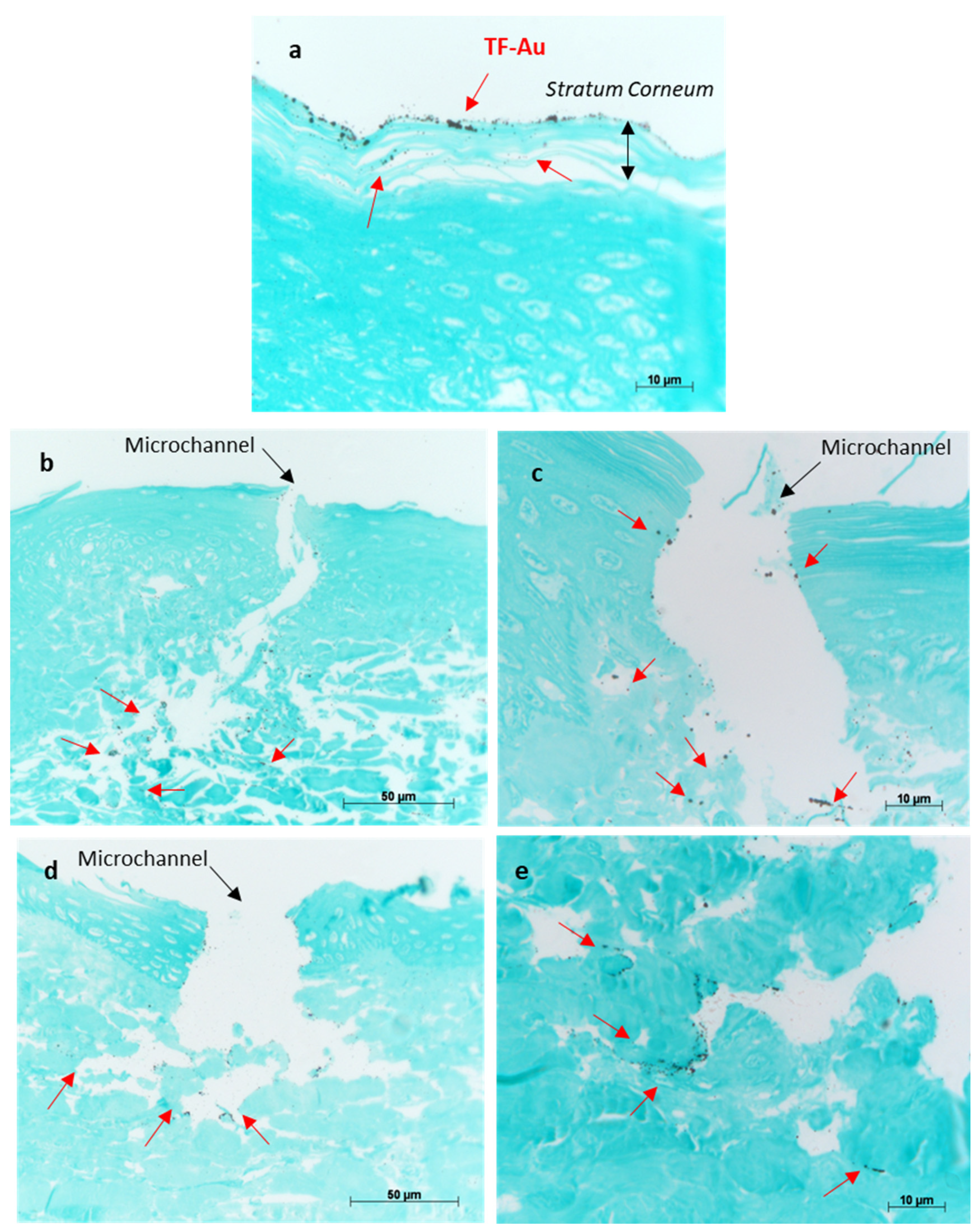
2.2.12. Application of TF-Encapsulated Au-NPs (TF–Au) for Qualitative Monitoring of Skin Permeation of TF
2.2.13. Franz Cells In Vitro Permeation Studies—Using Transfersomes and Microneedles
2.2.14. Cytotoxicity Tests of TFs, with and without Aspirin
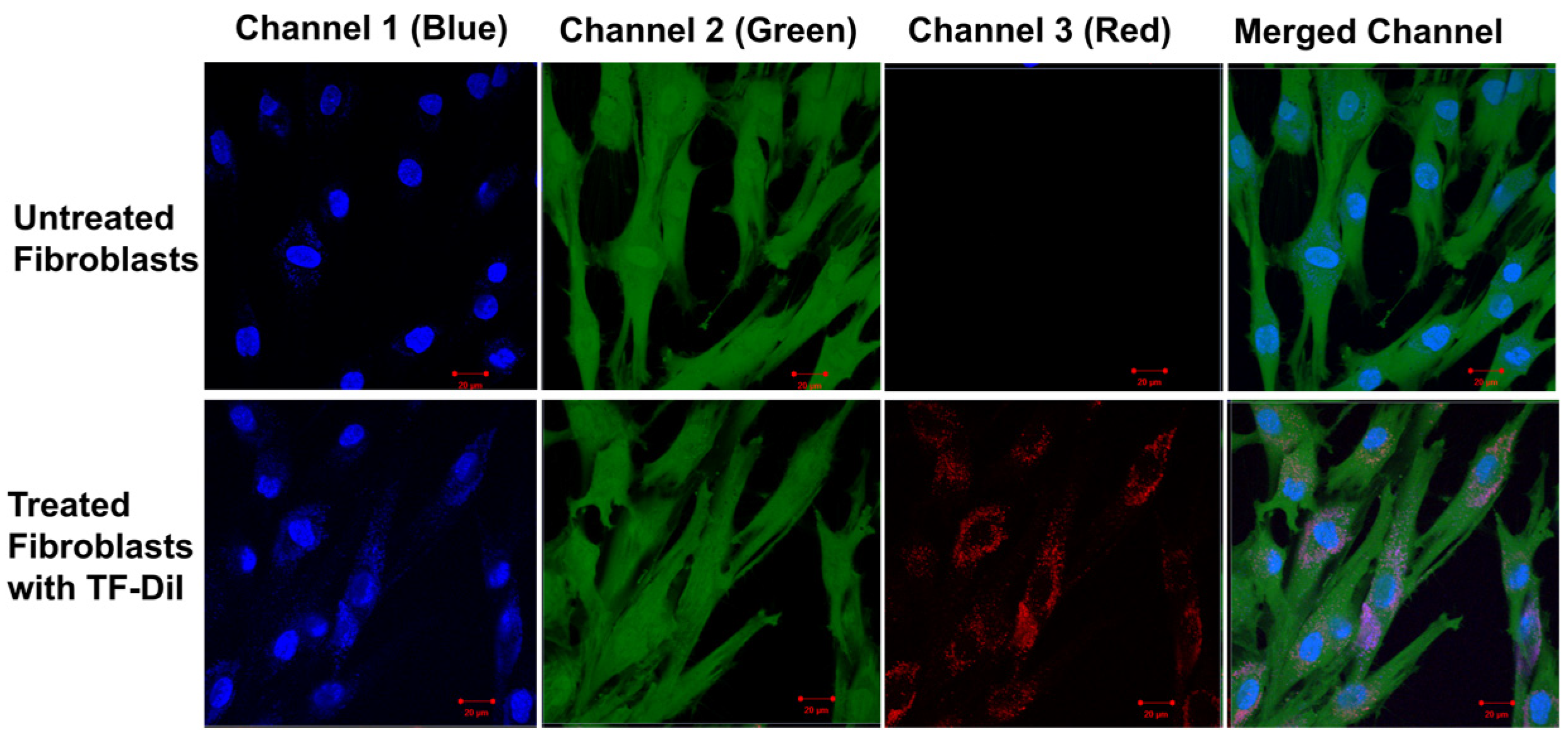
2.2.15. Intracellular Uptake of TFs by Human Fibroblast Skin Cells
2.2.16. Statistical Analysis
3. Results
3.1. Characterisation TF
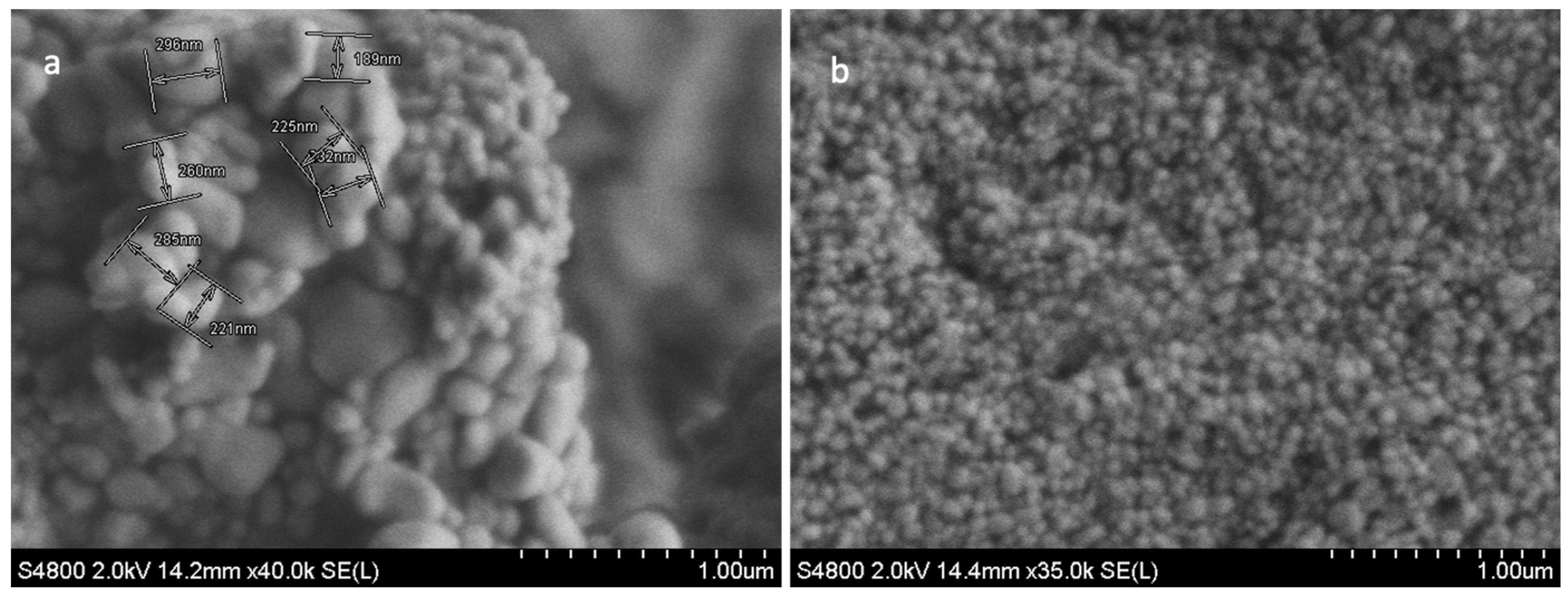
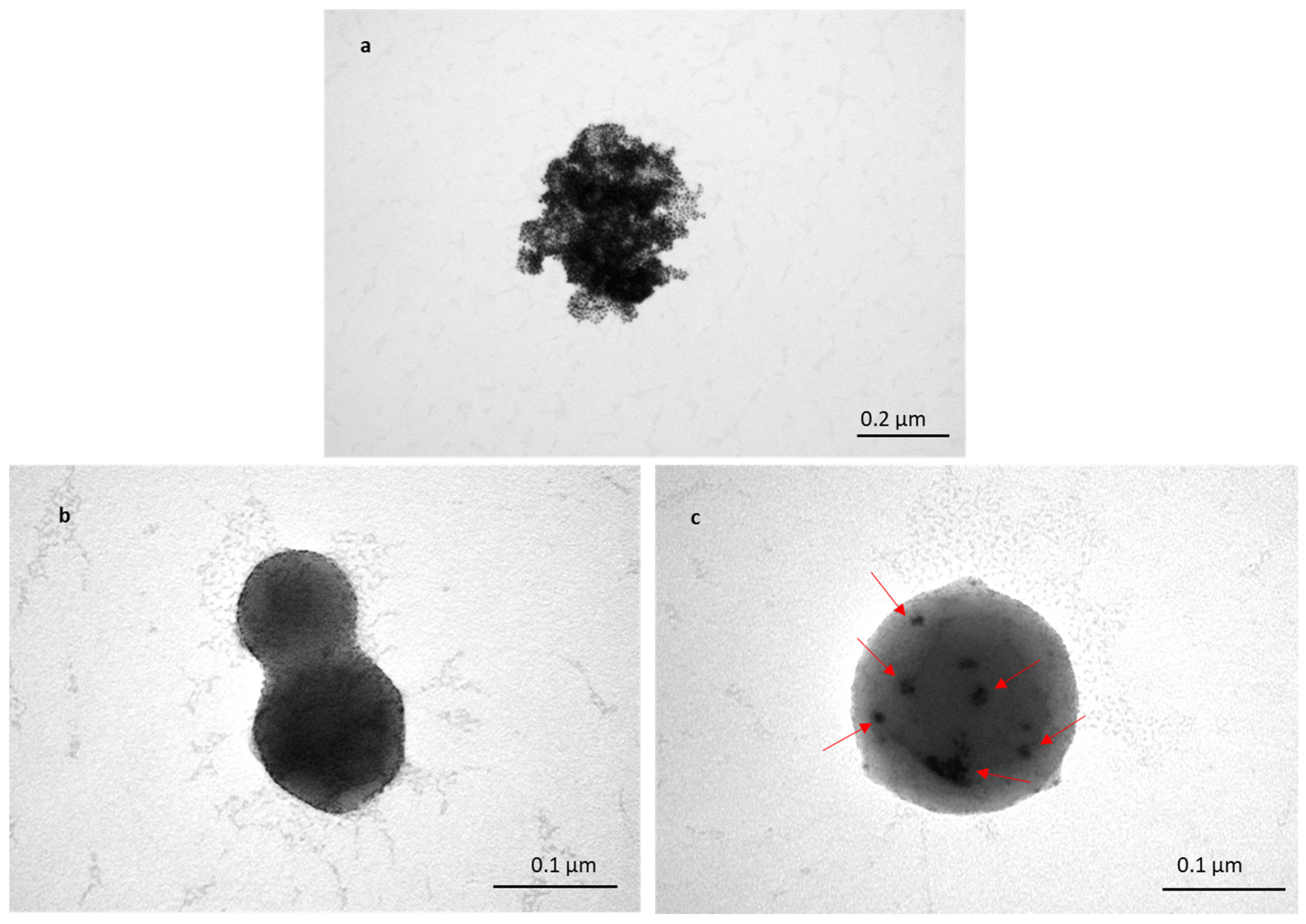
3.1.1. SEM Characterisation of TFs
3.1.2. Size Analysis
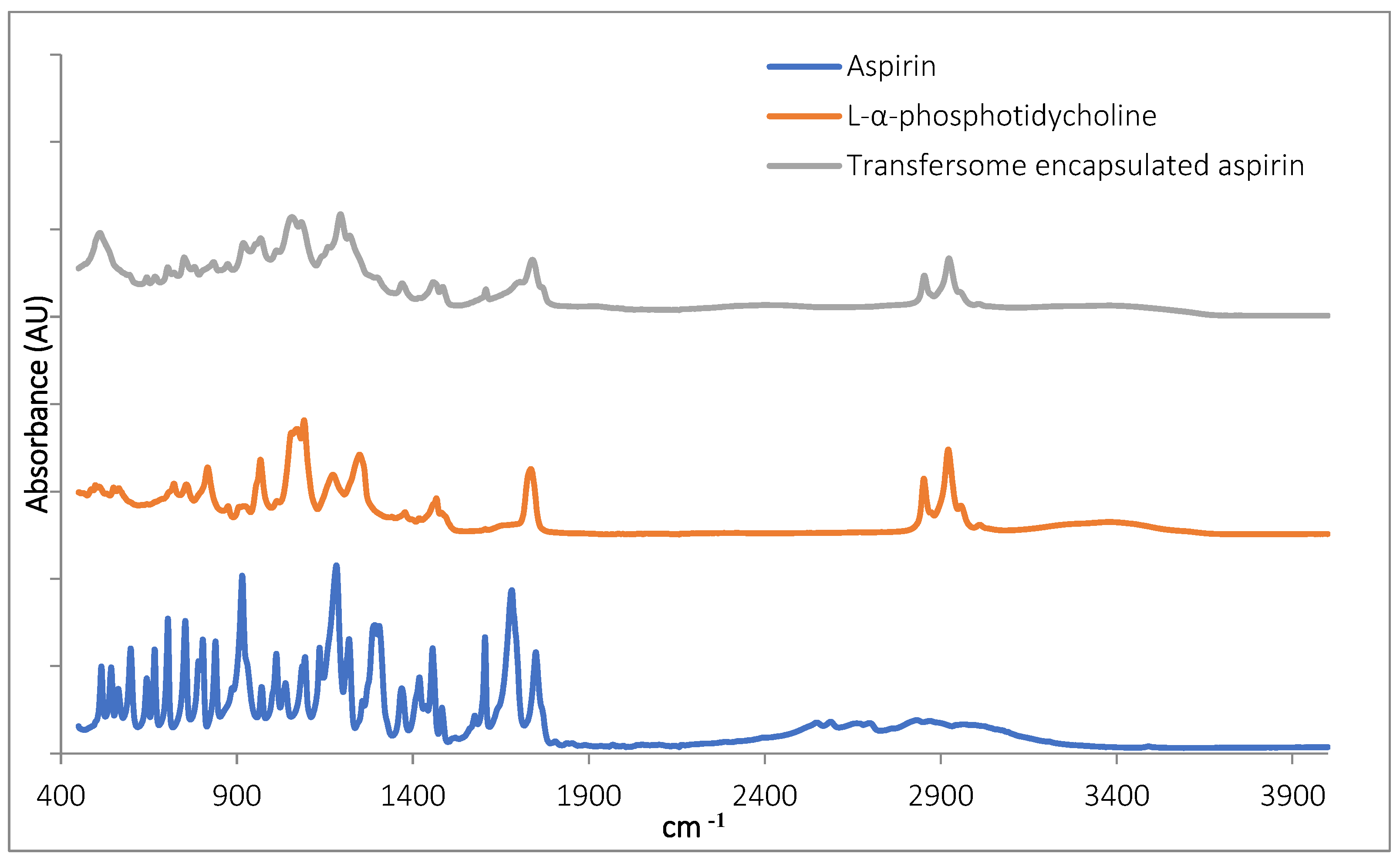
3.1.3. FTIR Characterisation of TF–Asp
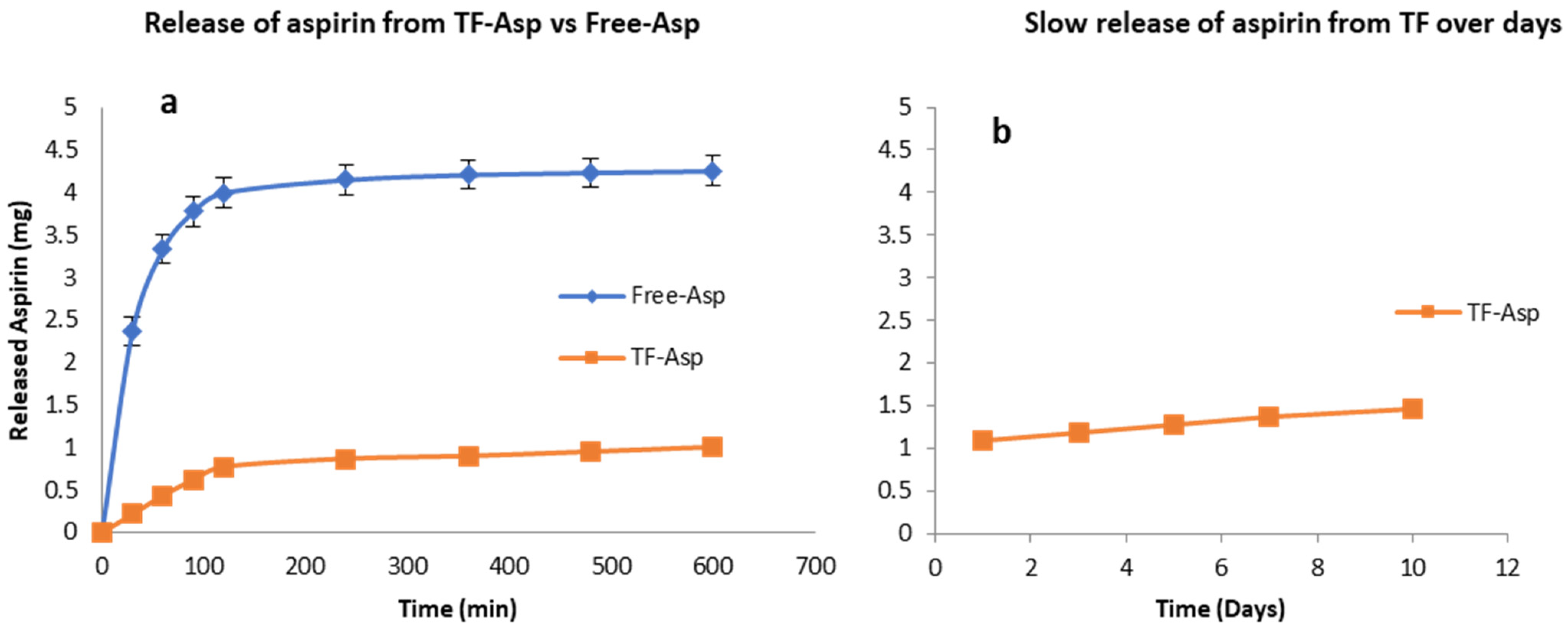
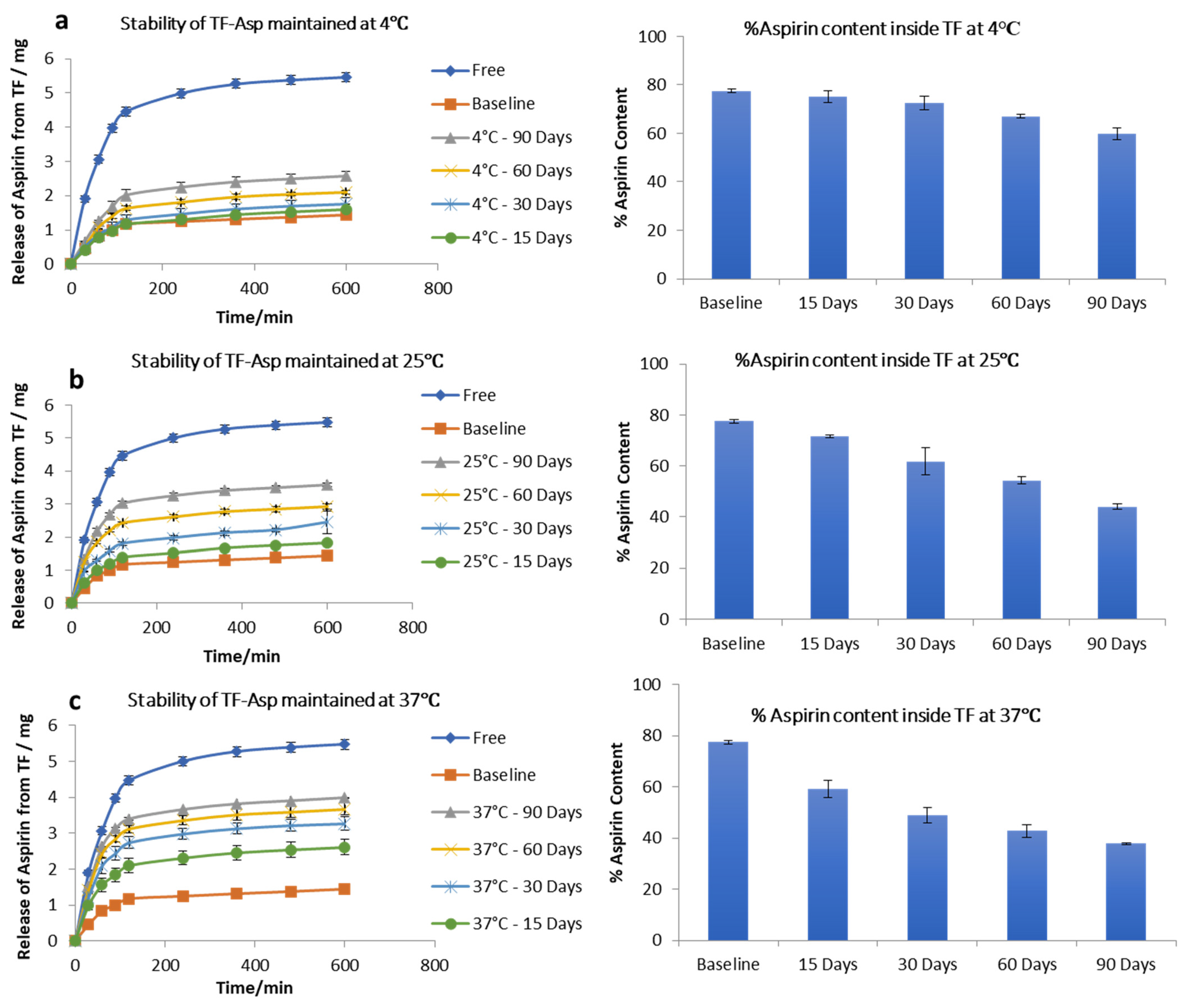
3.1.4. Drug-Load, Encapsulation Efficiency, Drug Release and Stability Study of TF–Asp
3.1.5. Encapsulation of Au-NPs inside TFs for Characterisation
3.2. Microneedle Characterisation (Silicon and Polycarbonate)
3.2.1. Morphology of Microneedles
3.2.2. Penetration Efficacy of Microneedles Using Optical Coherence Tomography (OCT)
3.2.3. Characterisation of Permeation of TF–Au into Skin Layers
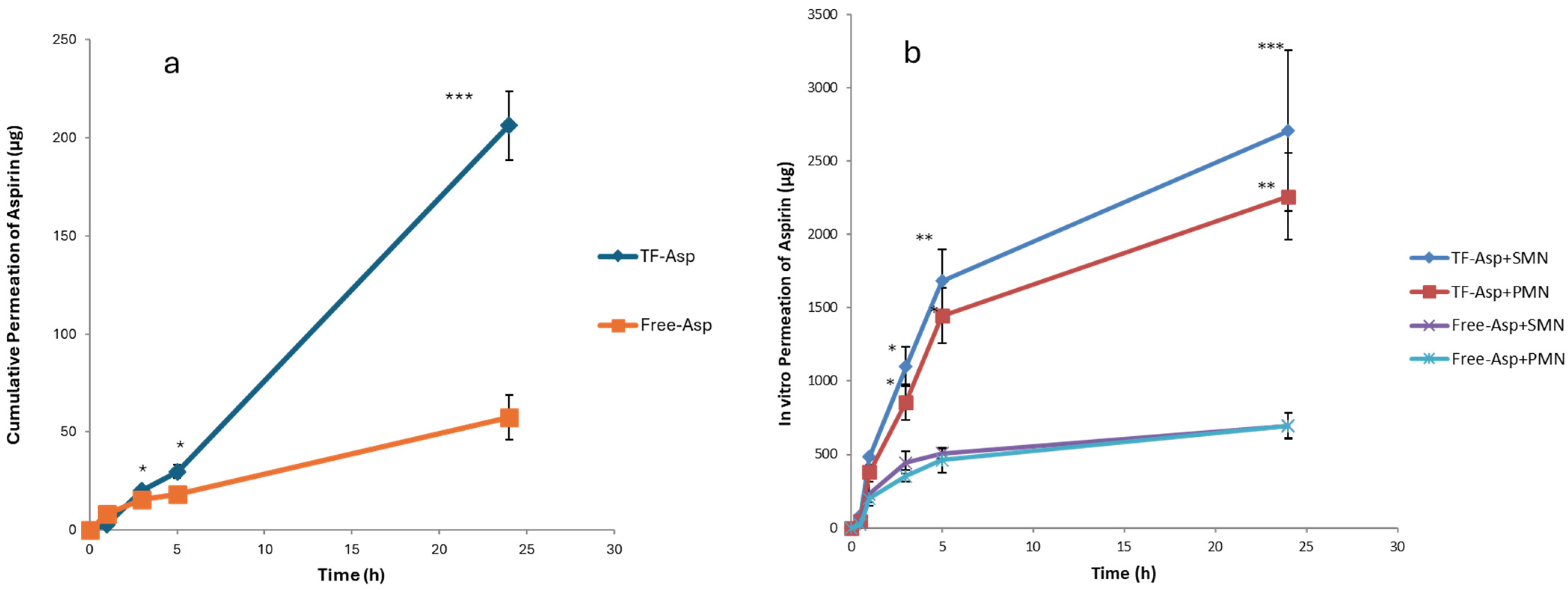
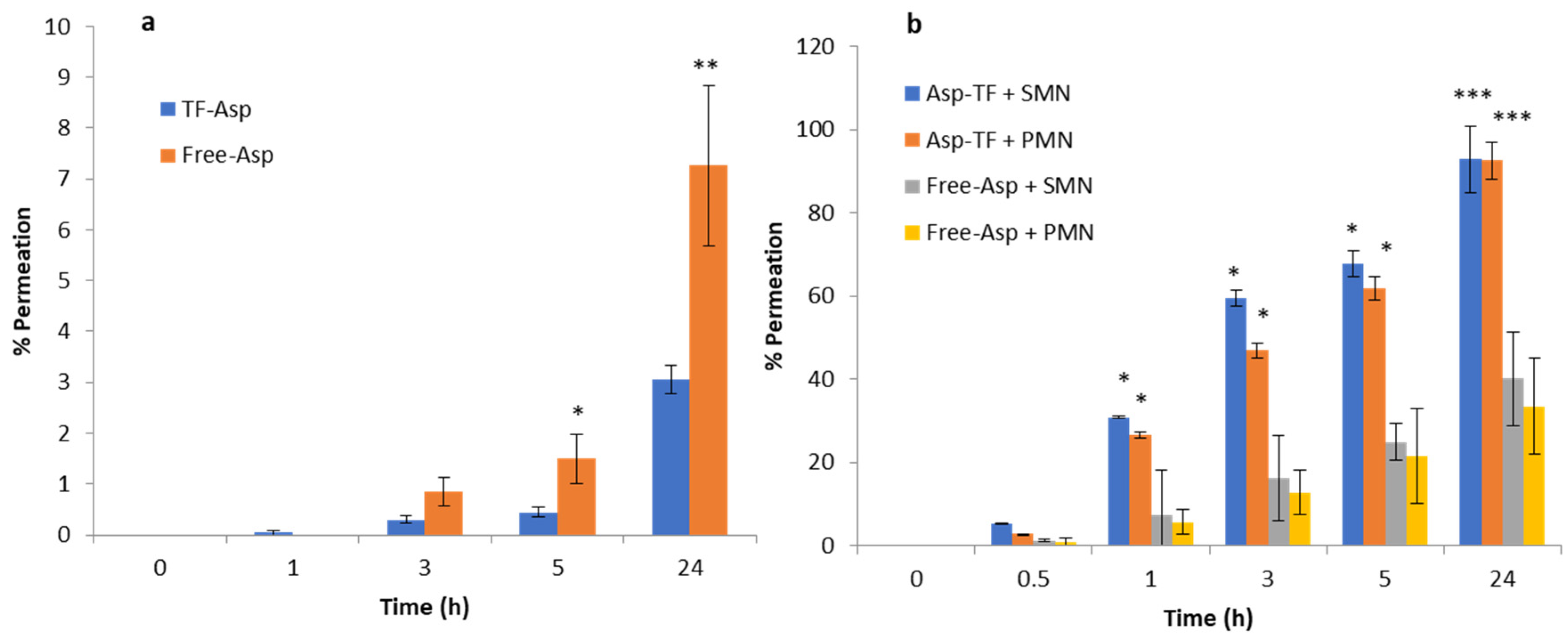
3.3. In Vitro Permeation Studies
3.4. Viability of Human Skin Fibroblasts Exposed to Different Concentrations of TFs, Free-Asp and TF–Asp
3.5. Intracellular Uptake of TFs by Human Skin Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomaa, Y.; Prausnitz, M.R. Delivery of Drugs, Vaccines, and Cosmeceuticals to Skin Using Microneedle Patches. In Percutaneous Absorption; CRC Press: Boca Raton, FL, USA, 2021; pp. 585–608. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Jain, K. Dermal and Transdermal Drug Delivery through Vesicles and Particles: Preparation and Applications. Adv. Pharm. Bull. 2021, 12, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Andrew, P.V.; Kay, L.J.; Pinnock, A.; Chittock, J.; Brown, K.; Williams, S.F.; Cork, M.J. Enhancement of stratum corneum lipid structure improves skin barrier function and protects against irritation in adults with dry, eczema-prone skin. Br. J. Dermatol. 2022, 186, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Osseiran, S.; Cruz, J.D.; Jeong, S.; Wang, H.; Fthenakis, C.; Evans, C.L. Characterizing stratum corneum structure, barrier function, and chemical content of human skin with coherent Raman scattering imaging. Biomed. Opt. Express 2018, 9, 6425–6443. [Google Scholar] [CrossRef] [PubMed]
- Denet, A.-R.; Vanbever, R.; Préat, V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004, 56, 659–674. [Google Scholar] [CrossRef]
- Seah, B.C.-Q.; Teo, B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, J.; Kumar, A.; Jyothi Prasanna, G.; Sushmitha, A.; Keerthana, K. Recent Update on Transferosomes as Transdermal Drug Delivery System. 2021. Available online: www.aditum.org (accessed on 23 May 2022).
- Miele, D.; Xia, X.; Catenacci, L.; Sorrenti, M.; Rossi, S.; Sandri, G.; Ferrari, F.; Rossi, J.J.; Bonferoni, M.C. Chitosan Oleate Coated PLGA Nanoparticles as siRNA Drug Delivery System. Pharmaceutics 2021, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Pujara, N.; Giri, R.; Wong, K.Y.; Qu, Z.; Rewatkar, P.; Moniruzzaman; Begun, J.; Ross, B.P.; McGuckin, M.; Popat, A. pH—Responsive colloidal carriers assembled from β-lactoglobulin and Epsilon poly-L-lysine for oral drug delivery. J. Colloid Interface Sci. 2021, 589, 45–55. [Google Scholar] [CrossRef]
- Bhat, M.A.; Rather, R.A.; Yaseen, Z.; Shalla, A.H. Viscoelastic and smart swelling disposition of Carboxymethylcellulose based hydrogels substantiated by Gemini surfactant and in-vitro encapsulation and controlled release of Quercetin. Int. J. Biol. Macromol. 2022, 207, 374–386. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Baek, J.; Ramasamy, M.; Willis, N.C.; Kim, D.S.; Anderson, W.A.; Tam, K.C. Encapsulation and controlled release of vitamin C in modified cellulose nanocrystal/chitosan nanocapsules. Curr. Res. Food Sci. 2021, 4, 215–223. [Google Scholar] [CrossRef]
- Tran, T.T.D.; Tran, P.H.L. Nanoconjugation and Encapsulation Strategies for Improving Drug Delivery and Therapeutic Efficacy of Poorly Water-Soluble Drugs. Pharmaceutics 2019, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; Terreni, E.; et al. pH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Carpi, S.; Digiacomo, M.; Polini, B.; Fogli, S.; Burgalassi, S.; Macchia, M.; Nieri, P.; Manera, C.; Monti, D. Diclofenac-Derived Hybrids for Treatment of Actinic Keratosis and Squamous Cell Carcinoma. Molecules 2019, 24, 1793. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Swain, S.S.; Sekar, M.; Karupiah, S.; Porwal, O.; Sahoo, A.; et al. Ultraflexible Liposome Nanocargo as a Dermal and Transdermal Drug Delivery System. Nanomaterials 2021, 11, 2557. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate. Saudi Pharm. J. 2017, 25, 1040–1046. [Google Scholar] [CrossRef]
- Aboud, H.M.; Ali, A.A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of carvedilol for intranasal delivery: Formulation, characterization and in vivo evaluation. Drug Deliv. 2015, 23, 2471–2481. [Google Scholar] [CrossRef]
- Fernández-García, R.; Lalatsa, A.; Statts, L.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Transferosomes as nanocarriers for drugs across the skin: Quality by design from lab to industrial scale. Int. J. Pharm. 2020, 573, 118817. [Google Scholar] [CrossRef]
- Cevc, G.; Gebauer, D.; Stieber, J.; Schätzlein, A.; Blume, G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta-Biomembr. 1998, 1368, 201–215. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Unnisa, S.J.; Arvapalli, S.; Karunakar, B.; Reddy, P.R.; Vaishnavi, A.; Sharma, J.V.C. A narrative review on transfersomes: Vesicular transdermal delivery system for enhanced drug permeation. Int. Res. J. Pharm. 2021, 12, 1–4. [Google Scholar] [CrossRef]
- Rajan, R.; Jose, S.; Mukund, V.P.B.; Vasudevan, D.T. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Valenti, D.; Cencetti, C.; Diez-Sales, O.; Nacher, A.; Mir-Palomo, S.; Terencio, M.C.; Demurtas, D.; et al. Nanodesign of new self-assembling core-shell gellan-transfersomes loading baicalin and in vivo evaluation of repair response in skin. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 569–579. [Google Scholar] [CrossRef]
- Gupta, A.; Aggarwal, G.; Singla, S.; Arora, R. Transfersomes: A Novel vesicular carrier for enhanced transdermal delivery of sertraline: Development, characterization, and performance evaluation. Sci. Pharm. 2012, 80, 1061–1080. [Google Scholar] [CrossRef]
- Rahbari, R.; Ichim, I.; Bamsey, R.; Burridge, J.; Guy, O.J.; Bolodeoku, J.; Graz, M. Characterisation of Drug Delivery Efficacy Using Microstructure-Assisted Application of a Range of APIs. Pharmaceutics 2020, 12, 1213. [Google Scholar] [CrossRef]
- Chaurasiya, P.; Ganju, E.; Upmanyu, N.; Ray, S.K.; Jain, P. Transfersomes: A novel technique for transdermal drug delivery. J. Drug Deliv. Ther. 2019, 9, 279–285. [Google Scholar] [CrossRef]
- Podlipec, R. Interaction of Liposomes on Endothelial Cells. Semin. IV, Univerza v Ljubljani. 2010, pp. 1–15. Available online: http://mafija.fmf.uni-lj.si/seminar/files/2010_2011/seminar2_pop.pdf (accessed on 1 February 2022).
- Yang, J.; Bahreman, A.; Daudey, G.; Bussmann, J.; Olsthoorn, R.C.L.; Kros, A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Central Sci. 2016, 2, 621–630. [Google Scholar] [CrossRef]
- Sabri, A.H.; Kim, Y.; Marlow, M.; Scurr, D.J.; Segal, J.; Banga, A.K.; Kagan, L.; Lee, J.B. Intradermal and transdermal drug delivery using microneedles—Fabrication, performance evaluation and application to lymphatic delivery. Adv. Drug Deliv. Rev. 2020, 153, 195–215. [Google Scholar] [CrossRef]
- Petchsangsai, M.; Wonglertnirant, N.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Application of Hollow Microneedle for Transdermal Delivery of Bovine Serum Albumin-Fluorescein Isothiocyanate Conjugate. Adv. Mater. Res. 2011, 338, 365–368. [Google Scholar] [CrossRef]
- Caudill, C.; Perry, J.L.; Iliadis, K.; Tessema, A.T.; Lee, B.J.; Mecham, B.S.; Tian, S.; DeSimone, J.M. Transdermal vaccination via 3D-printed microneedles induces potent humoral and cellular immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2102595118. [Google Scholar] [CrossRef]
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 2022, 12, 3372–3406. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Lim, D.-J.; Kim, H.-J. Microneedles in Action: Microneedling and Microneedles-Assisted Transdermal Delivery. Polymers 2022, 14, 1608. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Bolton, C.J.W.; Howells, O.; Blayney, G.J.; Eng, P.F.; Birchall, J.C.; Gualeni, B.; Roberts, K.; Ashraf, H.; Guy, O.J. Hollow silicon microneedle fabrication using advanced plasma etch technologies for applications in transdermal drug delivery. Lab A Chip 2020, 20, 2788–2795. [Google Scholar] [CrossRef]
- Kaushik, S.; Hord, A.H.; Denson, D.D.; McAllister, D.V.; Smitra, S.; Allen, M.G.; Prausnitz, M.R. Lack of pain associated with microfabricated microneedles. Anesth. Analg. 2001, 92, 502–504. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11159258 (accessed on 18 April 2019). [CrossRef]
- Nguyen, J.; Ita, K.B.; Morra, M.J.; Popova, I.E. The Influence of Solid Microneedles on the Transdermal Delivery of Selected Antiepileptic Drugs. Pharmaceutics 2016, 8, 33. [Google Scholar] [CrossRef]
- Olatunji, O.; Olubowale, M.; Okereke, C. Microneedle-assisted transdermal delivery of acetylsalicylic acid (aspirin) from biopolymer films extracted from fish scales. Polym. Bull. 2018, 75, 4103–4115. [Google Scholar] [CrossRef]
- Donnelly, R.; Douroumis, D. Microneedles for drug and vaccine delivery and patient monitoring. Drug Deliv. Transl. Res. 2015, 5, 311–312. [Google Scholar] [CrossRef]
- Wollina, U.; Tirant, M.; Vojvodic, A.; Lotti, T. Treatment of Psoriasis: Novel Approaches to Topical Delivery. Open Access Maced. J. Med. Sci. 2019, 7, 3018–3025. [Google Scholar] [CrossRef]
- Jing, Q.; Ruan, H.; Li, J.; Wang, Z.; Pei, L.; Hu, H.; He, Z.; Wu, T.; Ruan, S.; Guo, T.; et al. Keratinocyte membrane-mediated nanodelivery system with dissolving microneedles for targeted therapy of skin diseases. Biomaterials 2021, 278, 121142. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hou, X.; Li, J.; Ruan, H.; Pei, L.; Guo, T.; Wang, Z.; Ci, T.; Ruan, S.; He, Y.; et al. Microneedle-Mediated Biomimetic Cyclodextrin Metal Organic Frameworks for Active Targeting and Treatment of Hypertrophic Scars. ACS Nano 2021, 15, 20087–20104. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Chen, X.; Zhou, Z.; Pang, J.; Luo, X.; Kong, M. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunization. J. Mater. Chem. B 2019, 7, 4854–4866. [Google Scholar] [CrossRef] [PubMed]
- Wluka, A.; Ding, C.; Wang, Y.; Jones, G.; Cicuttini, F. Aspirin is associated with reduced cartilage loss in knee osteoarthritis: Data from a cohort study. Osteoarthr. Cartil. 2014, 22, S400. [Google Scholar] [CrossRef]
- Khodayar, S.; Bardania, H.; Shojaosadati, S.A.; Bagheri, F. Optimization and Characterization of Aspirin Encapsulated Nano-liposomes. Iran. J. Pharm. Res. 2018, 17, 11–22. [Google Scholar] [PubMed]
- Yuan, M.; Zhan, Y.; Hu, W.; Li, Y.; Xie, X.; Miao, N.; Jin, H.; Zhang, B. Aspirin promotes osteogenic differentiation of human dental pulp stem cells. Int. J. Mol. Med. 2018, 42, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, Y.; Luo, R.-C.; Li, A.-M. Aspirin for the primary prevention of skin cancer: A meta-analysis. Oncol. Lett. 2015, 9, 1073–1080. [Google Scholar] [CrossRef]
- Bubna, A.K. Aspirin in dermatology: Revisited. Indian Dermatol. Online J. 2015, 6, 428–435. [Google Scholar] [CrossRef]
- Khan, I.; Needham, R.; Yousaf, S.; Houacine, C.; Islam, Y.; Bnyan, R.; Sadozai, S.K.; Elrayess, M.A.; Elhissi, A. Impact of phospholipids, surfactants and cholesterol selection on the performance of transfersomes vesicles using medical nebulizers for pulmonary drug delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102822. [Google Scholar] [CrossRef]
- Kamkar, A.; Molaee-Aghaee, E.; Khanjari, A.; Akhondzadeh-Basti, A.; Noudoost, B.; Shariatifar, N.; Sani, M.A.; Soleimani, M. Nanocomposite active packaging based on chitosan biopolymer loaded with nano-liposomal essential oil: Its characterizations and effects on microbial, and chemical properties of refrigerated chicken breast fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef]
- Lamparelli, E.; Ciardulli, M.; Scala, P.; Scognamiglio, M.; Charlier, B.; Di Pietro, P.; Izzo, V.; Vecchione, C.; Maffulli, N.; Della Porta, G. Lipid nano-vesicles for thyroid hormone encapsulation: A comparison between different fabrication technologies, drug loading, and an in vitro delivery to human tendon stem/progenitor cells in 2D and 3D culture. Int. J. Pharm. 2022, 624, 122007. [Google Scholar] [CrossRef] [PubMed]
- Talebi, V.; Ghanbarzadeh, B.; Hamishehkar, H.; Pezeshki, A.; Ostadrahimi, A. Effects of different stabilizers on colloidal properties and encapsulation efficiency of vitamin D3 loaded nano-niosomes. J. Drug Deliv. Sci. Technol. 2021, 61, 101284. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Arsov, Z.; González-Ramírez, E.J.; Goñi, F.M.; Tristram-Nagle, S.; Nagle, J.F. Phase behavior of palmitoyl and egg sphingomyelin. Chem. Phys. Lipids 2018, 213, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.R.; Jasani, B. Silver Development In Microscopy And Bioanalysis: A New Versatile Formulation For Modern Needs. Histochem. J. 1998, 30, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Jin, S.-P.; Doh, E.J.; Lee, D.H.; Chung, J.H. Regional Variation of Human Skin Surface Temperature. Ann. Dermatol. 2019, 31, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Aggarwal, S. Salicylic Acid (Aspirin). StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519032/ (accessed on 22 December 2023).
- Jong, A.A.; Nangare-Patil, P.; Patil, S.S.; Vakhariya, R.R.; Mohite, S.K. Transfersomes: A Peculiar and Promising Technique for Transdermal Drug Delivery. Int. J. Pharm. Sci. Med. 2021, 6, 67–82. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Sulkowski, W.; Pentak, D.; Korus, W.; Sulkowska, A. Effect of temperature on liposome structures studied using EPR spectroscopy. Spectroscopy 2005, 19, 37–42. [Google Scholar] [CrossRef]
- Hadidi, N.; Saffari, M.; Faizi, M. Optimized transferosomal bovine lactoferrin (BLF) as a promising novel non-invasive topical treatment for genital warts caused by human papiluma virus (HPV). Iran. J. Pharm. Res. 2018, 17, 12–23. [Google Scholar]
- Sou, K.; Tsuchida, E. Electrostatic interactions and complement activation on the surface of phospholipid vesicle containing acidic lipids: Effect of the structure of acidic groups. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1035–1041. [Google Scholar] [CrossRef]
- Ducat, E.; Evrard, B.; Peulen, O.; Piel, G. Cellular uptake of liposomes monitored by confocal microscopy and flow cytometry. J. Drug Deliv. Sci. Technol. 2011, 21, 469–477. [Google Scholar] [CrossRef]
- Lammertink, B.; Deckers, R.; Derieppe, M.; De Cock, I.; Lentacker, I.; Storm, G.; Moonen, C.; Bos, C. Dynamic Fluorescence Microscopy of Cellular Uptake of Intercalating Model Drugs by Ultrasound-Activated Microbubbles. Mol. Imaging Biol. 2017, 19, 683–693. [Google Scholar] [CrossRef]
- Kettler, K.; Veltman, K.; van de Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Nojoki, F.; Ebrahimi-Hosseinzadeh, B.; Hatamian-Zarmi, A.; Khodagholi, F.; Khezri, K. Design and development of chitosan-insulin-transfersomes (Transfersulin) as effective intranasal nanovesicles for the treatment of Alzheimer’s disease: In vitro, in vivo, and ex vivo evaluations. Biomed. Pharmacother. 2022, 153, 113450. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef]
- Guillot, A.J.; Merino-Gutiérrez, P.; Bocchino, A.; O’Mahony, C.; Giner, R.M.; Recio, M.C.; Garrigues, T.M.; Melero, A. Exploration of Microneedle-assisted skin delivery of cyanocobalamin formulated in ultraflexible lipid vesicles. Eur. J. Pharm. Biopharm. 2022, 177, 184–198. [Google Scholar] [CrossRef]
- Cui, Y.; Mo, Y.; Zhang, Q.; Tian, W.; Xue, Y.; Bai, J.; Du, S. Microneedle-Assisted Percutaneous Delivery of Paeoniflorin-Loaded Ethosomes. Molecules 2018, 23, 3371. [Google Scholar] [CrossRef]
- Liu, C.; Quan, P.; Fang, L. Effect of drug physicochemical properties on drug release and their relationship with drug skin permeation behaviors in hydroxyl pressure sensitive adhesive. Eur. J. Pharm. Sci. 2016, 93, 437–446. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Rani, R.S. Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian J. Pharm. Sci. 2008, 70, 94–96. [Google Scholar] [CrossRef]
- Kokate, A.; Li, X.; Jasti, B. Effect of drug lipophilicity and ionization on permeability across the buccal mucosa: A technical note. AAPS PharmSciTech 2008, 9, 501–504. [Google Scholar] [CrossRef][Green Version]
- Ng, K.W.; Lau, W.M. Skin Deep: The Basics of Human Skin Structure and Drug Penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar] [CrossRef]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.-W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of Size, Surface Charge, and Hydrophobicity of Poly(amidoamine) Dendrimers on Their Skin Penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Boonme, P.; Songkro, S.; Krauel, K.; Rades, T. Transdermal delivery of hydrophobic and hydrophilic local anesthetics from o/w and w/o Brij 97-based microemulsions. J. Pharm. Pharm. Sci. 2007, 10, 288–298. [Google Scholar]
- Xie, F.; Chai, J.-K.; Hu, Q.; Yu, Y.-H.; Ma, L.; Liu, L.-Y.; Zhang, X.-L.; Li, B.-L.; Zhang, D.-H. Transdermal permeation of drugs with differing lipophilicity: Effect of penetration enhancer camphor. Int. J. Pharm. 2016, 507, 90–101. [Google Scholar] [CrossRef]
- Muro, S. Mucosal Barriers. In Drug Delivery Across Physiological Barriers; Pan Stanford: Stanford, CA, USA, 2016; pp. 155–180. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Gomaa, Y.A.; Garland, M.J.; McInnes, F.J.; Donnelly, R.F.; El-Khordagui, L.K.; Wilson, C.G. Flux of ionic dyes across microneedle-treated skin: Effect of molecular characteristics. Int. J. Pharm. 2012, 438, 140–149. [Google Scholar] [CrossRef]
- Cross, S.E.; Magnusson, B.M.; Winckle, G.; Anissimov, Y.; Roberts, M.S. Determination of the Effect of Lipophilicity on the in vitro Permeability and Tissue Reservoir Characteristics of Topically Applied Solutes in Human Skin Layers. J. Investig. Dermatol. 2003, 120, 759–764. [Google Scholar] [CrossRef]
- N’Da, D.D. Prodrug Strategies for Enhancing the Percutaneous Absorption of Drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef]
- Williams, A. Transdermal and Topical Drug Delivery from Theory to Clinical Practice; Pharmaceutical Press: London, UK, 2003. [Google Scholar]
- Chantasart, D.; Chootanasoontorn, S.; Suksiriworapong, J.; Li, S.K. Investigation of pH Influence on Skin Permeation Behavior of Weak Acids Using Nonsteroidal Anti-Inflammatory Drugs. J. Pharm. Sci. 2015, 104, 3459–3470. [Google Scholar] [CrossRef]
- Knudsen, N.Ø.; Pedersen, G.P. pH and Drug Delivery; Karger Publishers: Basel, Switzerland, 2018; Volume 54, pp. 143–151. [Google Scholar] [CrossRef]
- Dowd, F.J. Pharmacokinetics: The absorption, distribution, and fate of drugs. In Pharmacology and Therapeutics for Dentistry, 7th ed.; Mosby, Inc.: St Louis, MO, USA, 2017; pp. 15–43. [Google Scholar] [CrossRef]
- Kumar, S.; Duke, J.C. Local Anesthetics. In Duke’s Anesthesia Secrets E-Book, 4th ed.; MOSBY ELSEVIER Publishers: Philadelphia, PA, USA, 2011; pp. 105–111. [Google Scholar] [CrossRef]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F.; Pearlman, E. General and ocular pharmacology. In The Eye, Basic Sciences and Practice, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 338–369.e1. [Google Scholar] [CrossRef]
- Allegaert, K.; Van Den Anker, J.N. Physicochemical and Structural Properties Regulating Placental Drug Transfer. Fetal Neonatal Physiol. 2017, 1, 208–221.e4. [Google Scholar] [CrossRef]
- Iriarte, C.; Awosika, O.; Rengifo-Pardo, M.; Ehrlich, A. Review of applications of microneedling in dermatology. Clin. Cosmet. Investig. Dermatol. 2017, 10, 289–298. [Google Scholar] [CrossRef]
- Gowda, A.; Healey, B.; Ezaldein, H.; Merati, M. A systematic review examining the potential adverse effects of microneedling. J. Clin. Aesthetic Dermatol. 2021, 14, 45–54. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7869810/ (accessed on 22 April 2023).
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef]
- Garnacho, C. Intracellular Drug Delivery: Mechanisms for Cell Entry. Curr. Pharm. Des. 2016, 22, 1210–1226. [Google Scholar] [CrossRef]
- Prabha, S.; Zhou, W.-Z.; Panyam, J.; Labhasetwar, V. Size-dependency of nanoparticle-mediated gene transfection: Studies with fractionated nanoparticles. Int. J. Pharm. 2002, 244, 105–115. [Google Scholar] [CrossRef]
- Salatin, S.; Khosroushahi, A.Y. Overviews on the cellular uptake mechanism of polysaccharide colloidal nanoparticles. J. Cell. Mol. Med. 2017, 21, 1668–1686. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Shen, L.-N.; Wu, Z.-H.; Zhao, J.-H.; Feng, N.-P. Evaluation of Skin Viability Effect on Ethosome and Liposome-Mediated Psoralen Delivery via Cell Uptake. J. Pharm. Sci. 2014, 103, 3120–3126. [Google Scholar] [CrossRef]
- Weiss, P. Microneedle Makers Seek to Engineer a Better Shot. Engineering 2021, 7, 1661–1664. [Google Scholar] [CrossRef]
- Shin, J.U.; Kim, J.D.; Kim, H.K.; Kang, H.K.; Joo, C.; Lee, J.H.; Jeong, D.H.; Song, S.; Chu, H.; Lee, J.S.; et al. The use of biodegradable microneedle patches to increase penetration of topical steroid for prurigo nodularis. Eur. J. Dermatol. 2018, 28, 71–77. [Google Scholar] [CrossRef]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]




| Samples | Concentration (µg/mL) | ||||
|---|---|---|---|---|---|
| Free-Asp | 400 | 200 | 100 | 50 | 25 |
| TF–Asp | 400 | 200 | 100 | 50 | 25 |
| Sample | Concentration (mg/mL) | ||||
|---|---|---|---|---|---|
| TF | 0.8 | 0.4 | 0.2 | 0.1 | 0.05 |
| TF | Baseline | 4 °C | 25 °C | 37 °C |
|---|---|---|---|---|
| Size | 88.06 ± 8 | 89 ± 1.5 nm | 182 ± 3 nm | 247 ± 4 nm |
| PDI | 0.23 ± 0.07 | 0.226 ± 0.07 | 0.239 ± 0.09 | 0.589 ± 0.1 |
| Sample | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| TF | 88.06 ± 8 | 0.23 ± 0.07 | 2.64 ± 0.41 |
| TF–Asp | 106 ± 23 | 0.24 ± 0.01 | 9.96 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahbari, R.; Francis, L.; Guy, O.J.; Sharma, S.; Von Ruhland, C.; Xia, Z. Microneedle-Assisted Transfersomes as a Transdermal Delivery System for Aspirin. Pharmaceutics 2024, 16, 57. https://doi.org/10.3390/pharmaceutics16010057
Rahbari R, Francis L, Guy OJ, Sharma S, Von Ruhland C, Xia Z. Microneedle-Assisted Transfersomes as a Transdermal Delivery System for Aspirin. Pharmaceutics. 2024; 16(1):57. https://doi.org/10.3390/pharmaceutics16010057
Chicago/Turabian StyleRahbari, Raha, Lewis Francis, Owen J. Guy, Sanjiv Sharma, Christopher Von Ruhland, and Zhidao Xia. 2024. "Microneedle-Assisted Transfersomes as a Transdermal Delivery System for Aspirin" Pharmaceutics 16, no. 1: 57. https://doi.org/10.3390/pharmaceutics16010057
APA StyleRahbari, R., Francis, L., Guy, O. J., Sharma, S., Von Ruhland, C., & Xia, Z. (2024). Microneedle-Assisted Transfersomes as a Transdermal Delivery System for Aspirin. Pharmaceutics, 16(1), 57. https://doi.org/10.3390/pharmaceutics16010057







