Preparation and Characterization of a Novel Salicin–Cyclodextrin Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Liquid Phase Complexation
2.4. Obtaining the Inclusion Complex in Solid State
2.5. Obtaining the Physical Mixture
2.6. Solubility of Free and Complexed Salicin
2.7. Computational Details
3. Results and Discussion
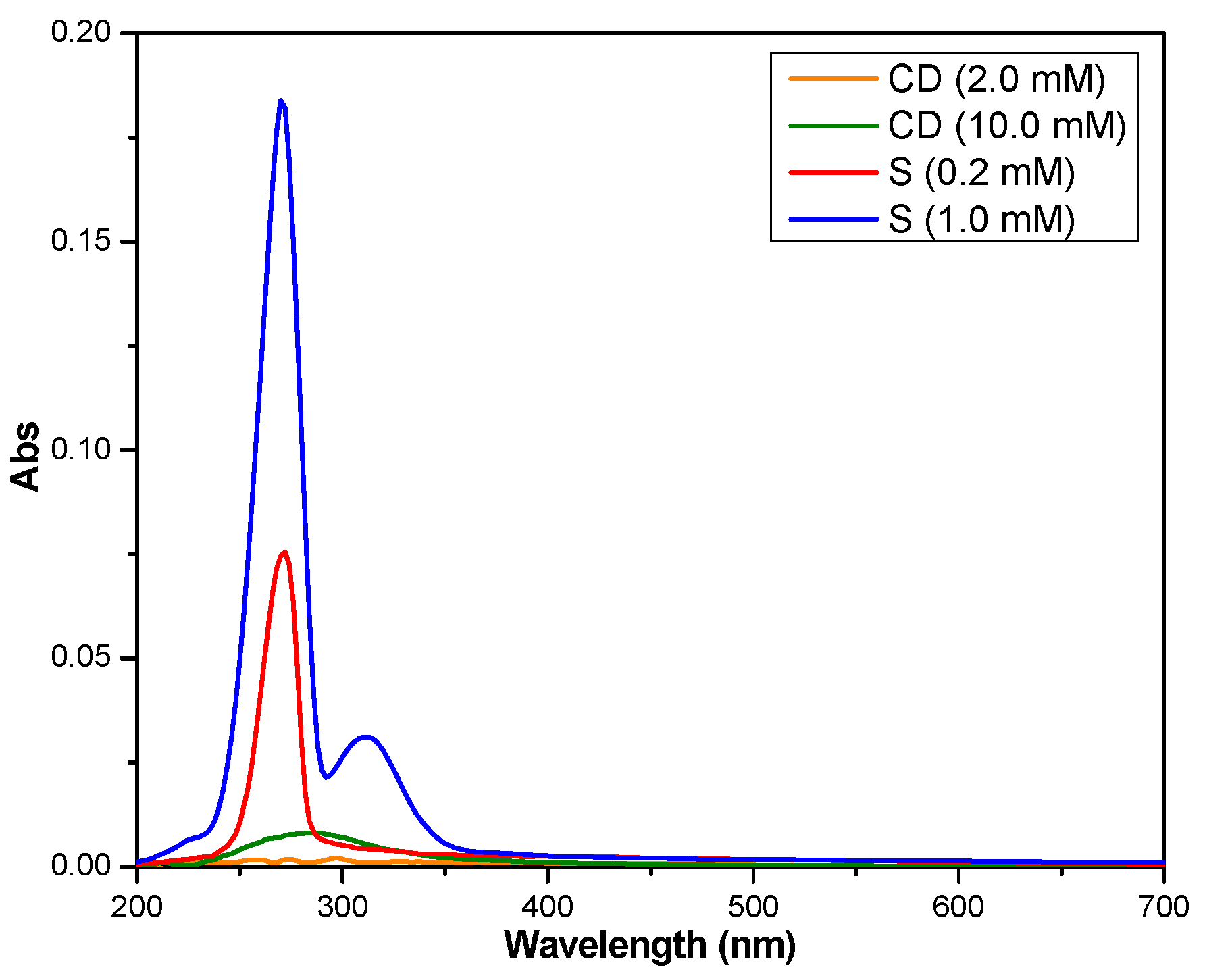
3.1. Stoichiometry and Association Constant Determination
- −
- ∆A is the difference between the absorbance of the complex and the absorbance of the salicin;
- −
- ∆ε is the difference between the molar absorptivity of the complex and the molar absorptivity of the salicin;
- −
- [G]0, K, [CD], are the initial concentration of salicin, the apparent formation constant of the inclusion complex and the concentration of β-cyclodextrin, respectively.
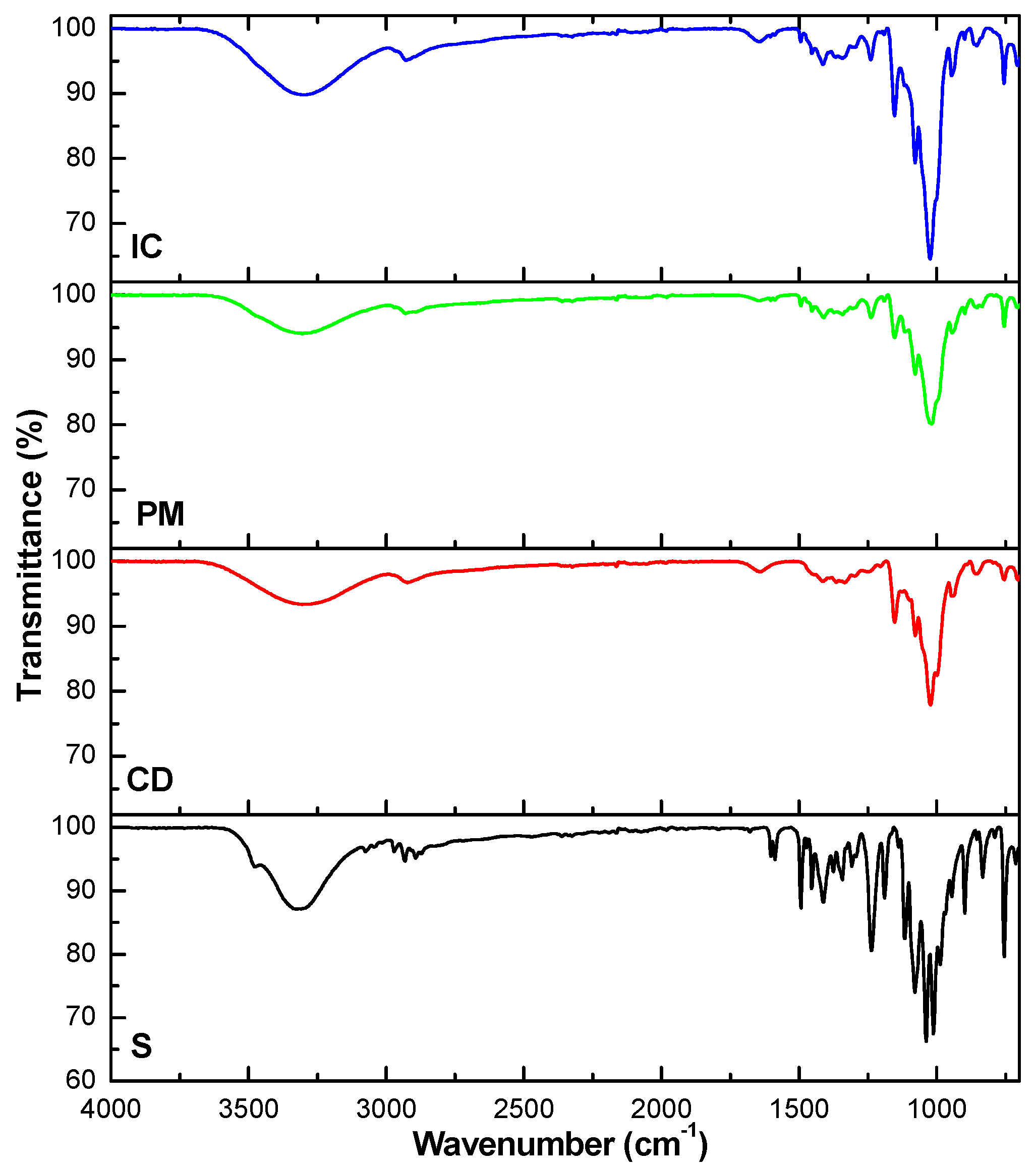
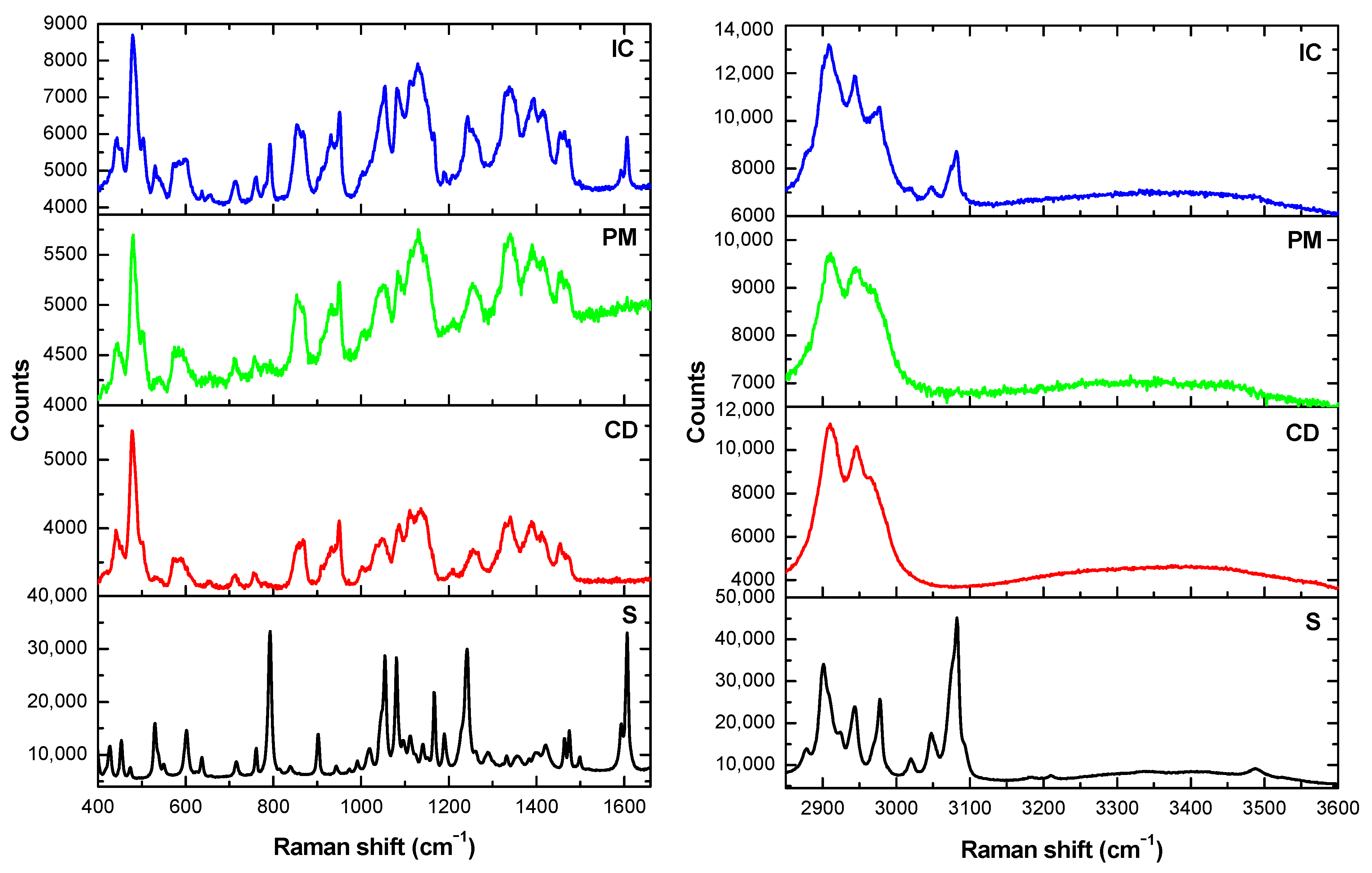
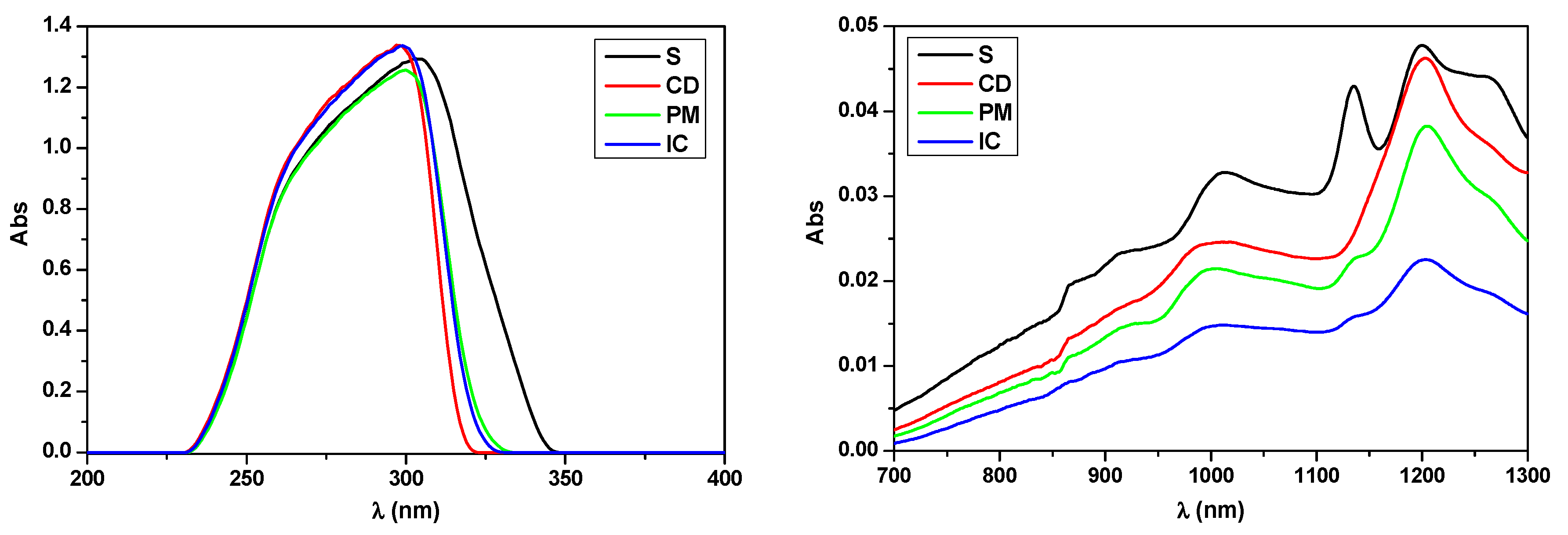
3.2. Characterization of the Solid State Inclusion Complex
3.3. Evaluation of the Complexation Effect on Salicin Solubility
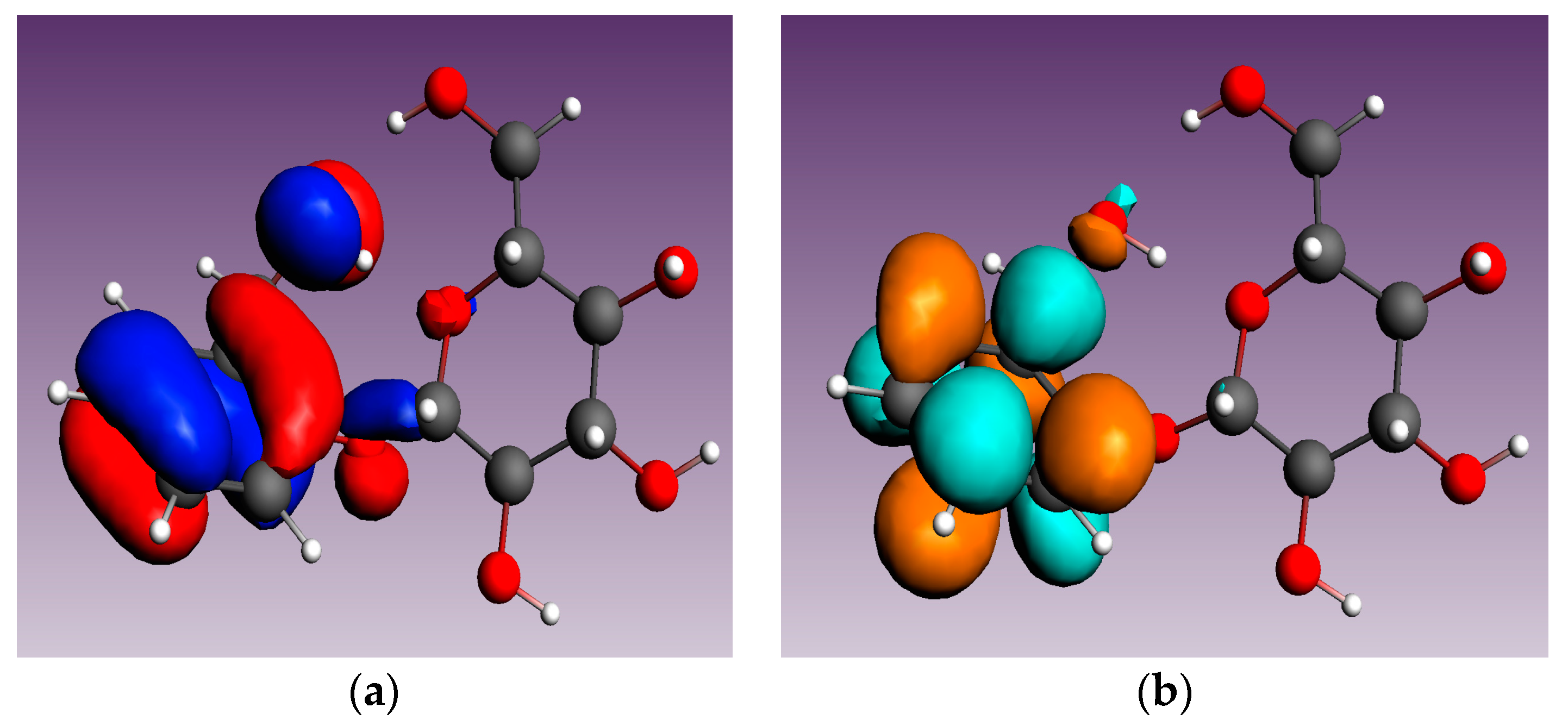
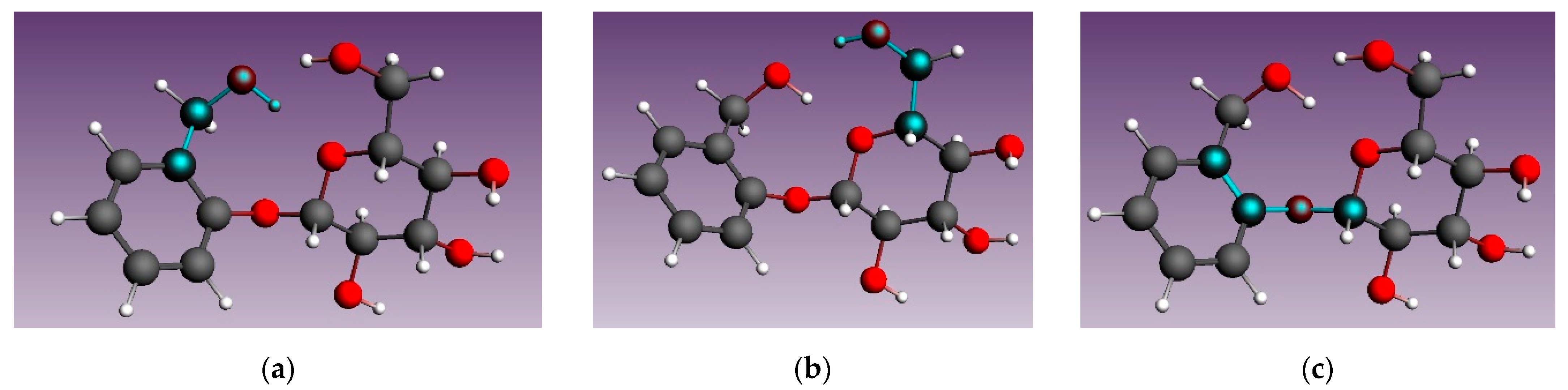
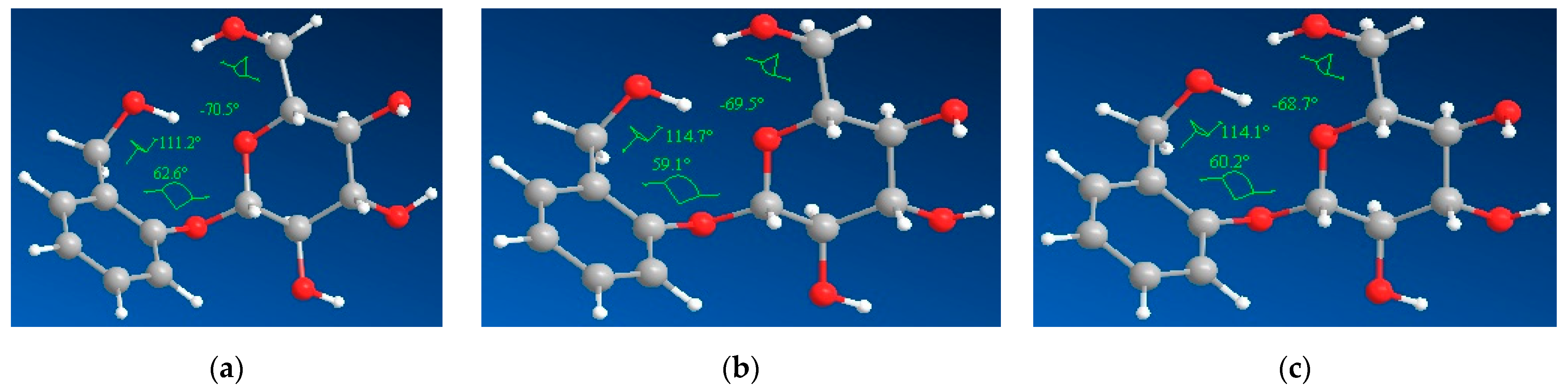
3.4. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aridoǧan, B.C.; Baydar, H.; Kaya, S.; Demirci, M.; Özbaşar, D.; Mumcu, E. Antimicrobial Activity and Chemical Composition of Some Essential Oils. Arch. Pharm. Res. 2002, 25, 860–864. [Google Scholar] [CrossRef]
- Frum, A.; Dobrea, C.M.; Rus, L.L.; Virchea, L.I.; Morgovan, C.; Chis, A.A.; Arseniu, A.M.; Butuca, A.; Gligor, F.G.; Vicas, L.G.; et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients 2022, 14, 3065. [Google Scholar] [CrossRef] [PubMed]
- Malek, S.N.A.; Phang, C.W.; Ibrahim, H.; Wahab, N.A.; Sim, K.S. Phytochemical and Cytotoxic Investigations of Alpinia Mutica Rhizomes. Molecules 2011, 16, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.; Popescu, C.; Popescu, B.; Daas, D.; Morgovan, C.; Olah, N.K. Antimicrobial Efficacy of the Organic Greasy Oils Combination-Sea Buckthorn Oil and Maize Germs Oil. Farmacia 2014, 62, 743–752. [Google Scholar]
- Gou, J.; Zou, Y.; Ahn, J. Enhancement of Antioxidant and Antimicrobial Activities of Dianthus Superbus, Polygonum Aviculare, Sophora Flavescens, and Lygodium Japonicum by Pressure-Assisted Water Extraction. Food Sci. Biotechnol. 2011, 20, 283–287. [Google Scholar] [CrossRef]
- Belšćak-Cvitanović, A.; Stojanović, R.; Manojlović, V.; Komes, D.; Cindrić, I.J.; Nedović, V.; Bugarski, B. Encapsulation of Polyphenolic Antioxidants from Medicinal Plant Extracts in Alginate–Chitosan System Enhanced with Ascorbic Acid by Electrostatic Extrusion. Food Res. Int. 2011, 44, 1094–1101. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Mureşan, M.; Olteanu, D.; Filip, G.A.; Clichici, S.; Baldea, I.; Jurca, T.; Pallag, A.; Marian, E.; Frum, A.; Gligor, F.G.; et al. Comparative Study of the Pharmacological Properties and Biological Effects of Polygonum Aviculare L. Herba Extract-Entrapped Liposomes versus Quercetin-Entrapped Liposomes on Doxorubicin-Induced Toxicity on HUVECs. Pharmaceutics 2021, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.; Weyers, M.; Steenekamp, J.H.; Steyn, J.D.; Gouws, C.; Hamman, J.H. Drug Bioavailability Enhancing Agents of Natural Origin (Bioenhancers) That Modulate Drug Membrane Permeation and Pre-Systemic Metabolism. Pharmaceutics 2019, 11, 33. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as Encapsulation Agents for Plant Bioactive Compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Rasheed, A.; Kumar, C.K.A.; Sravanthi, V.V.N.S.S. Cyclodextrins as Drug Carrier Molecule: A Review. Sci. Pharm. 2008, 76, 567–598. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M.d.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Cal, K.; Centkowska, K. Use of Cyclodextrins in Topical Formulations: Practical Aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Cabral Marques, H.M. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- De Gaetano, F.; Margani, F.; Barbera, V.; D’Angelo, V.; Germanò, M.P.; Pistarà, V.; Ventura, C.A. Characterization and In Vivo Antiangiogenic Activity Evaluation of Morin-Based Cyclodextrin Inclusion Complexes. Pharmaceutics 2023, 15, 2209. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.d.S.; Campos, C.d.A.; da Silva Santos, A.C.R.; Santos, V.C.N.; Trindade, G.d.G.G.; Shanmugam, S.; Pereira, E.W.M.; Marreto, R.N.; Duarte, M.C.; da Silva Almeida, J.R.G.; et al. Development of Morin/Hydroxypropyl-β-Cyclodextrin Inclusion Complex: Enhancement of Bioavailability, Antihyperalgesic and Anti-Inflammatory Effects. Food Chem. Toxicol. 2019, 126, 15–24. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, H.; Hu, H.; Ruan, J.; Shi, C.; Cao, J.; Zhang, X. Preparation, Characterization and Antioxidant Activity of Inclusion Complex Loaded with Puerarin and Corn Peptide. Food Biosci. 2022, 49, 101886. [Google Scholar] [CrossRef]
- Minda, D.; Pavel, I.Z.; Borcan, F.; Coricovac, D.; Pinzaru, I.; Andrica, F.; Morgovan, C.; Nita, L.D.; Soica, C.; Muntean, D.; et al. Beneficial Effects of a Lupeol-Cyclodextrin Complex in a Murine Model of Photochemical Skin Carcinoma. Rev. Chim. 2015, 66, 373–377. [Google Scholar]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of Cyclodextrin Complexation of Curcumin on Its Solubility and Antiangiogenic and Anti-Inflammatory Activity in Rat Colitis Model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef]
- Atzeni, F.; Rodríguez-Carrio, J.; Popa, C.D.; Nurmohamed, M.T.; Szűcs, G.; Szekanecz, Z. Cardiovascular Effects of Approved Drugs for Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2021, 17, 270–290. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Du, H.; Liu, D.; Ma, Z. Editorial: The Role of Natural Products in Chronic Inflammation. Front. Pharmacol. 2022, 13, 901538. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Voştinaru, O.; Mogoşan, C.; Ghibu, S. Phytochemical and Pharmacological Research on Some Extracts Obtained from Serpylli Herba. Farmacia 2011, 59, 77–84. [Google Scholar]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A.; Marles, R.J.; Jordan, S.A.; Low Dog, T. United States Pharmacopeia Safety Review of Willow Bark. Planta Med. 2019, 85, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Shara, M.; Stohs, S.J. Efficacy and Safety of White Willow Bark (Salix Alba) Extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef]
- Schmid, B.; Kötter, I.; Heide, L. Pharmacokinetics of Salicin after Oral Administration of a Standardised Willow Bark Extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef]
- Schmid, B.; Lüdtke, R.; Selbmann, H.K.; Kötter, I.; Tschirdewahn, B.; Schaffner, W.; Heide, L. Efficacy and Tolerability of a Standardized Willow Bark Extract in Patients with Osteoarthritis: Randomized Placebo-Controlled, Double Blind Clinical Trial. Phytother. Res. 2001, 15, 344–350. [Google Scholar] [CrossRef]
- WHO. WHO Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Mahdi, J.G. Medicinal Potential of Willow: A Chemical Perspective of Aspirin Discovery. J. Saudi Chem. Soc. 2010, 14, 317–322. [Google Scholar] [CrossRef]
- Use of Salicin as an Anti-Irritative Active Compound in Cosmetic and Topical Dermatological Preparations. U.S. Patent 5,876,737, 2 March 1997.
- Gopaul, R.; Knaggs, H.E.; Lephart, J.F.; Holley, K.C.; Gibson, E.M. An Evaluation of the Effect of a Topical Product Containing Salicin on the Visible Signs of Human Skin Aging. J. Cosmet. Dermatol. 2010, 9, 196–201. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, S. Salicin Inhibits AGE-Induced Degradation of Type II Collagen and Aggrecan in Human SW1353 Chondrocytes: Therapeutic Potential in Osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1043–1049. [Google Scholar] [CrossRef]
- Adamiak, K.; Lewandowska, K.; Sionkowska, A. The Infuence of Salicin on Rheological and Film-Forming Properties of Collagen. Molecule 2021, 26, 1661. [Google Scholar] [CrossRef]
- Sambasevam, K.P.; Mohamad, S.; Sarih, N.M.; Ismail, N.A. Synthesis and Characterization of the Inclusion Complex of β-Cyclodextrin and Azomethine. Int. J. Mol. Sci. 2013, 14, 3671–3682. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-Type Basis Sets for the Elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Baerends, E.J.; Ziegler, T.; Autschbach, J.; Bashford, D.; Bérces, A.; Bickelhaupt, F.M.; Swerhone, D. Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zyrianov, Y. Distribution-Based Descriptors of the Molecular Shape. J. Chem. Inf. Model. 2005, 45, 657–672. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Sivakumar, K.; Parameswari, M. Salicylanilide/Cyclodextrin Inclusion Complex: Preparation, Characterization and Molecular Docking Studies. SOJ Mater. Sci. Eng. 2015, 3, 1–4. [Google Scholar] [CrossRef]
- Ienaşcu, I.M.C.; Ştefănuţ, M.N.; Pascariu, M.-C.; Popescu, I.M.; Căta, A.; Pop, R. Complexation of [2-(2-Bromophenylcarbamoyl)Phenoxy]Acetic Acid Ethyl Ester With β-Cyclodextrin. Rev. Roum. Chim 2019, 64, 849–857. [Google Scholar] [CrossRef]
- Belyakova, L.A.; Varvarin, A.M.; Lyashenko, D.Y.; Khora, O.V.; Oranskaya, E.I. Complexation in a β-Cyclodextrin-Salicylic Acid System. Colloid J. 2007, 69, 546–551. [Google Scholar] [CrossRef]
- Valdebenito, S.; Zapata-Torres, G.; Lemp, E.; Zanocco, A.L. Experimental and Theoretical Studies of the Photophysics of 7-Amino-3-Phenyl-2H-Benzo[b] [1,4]Oxazin-2-One in Homogeneous Solvents and β-Cyclodextrin Aqueous Solutions. Afinidad 2015, 72, 53–59. [Google Scholar]
- Malapert, A.; Reboul, E.; Tourbin, M.; Dangles, O.; Thiéry, A.; Ziarelli, F.; Tomao, V. Characterization of Hydroxytyrosol-β-Cyclodextrin Complexes in Solution and in the Solid State, a Potential Bioactive Ingredient. LWT 2019, 102, 317–323. [Google Scholar] [CrossRef]
- Eteer, S.A. UV-Vis Spectroscopic Characterization of β-Cyclodextrin-Vanillin Inclusion Complex. Mediterr. J. Chem. 2022, 12, 175. [Google Scholar] [CrossRef]
- Prabu, S.; Samad, N.A.; Ahmad, N.A.; Jumbri, K.; Raoov, M.; Rahim, N.Y.; Kanagesan Samikannu, S.M. Studies on the Supramolecular Complex of a Guanosine with Beta-Cyclodextrin and Evaluation of Its Anti-Proliferative Activity. Carbohydr. Res. 2020, 497, 108138. [Google Scholar] [CrossRef]
- Pradhan, P.C.; Mandal, A.; Dutta, A.; Sarkar, R.; Kundu, A.; Saha, S. Delineating the Behavior of Berberis Anthocyanin/β-Cyclodextrin Inclusion Complex in Vitro: A Molecular Dynamics Approach. LWT 2022, 157, 113090. [Google Scholar] [CrossRef]
- Ienașcu, I.M.C.; Căta, A.; Ştefănuț, M.N.; Popescu, I.; Rusu, G.; Sfîrloagă, P.; Ursu, D.; Moşoarcă, C.; Dabici, A.; Danciu, C.; et al. Novel Chloro-Substituted Salicylanilide Derivatives and Their β-Cyclodextrin Complexes: Synthesis, Characterization, and Antibacterial Activity. Biomedicines 2022, 10, 71740. [Google Scholar] [CrossRef]
- Tang, B.; Chen, Z.Z.; Zhang, N.; Zhang, J.; Wang, Y. Synthesis and Characterization of a Novel Cross-Linking Complex of Beta-Cyclodextrin-o-Vanillin Furfuralhydrazone and Highly Selective Spectrofluorimetric Determination of Trace Gallium. Talanta 2006, 68, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water Soluble Complexes of Curcumin with Cyclodextrins: Characterization by FT-Raman Spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Mrozek, M.F.; Zhang, D.; Ben-Amotz, D. Oligosaccharide Identification and Mixture Quantification Using Raman Spectroscopy and Chemometric Analysis. Carbohydr. Res. 2004, 339, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Koenig, J.L. Vibrational Spectra of Carbohydrates. Adv. Carbohydr. Chem. Biochem. 1987, 44, 7–89. [Google Scholar] [CrossRef]
- Dudek, M.; Zajac, G.; Szafraniec, E.; Wiercigroch, E.; Tott, S.; Malek, K.; Kaczor, A.; Baranska, M. Raman Optical Activity and Raman Spectroscopy of Carbohydrates in Solution. Mol. Biomol. Spectrosc. 2019, 206, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Tita, B.; Morgovan, C.; Tita, D.; Neag, T.A. Anti-Inflammatory Drugs Interacting with Zn(II) Metal Ion Synthesis, Characterization and Thermal Behaviour of the Complex with Ketoprofen. Rev. Chim. 2016, 67, 38–41. [Google Scholar]
- Zhu, G.; Xiao, Z.; Zhou, R.; Niu, Y. Pyrolysis Characteristics and Kinetics of β-Cyclodextrin and Its Two Derivatives. Pol. J. Chem. Technol. 2015, 17, 1–4. [Google Scholar] [CrossRef]
- Szejtli, J.; Szente, L. Elimination of Bitter, Disgusting Tastes of Drugs and Foods by Cyclodextrins. Eur. J. Pharm. Biopharm. 2005, 61, 115–125. [Google Scholar] [CrossRef]









| Compound | Solubility (g/L) |
|---|---|
| salicin (free) | 43.19 ± 1.06 |
| salicin (complexed) | 85.80 ± 1.26 |
| Compound | Connolly Accessible Area (Å2) | Connolly Solvent-Excluded Volume (Å3) | Ovality | logP |
|---|---|---|---|---|
| Salicin (gas) | 483.013 | 245.948 | 1.344 | −0.471 |
| Salicin (aq) | 486.480 | 247.799 | 1.345 | |
| Salicin (EtOH) | 485.620 | 247.613 | 1.343 |
| Compound | E (kcal/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | E | |
| Salicin (gas) | −5.2 | −5.1 | −5.1 | −5.0 | −4.9 | −4.9 | −4.9 | −4.8 | −4.8 | −4.97 |
| Salicin (aq) | −5.2 | −5.0 | −4.9 | −4.9 | −4.9 | −4.8 | −4.8 | −4.7 | −4.7 | −4.88 |
| Salicin (EtOH) | −5.2 | −5.0 | −4.9 | −4.9 | −4.9 | −4.8 | −4.8 | −4.8 | −4.8 | −4.90 |
| Compound | Interactions with β-Cyclodextrin |
|---|---|
| Salicin (gas-phase) | Atoms in close contact |
| Salicin (aq) | Atoms in close contact 1 hydrogen bond (1.981 Å) (phenyl OH–CD) |
| Salicin (EtOH) | Atoms in close contact |
| Best Ligand Conformation | Dihedral Angle 1 (°) | Dihedral Angle 2 (°) | Dihedral Angle 3 (°) |
|---|---|---|---|
| Salicin (gas) | −65.9 | −153.1 | −130.9 |
| Salicin (aq) | 160.8 | 167.9 | −137.2 |
| Salicin (EtOH) | −1.7 | −32.3 | 64.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căta, A.; Ienaşcu, I.M.C.; Frum, A.; Ursu, D.; Svera, P.; Orha, C.; Rusu, G.; Chiș, A.A.; Dobrea, C.M.; Morgovan, C.; et al. Preparation and Characterization of a Novel Salicin–Cyclodextrin Complex. Pharmaceutics 2024, 16, 369. https://doi.org/10.3390/pharmaceutics16030369
Căta A, Ienaşcu IMC, Frum A, Ursu D, Svera P, Orha C, Rusu G, Chiș AA, Dobrea CM, Morgovan C, et al. Preparation and Characterization of a Novel Salicin–Cyclodextrin Complex. Pharmaceutics. 2024; 16(3):369. https://doi.org/10.3390/pharmaceutics16030369
Chicago/Turabian StyleCăta, Adina, Ioana Maria Carmen Ienaşcu, Adina Frum, Daniel Ursu, Paula Svera, Corina Orha, Gerlinde Rusu, Adriana Aurelia Chiș, Carmen Maximiliana Dobrea, Claudiu Morgovan, and et al. 2024. "Preparation and Characterization of a Novel Salicin–Cyclodextrin Complex" Pharmaceutics 16, no. 3: 369. https://doi.org/10.3390/pharmaceutics16030369






