Experimental and Modelling Study of Controlled Release from Dextran-Based Cryogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Dextran Methacrylate
2.3. Synthesis of Dextran Derivatives
2.4. Cryogel Preparation
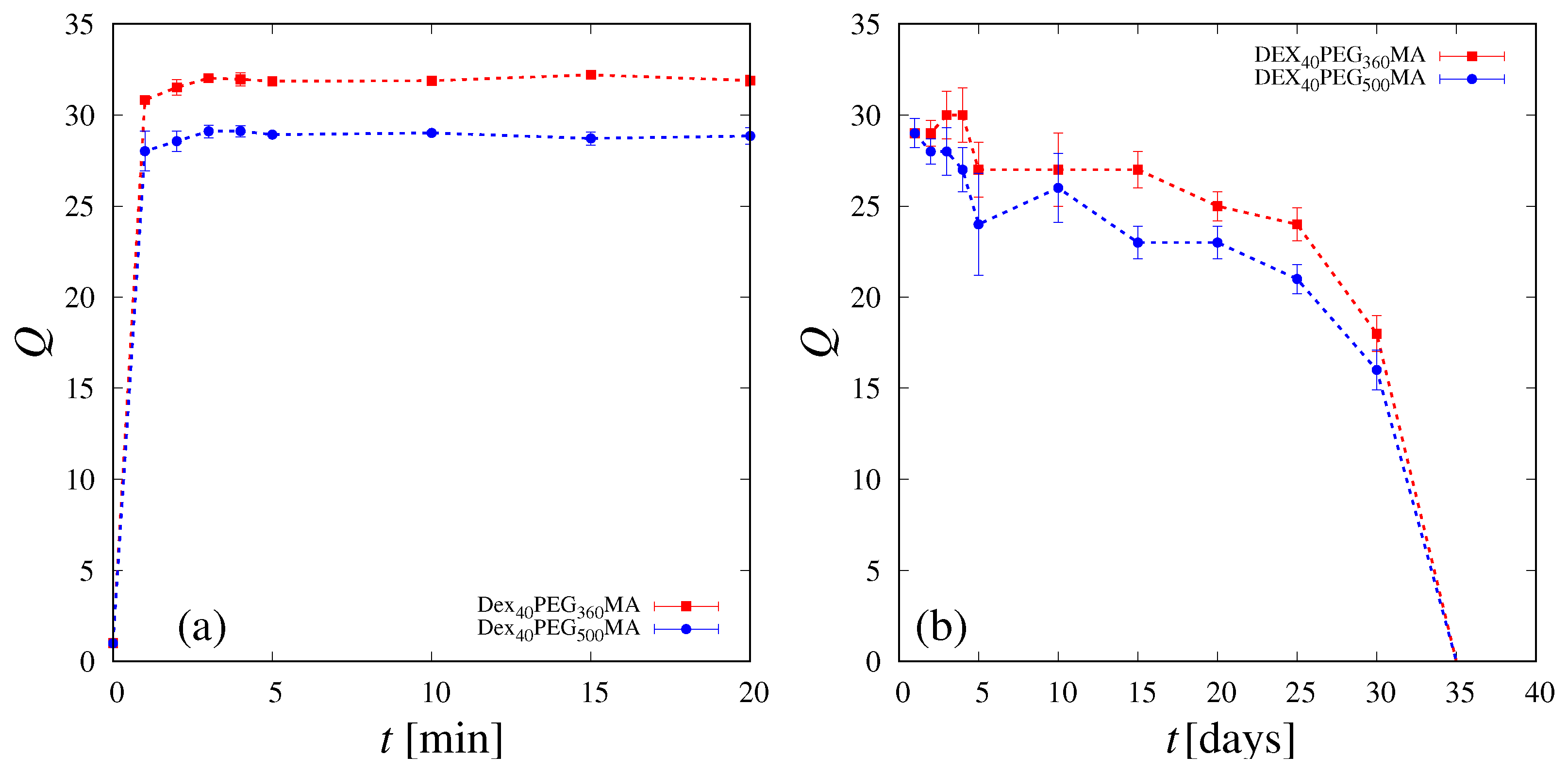
2.5. Absorption, Swelling and Degradation Studies
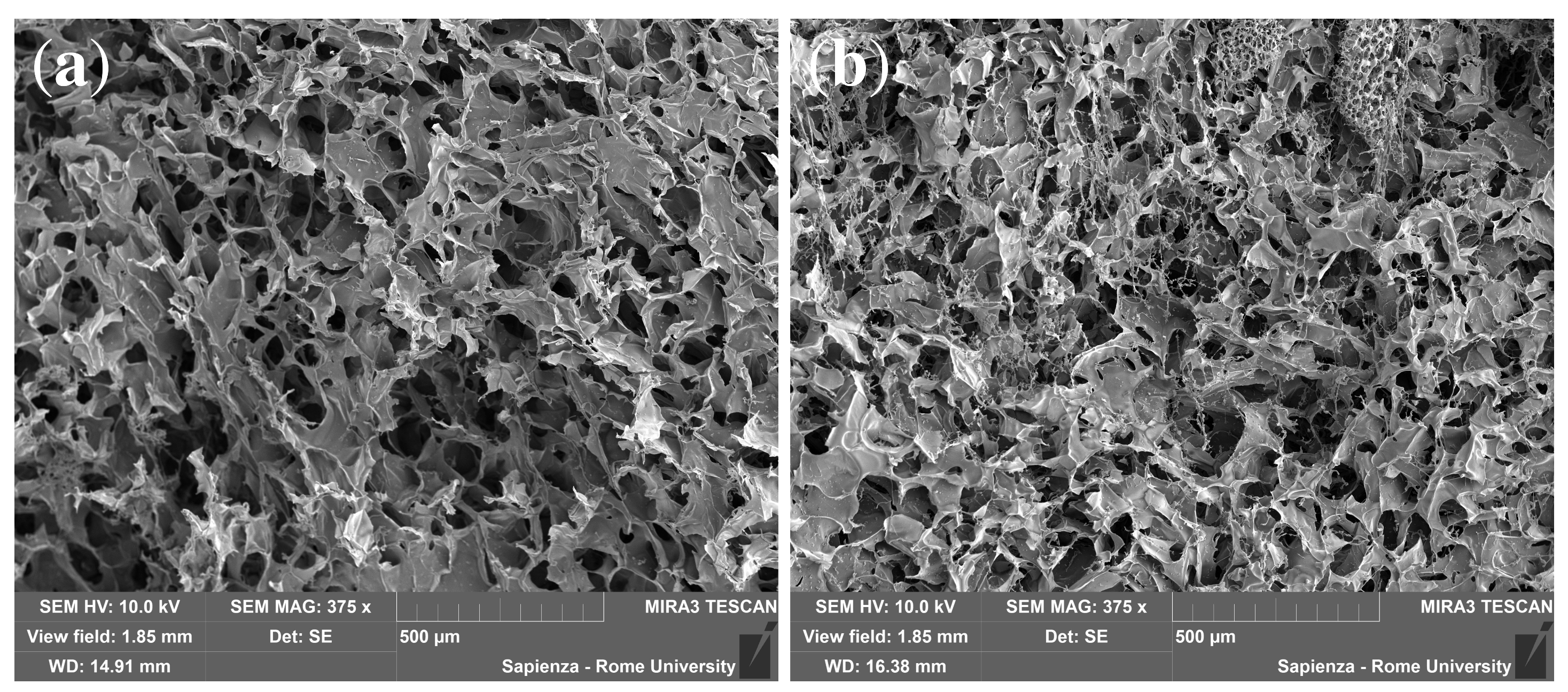
2.6. Morphological Characterization
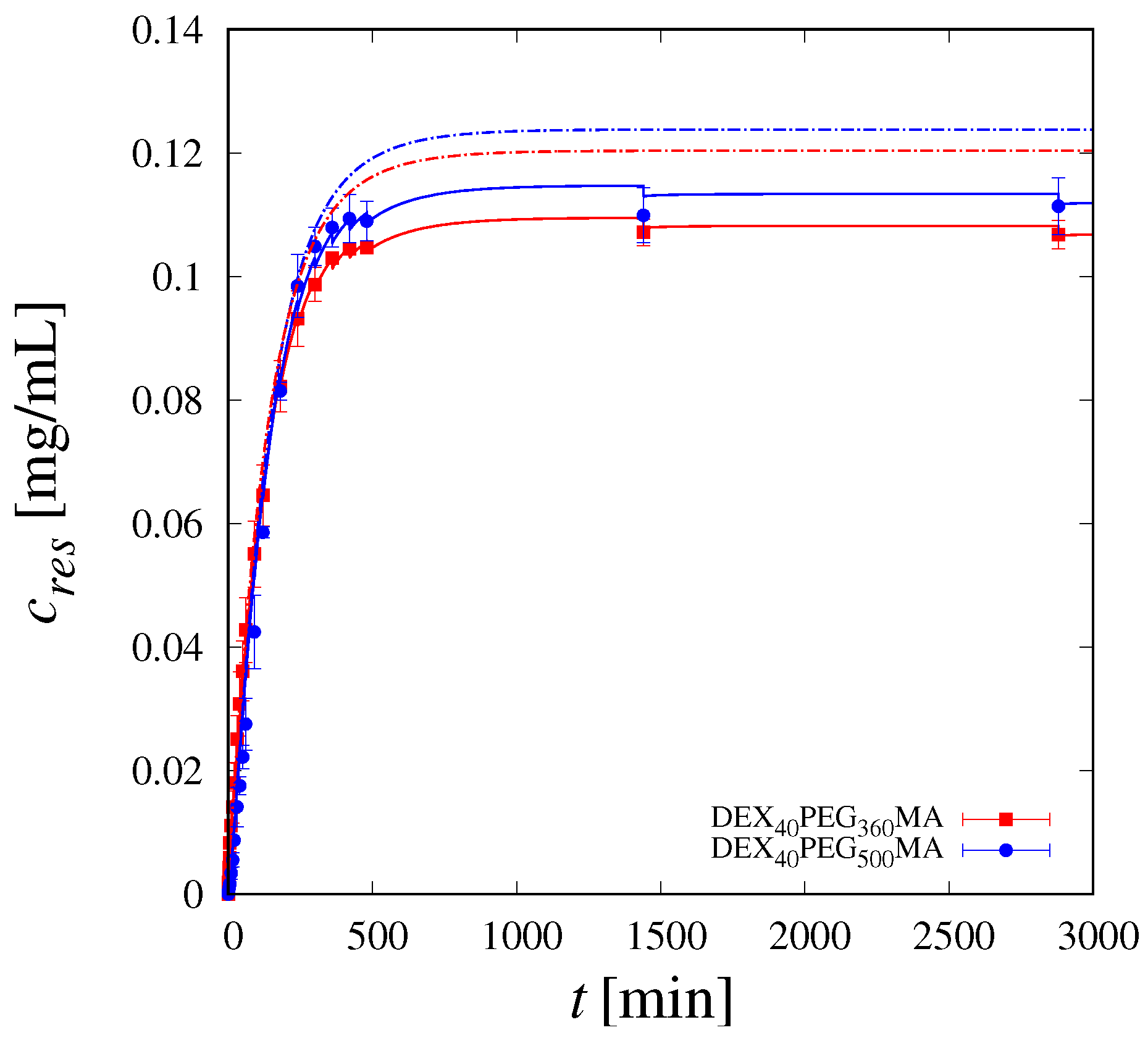
2.7. Release of Vitamin B12
- 1.
- Loading before cryogel formation (pre-loading)Cryogels were loaded with 2.5 mg of vitamin B12 by introducing a solution of vitamin B12 in distilled water (2.5 mg/mL) directly into the system before the low-temperature crosslinking step, which was carried out as reported in Section 2.4, leading to the cryogel formation.
- 2.
- Loading on preformed cryogel (after loading)Freeze-dried cryogel samples were infused with a solution of vitamin B12. The concentration of the solution was arranged so that, on the base of absorption volumes, the amount of vitamin B12 in the cryogel was 2.5 mg. After loading, the specimens were lyophilized.
3. Results and Discussion
3.1. Swelling and SEM Analysis
3.2. Release Studies
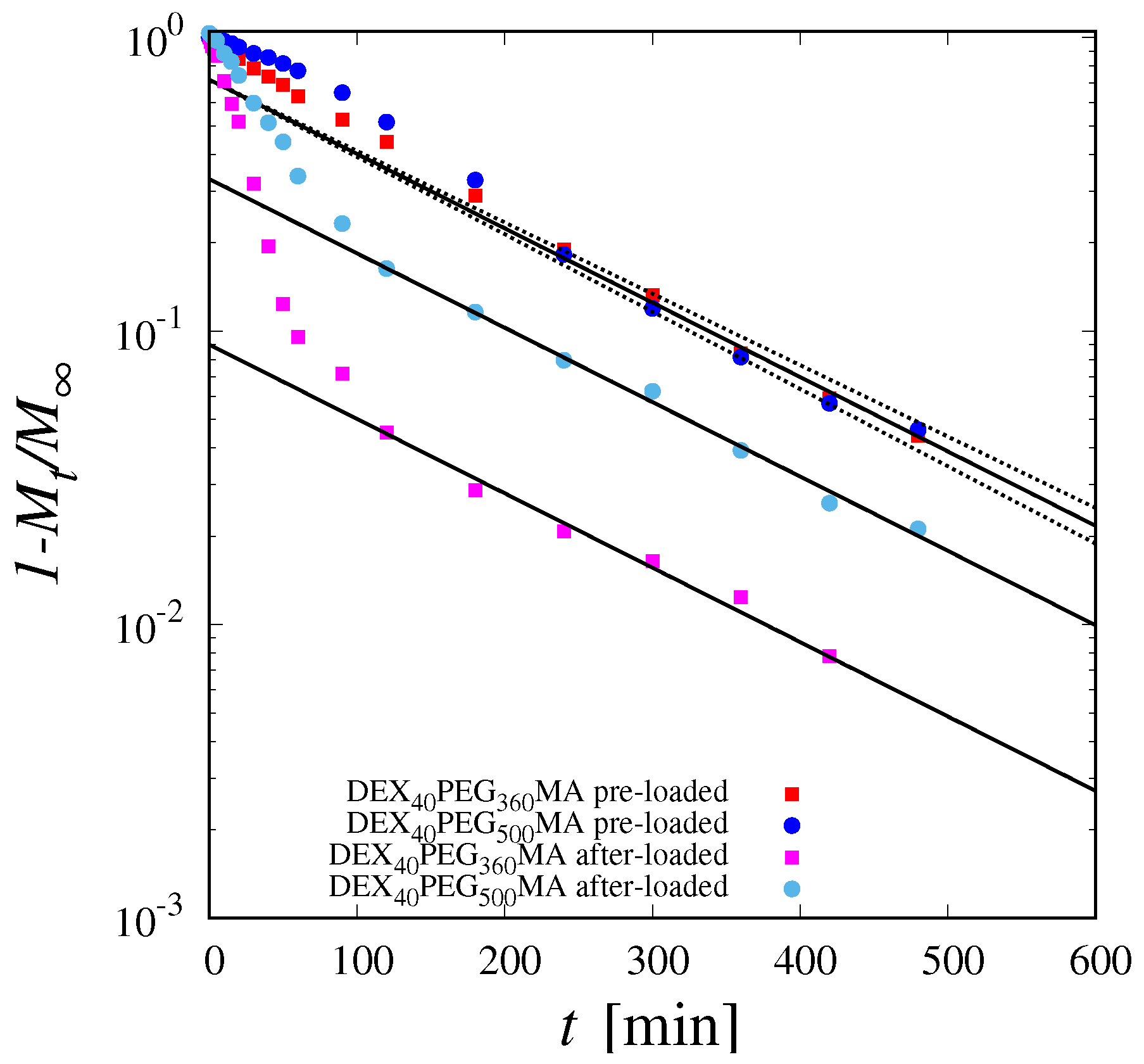
3.3. Theoretical Model of Drug Release in the Pre-Loading Case
3.4. Estimation of the Diffusion Coefficient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- de Castro, J.M.C.K.C.; Campos, M.G.N. Drug-loaded polymeric nanoparticles: A review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–13. [Google Scholar] [CrossRef]
- Abasian, P.; Ghanavati, S.; Rahebi, S.; Khorasani, S.N.; Khalili, S. Polymeric nanocarriers in targeted drug delivery systems: A review. Polym. Adv. Technol. 2020, 31, 2939–2954. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Gholamali, I.; Yadollahi, M. Bio-nanocomposite polymer hydrogels containing nanoparticles for drug delivery: A review. Regen. Eng. Transl. Med. 2021, 7, 129–146. [Google Scholar] [CrossRef]
- Laracuente, M.L.; Marina, H.Y.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef] [PubMed]
- Öncel, P.; Çetin, K.; Topçu, A.A.; Yavuz, H.; Denizli, A. Molecularly imprinted cryogel membranes for mitomycin C delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 519–531. [Google Scholar] [CrossRef]
- Newland, B.; Varricchio, C.; Körner, Y.; Hoppe, F.; Taplan, C.; Newland, H.; Eigel, D.; Tornillo, G.; Pette, D.; Brancale, A.; et al. Focal drug administration via heparin-containing cryogel microcarriers reduces cancer growth and metastasis. Carbohydr. Polym. 2020, 245, 116504. [Google Scholar] [CrossRef]
- Evans, C.; Morimitsu, Y.; Hisadome, T.; Inomoto, F.; Yoshida, M.; Takei, T. Optimized hydrophobically modified chitosan cryogels for strength and drug delivery systems. J. Biosci. Bioeng. 2021, 132, 81–87. [Google Scholar] [CrossRef]
- Ari, B.; Sahiner, M.; Suner, S.S.; Demirci, S.; Sahiner, N. Super-macroporous pulluan cryogels as controlled active delivery systems with controlled degradability. Micromachines 2023, 14, 1323. [Google Scholar] [CrossRef]
- Hakami, A.; Narasimhan, K.; Comini, G.; Thiele, J.; Werner, C.; Dowd, E.; Newland, B. Cryogel microcarriers for sustained local delivery of growth factors to the brain. J. Control. Release 2024, 369, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Jahanmir, G.; Chau, Y. Chapter 9—Mathematical models of drug release from degradable hydrogels. In Biomedical Applications of Nanoparticles; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 233–269. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and application. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. TRENDS Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, A. Multi-featured macroporous agarose–alginate cryogel: Synthesis and characterization for bioengineering applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of aerogels, cryogels and xerogels of alginate: Effect of molecular weight, gelation conditions and drying method on particles’ micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef]
- Bauleth-Ramos, T.; Shih, T.Y.; Shahbazi, M.A.; Najibi, A.J.; Mao, A.S.; Liu, D.; Granja, P.; Santos, H.A.; Sarmento, B.; Mooney, D.J. Acetalated dextran nanoparticles loaded into an injectable alginate cryogel for combined chemotherapy and cancer vaccination. Adv. Funct. Mater. 2019, 29, 1903686. [Google Scholar] [CrossRef]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef]
- Buchtová, N.; Pradille, C.; Bouvard, J.L.; Budtova, T. Mechanical properties of cellulose aerogels and cryogels. Soft Matter. 2019, 15, 7901–7908. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, N.; Tripathi, A.; Kar, K.K.; Kumar, A. Synthesis and characterization of elastic and macroporous chitosan–gelatin cryogels for tissue engineering. Acta Biomater. 2009, 5, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Tripathi, A.; Kumar, A. Supermacroprous chitosan–agarose–gelatin cryogels: In vitro characterization and in vivo assessment for cartilage tissue engineering. J. R. Soc. Interface 2011, 8, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Konovalova, M.V.; Markov, P.A.; Durnev, E.A.; Kurek, D.V.; Popov, S.V.; Varlamov, V.P. Preparation and biocompatibility evaluation of pectin and chitosan cryogels for biomedical application. J. Biomed. Mater. Res. Part A 2017, 105, 547–556. [Google Scholar] [CrossRef]
- Han, M.E.; Kim, S.H.; Kim, H.D.; Yim, H.G.; Bencherif, S.A.; Kim, T.I.; Hwang, N.S. Extracellular matrix-based cryogels for cartilage tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1410–1419. [Google Scholar] [CrossRef]
- Yu, X.; Qian, G.; Chen, S.; Xu, D.; Zhao, X.; Du, C. A tracheal scaffold of gelatin-chondroitin sulfate-hyaluronan-polyvinyl alcohol with orientated porous structure. Carbohydr. Polym. 2017, 159, 20–28. [Google Scholar] [CrossRef]
- Inci, I.; Kirsebom, H.; Galaev, I.Y.; Mattiasson, B.; Piskin, E. Gelatin cryogels crosslinked with oxidized dextran and containing freshly formed hydroxyapatite as potential bone tissue-engineering scaffolds. J. Tissue Eng. Regen. Med. 2013, 7, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Di Muzio, L.; Paolicelli, P.; Fortunati, V.; Petralito, S.; Trilli, J.; Casadei, M.A. Dextran-polyethylene glycol cryogels as spongy scaffolds for drug delivery. Int. J. Biol. Macromol. 2021, 166, 1292–1300. [Google Scholar] [CrossRef]
- Di Muzio, L.; Sergi, C.; Carriero, V.C.; Tirillò, J.; Adrover, A.; Messina, E.; Gaetani, R.; Petralito, S.; Casadei, M.A.; Paolicelli, P. Gelatin-based spongy and compressive resistant cryogels with shape recovery ability as ideal scaffolds to support cell adhesion for tissue regeneration. React. Funct. Polym. 2023, 189, 105607. [Google Scholar] [CrossRef]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, L.; Yang, Y.; Yin, Z.; Guo, B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2022, 608, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Kumar, A. Cell proliferation on three-dimensional chitosan–agarose–gelatin cryogel scaffolds for tissue engineering applications. J. Biosci. Bioeng. 2012, 114, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, A.; Weisser, J.; Schnabelrauch, M. Glycosaminoglycan-Based Cryogels as Scaffolds for Cell Cultivation and Tissue Regeneration. Molecules 2021, 26, 5597. [Google Scholar] [CrossRef]
- Yılmaz, F.; Bereli, N.; Yavuz, H.; Denizli, A. Supermacroporous hydrophobic affinity cryogels for protein chromatography. Biochem. Eng. J. 2009, 43, 272–279. [Google Scholar] [CrossRef]
- Srivastava, A.; Shakya, A.K.; Kumar, A. Boronate affinity chromatography of cells and biomacromolecules using cryogel matrices. Enzym. Microb. Technol. 2012, 51, 373–381. [Google Scholar] [CrossRef]
- Bereli, N.; Yavuz, H.; Denizli, A. Protein chromatography by molecular imprinted cryogels. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 657–670. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Singh, M.; Lumpkin, J.A.; Rosenblatt, J. Mathematical modeling of drug release from hydrogel matrices via a diffusion coupled with desorption mechanism. J. Control. Release 1994, 32, 17–25. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Controlled drug release from hydrogel-based matrices: Experiments and modeling. Int. J. Pharm. 2015, 486, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Paolicelli, P.; Avitabile, M.; Varani, G.; Di Muzio, L.; Cesa, S.; Tirillò, J.; Bartuli, C.; Nardoni, M.; Petralito, S.; et al. Design of a tunable nanocomposite double network hydrogel based on gellan gum for drug delivery applications. Eur. Polym. J. 2018, 104, 184–193. [Google Scholar] [CrossRef]
- Caccavo, D.; Lamberti, G.; Barba, A.A. Mechanics and drug release from poroviscoelastic hydrogels: Experiments and modeling. Eur. J. Pharm. Biopharm. 2020, 152, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A.; Lazar, M.M.; Doroftei, F. Preparation and characterization of semi-IPN cryogels based on polyacrylamide and poly (N, N-dimethylaminoethyl methacrylate); Functionalization of carrier with monochlorotriazinyl-β-cyclodextrin and release kinetics of curcumin. Molecules 2021, 26, 6975. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Akbari, A.; Jafari, H.; Gohari, G.; Kheiri, G.; Mahdavinia, G.R. Fulvic acid-embedded poly (vinyl alcohol)–zinc oxide hydrogel nanocomposite: Synthesis, characterization, swelling and release kinetic. Int. Nano Lett. 2021, 11, 347–354. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Wróblewska, M.; Basa, A.; Winnicka, K. Does the freeze–thaw technique affect the properties of the alginate/chitosan glutamate gels with posaconazole as a model antifungal drug? Int. J. Mol. Sci. 2022, 23, 6775. [Google Scholar] [CrossRef]
- Zagni, C.; Coco, A.; Mecca, T.; Curcuruto, G.; Patamia, V.; Mangano, K.; Rescifina, A.; Carroccio, S.C. Sponge-like macroporous cyclodextrin-based cryogels for controlled drug delivery. Mater. Chem. Front. 2023, 7, 2693–2705. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Georgiev, G.L.; Trzebicka, B.; Kostova, B.; Petrov, P.D. Super-macroporous dextran cryogels via UV-induced crosslinking: Synthesis and characterization. Polym. Int. 2017, 66, 1306–1311. [Google Scholar] [CrossRef]
- Ni, H.; Qian, S.; Lu, J.; Feng, J.; Mou, X.z.; Zhang, J. Natural Polysaccharide Delivery Platforms with Multiscale Structure Used for Cancer Chemoimmunotherapy. Mol. Pharm. 2023, 20, 5778–5789. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, P.A.; Andrabi, S.M.; Singh, A.; Majumder, S.; Kumar, A. Designing cryogels through cryostructuring of polymeric matrices for biomedical applications. Eur. Polym. J. 2021, 144, 110234. [Google Scholar] [CrossRef]
- Bölgen, N.; Aguilar, M.R.; Fernández, M.D.M.; Gonzalo-Flores, S.; Villar-Rodil, S.; San Román, J.; Pişkin, E. Thermoresponsive biodegradable HEMA–Lactate–Dextran-co-NIPA cryogels for controlled release of simvastatin. Artif. Cells Nanomed. Biotechnol. 2015, 43, 40–49. [Google Scholar] [CrossRef]
- Slavkova, M.I.; Momekova, D.; Kostova, B.; Momekov, G.T.; Petrov, P. Novel dextran/β-cyclodextrin and dextran macroporous cryogels for topical delivery of curcumin in the treatment of cutaneous T-cell lymphoma. Bulg. Chem. Commun 2017, 49, 792–799. [Google Scholar]
- Lei, D.; Zhao, J.; Zhu, C.; Jiang, M.; Ma, P.; Mi, Y.; Fan, D. Multifunctional Oxidized Dextran Cross-Linked Alkylated Chitosan/Drug-Loaded and Silver-Doped Mesoporous Bioactive Glass Cryogel for Hemostasis of Noncompressible Wounds. Gels 2023, 9, 455. [Google Scholar] [CrossRef]
- Simon, L. A computational procedure for assessing the dynamic performance of diffusion-controlled transdermal delivery devices. Pharmaceutics 2011, 3, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Hauck, M.; Dittmann, J.; Zeller-Plumhoff, B.; Madurawala, R.; Hellmold, D.; Kubelt, C.; Synowitz, M.; Held-Feindt, J.; Adelung, R.; Wulfinghoff, S.; et al. Fabrication and Modelling of a Reservoir-Based Drug Delivery System for Customizable Release. Pharmaceutics 2022, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Kalosakas, G.; Panagopoulou, E. Lag Time in Diffusion-Controlled Release Formulations Containing a Drug-Free Outer Layer. Processes 2022, 10, 2592. [Google Scholar] [CrossRef]
- Van Dijk-Wolthuis, W.; Franssen, O.; Talsma, H.; Van Steenbergen, M.; Kettenes-Van Den Bosch, J.; Hennink, W. Synthesis, characterization, and polymerization of glycidyl methacrylate derivatized dextran. Macromolecules 1995, 28, 6317–6322. [Google Scholar] [CrossRef]
- Di Muzio, L.; Paolicelli, P.; Brandelli, C.; Cesa, S.; Trilli, J.; Petralito, S.; Casadei, M.A. Injectable and in situ gelling dextran derivatives containing hydrolyzable groups for the delivery of large molecules. Gels 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk-Wolthuls, W.; Tsang, S.; Kettenes-van den Bosch, W.E.; Hennink, J. A new class of polymerizable dextrans with hydrolyzable groups: Hydroxyethyl methacrylated dextran with and without oligolactate spacer. Polymer 1997, 38, 6235–6242. [Google Scholar] [CrossRef]
- Maiti, S.; Maji, B.; Yadav, H. Progress on green crosslinking of polysaccharide hydrogels for drug delivery and tissue engineering applications. Carbohydr. Polym. 2023, 326, 121584. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Pumford, E.A.; Jackson Hoffman, B.A.; Kasko, A.M. Nontoxic Initiator Alternatives to TEMED for Redox Hydrogel Polymerization. ACS Appl. Bio Mater. 2024, 7, 2264–2271. [Google Scholar] [CrossRef]
- Heller, M.C.; Carpenter, J.F.; Randolph, T.W. Application of a Thermodynamic Model to the Prediction of Phase Separations in Freeze-Concentrated Formulations for Protein Lyophilization. Arch. Biochem. Biophys. 1999, 363, 191–201. [Google Scholar] [CrossRef]
- Adrover, A.; Varani, G.; Paolicelli, P.; Petralito, S.; Di Muzio, L.; Casadei, M.A.; Tho, I. Experimental and Modeling Study of Drug Release from HPMC-Based Erodible Oral Thin Films. Pharmaceutics 2018, 10, 222. [Google Scholar] [CrossRef]
- Adrover, A.; Paolicelli, P.; Petralito, S.; Di Muzio, L.; Trilli, J.; Cesa, S.; Tho, I.; Casadei, M.A. Gellan Gum/Laponite Beads for the Modified Release of Drugs: Experimental and Modeling Study of Gastrointestinal Release. Pharmaceutics 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauriola, C.; Di Muzio, L.; Paolicelli, P.; Casadei, M.A.; Sergi, C.; Tirillò, J.; Carriero, V.C.; Adrover, A. Experimental and Modelling Study of Controlled Release from Dextran-Based Cryogels. Pharmaceutics 2024, 16, 1256. https://doi.org/10.3390/pharmaceutics16101256
Lauriola C, Di Muzio L, Paolicelli P, Casadei MA, Sergi C, Tirillò J, Carriero VC, Adrover A. Experimental and Modelling Study of Controlled Release from Dextran-Based Cryogels. Pharmaceutics. 2024; 16(10):1256. https://doi.org/10.3390/pharmaceutics16101256
Chicago/Turabian StyleLauriola, Carolina, Laura Di Muzio, Patrizia Paolicelli, Maria Antonietta Casadei, Claudia Sergi, Jacopo Tirillò, Vito Cosimo Carriero, and Alessandra Adrover. 2024. "Experimental and Modelling Study of Controlled Release from Dextran-Based Cryogels" Pharmaceutics 16, no. 10: 1256. https://doi.org/10.3390/pharmaceutics16101256
APA StyleLauriola, C., Di Muzio, L., Paolicelli, P., Casadei, M. A., Sergi, C., Tirillò, J., Carriero, V. C., & Adrover, A. (2024). Experimental and Modelling Study of Controlled Release from Dextran-Based Cryogels. Pharmaceutics, 16(10), 1256. https://doi.org/10.3390/pharmaceutics16101256








