In Vitro Profile of Hydrocortisone Release from Three-Dimensionally Printed Paediatric Mini-Tablets
Abstract
:1. Introduction
2. Materials and Methods
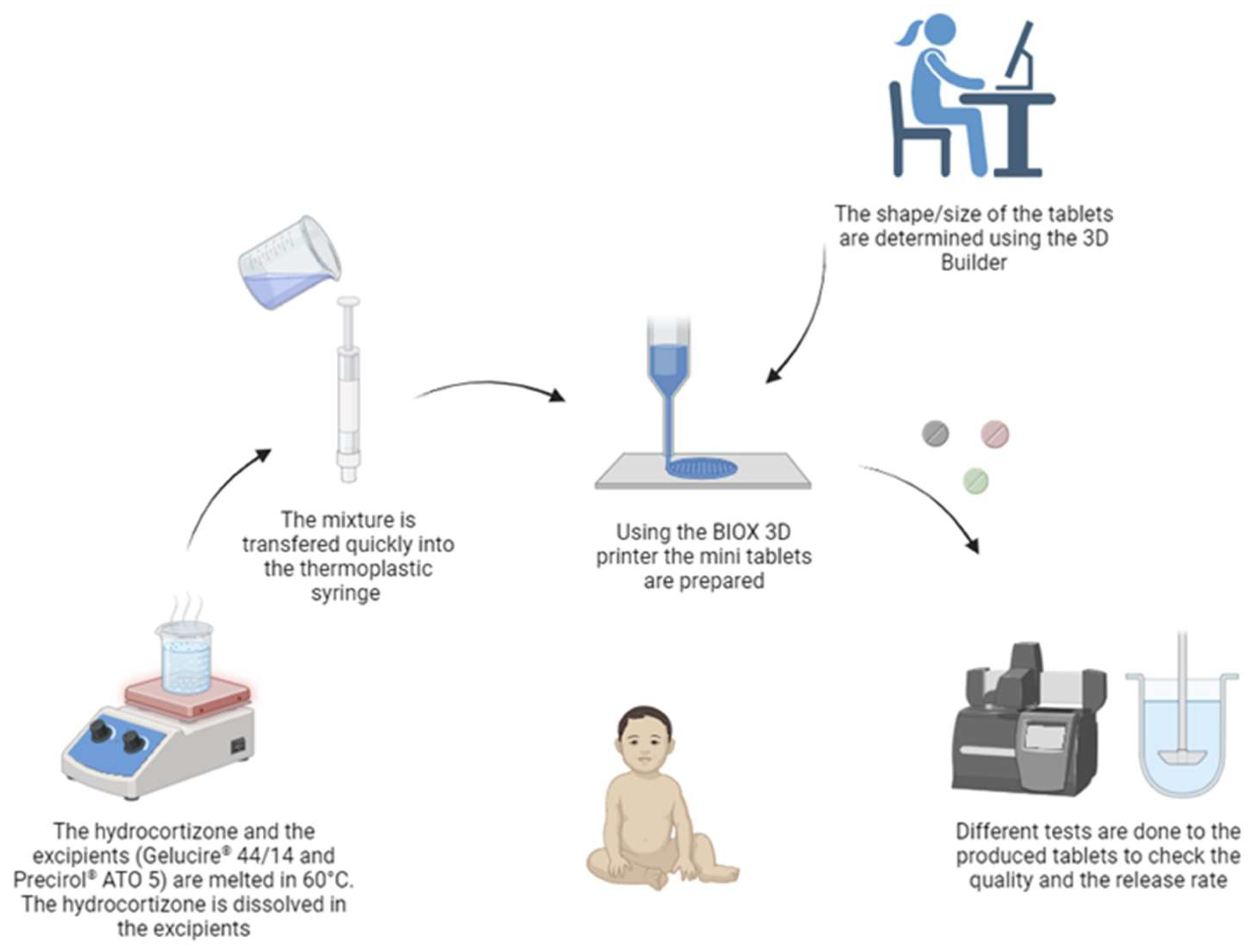
2.1. Three-Dimensional Printing
2.2. Mini-Tablets’ Physical Characteristics
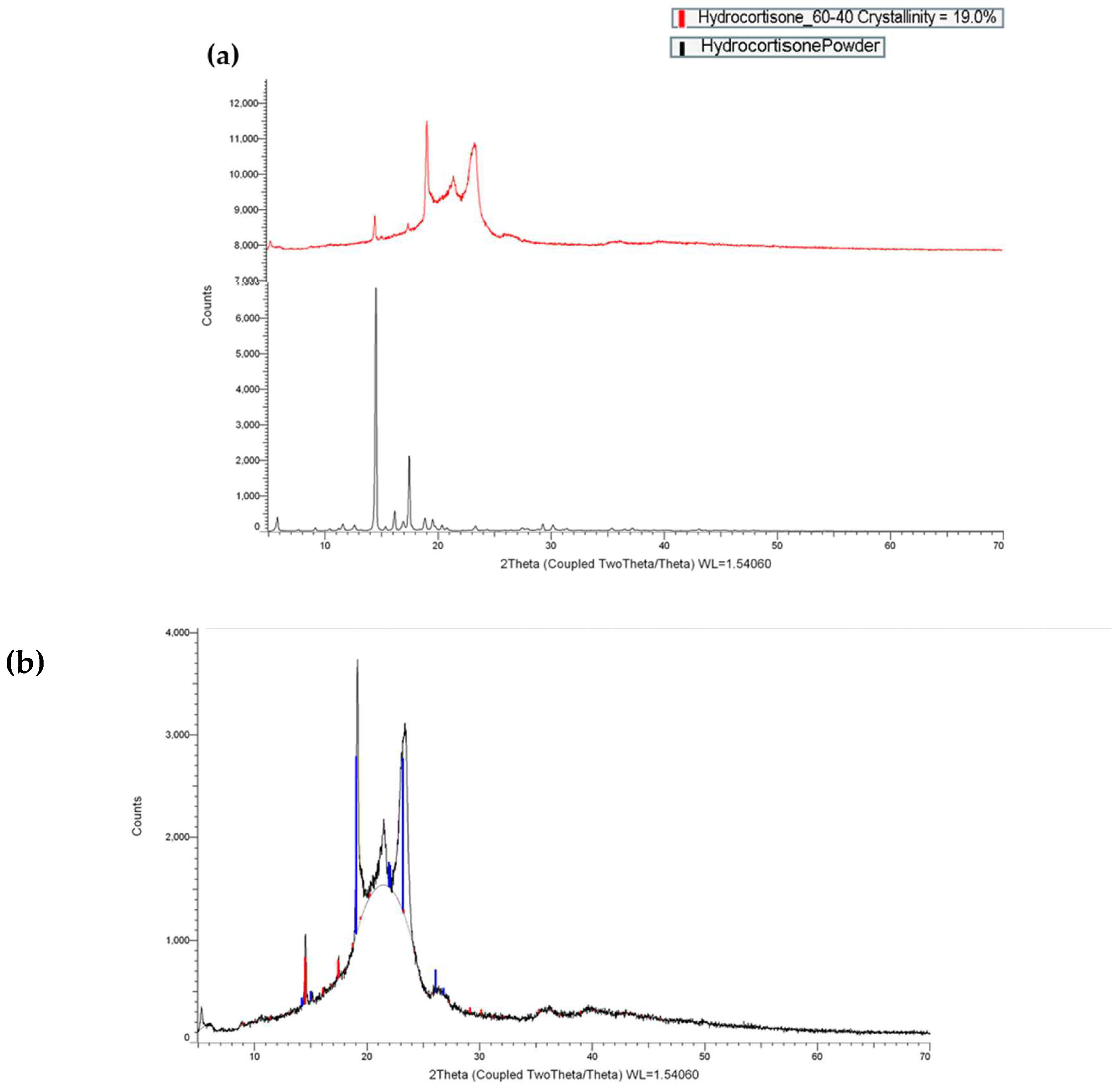
2.3. X-ray Powder Diffraction (XRPD)
2.4. Differential Scanning Calorimetry (DSC)
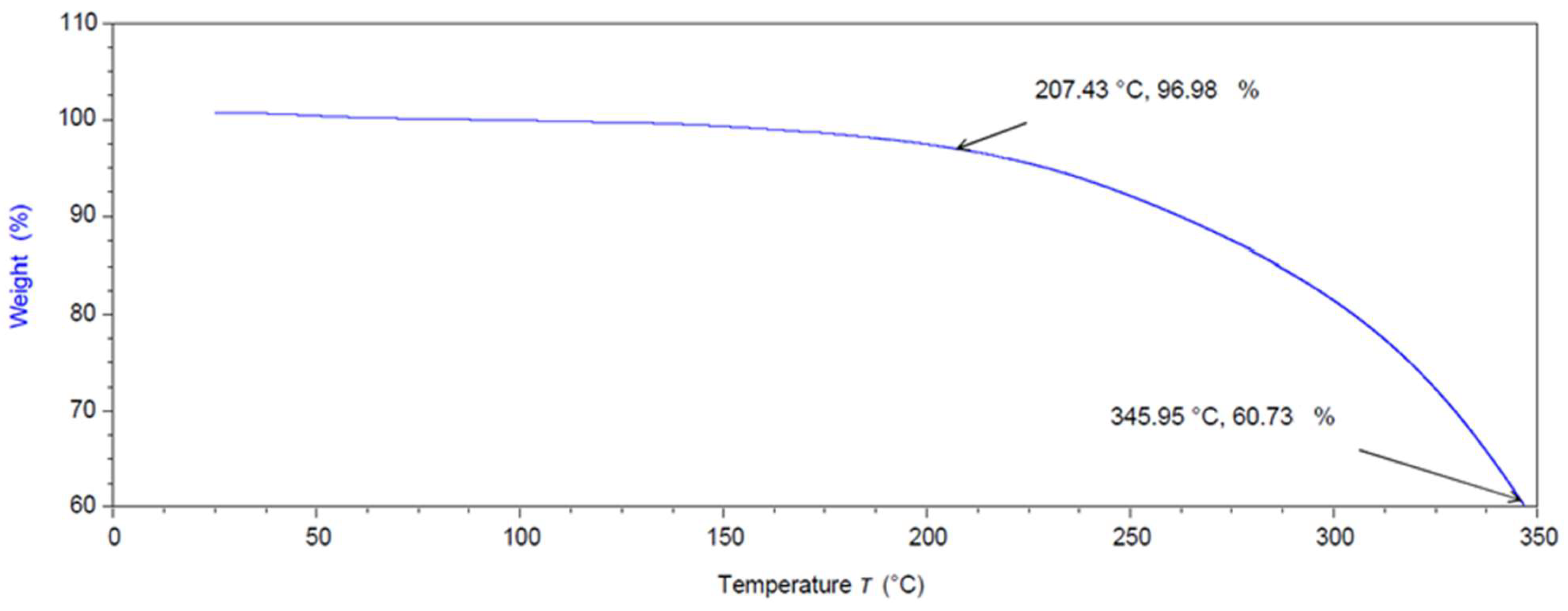
2.5. Thermal Gravimetric Analysis (TGA)
2.6. Dissolution Studies
2.7. Mathematical and Statistical Analysis
3. Results and Discussion
3.1. Printing Process
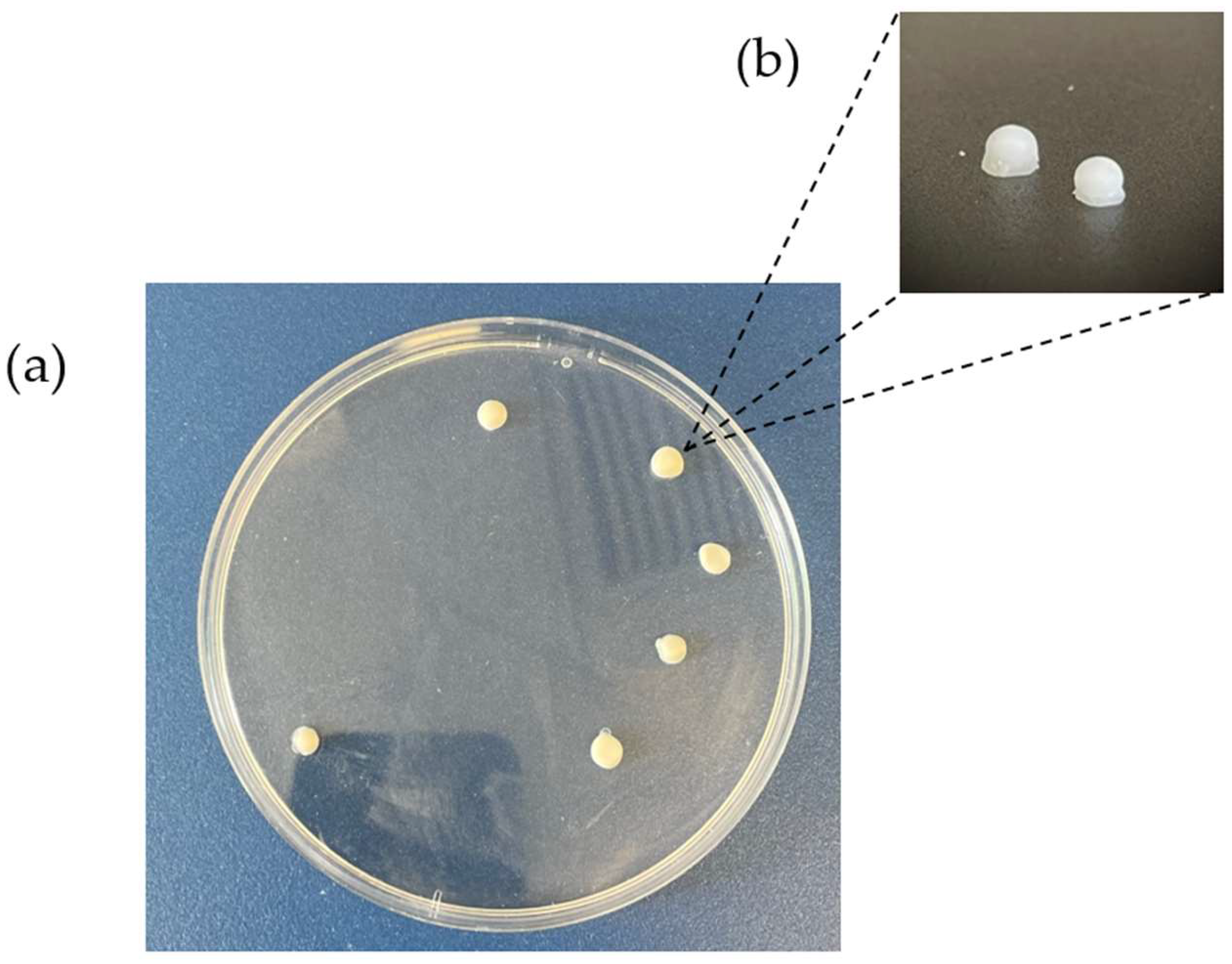
3.2. Mini-Tablets’ Physical Characteristics
3.2.1. X-ray Powder Diffraction (XRPD)
3.2.2. Thermal Gravimetric Analysis (TGA)
3.2.3. Mini-Tablets’ Dimensions and Weight Variations
3.2.4. Differential Scanning Calorimetry (DSC)
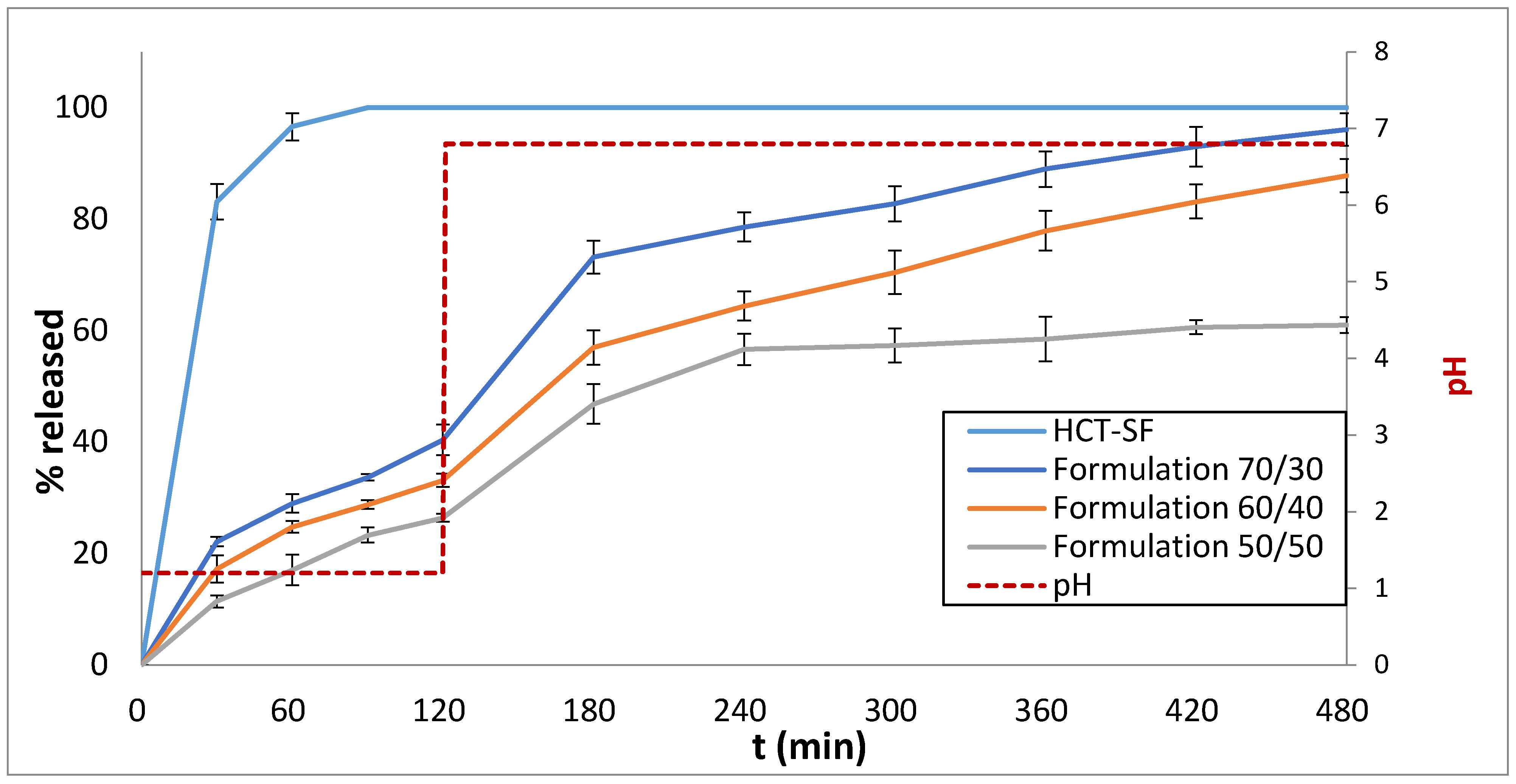
3.3. Drug Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreadis, I.I.; Gioumouxouzis, C.I.; Eleftheriadis, G.K.; Fatouros, D.G. The Advent of a New Era in Digital Healthcare: A Role for 3D Printing Technologies in Drug Manufacturing? Pharmaceutics 2022, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, E.; Protopapa, C. Machine Reading Comprehension and Expert System technologies for social innovation in the drug excipient selection process. Artif. Intell. Soc. Comput. 2023, 72, 48–57. [Google Scholar] [CrossRef]
- Vlachou, M.; Siamidi, A.; Protopapa, C.; Sotiropoulou, I. A review on the colours, flavours and shapes used in paediatric 3D printed oral solid dosage forms. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad009. [Google Scholar] [CrossRef]
- Vlachou, M.; Siamidi, A.; Protopapa, C. Extrusion-Based 3D Printing Methods for Oral Solid Dosage Forms. In 3D & 4D Printing Methods for Pharmaceutical Manufacturing and Personalised Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2023; pp. 195–218. [Google Scholar]
- Kodama, H. Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev. Sci. Instrum. 1981, 52, 1770–1773. [Google Scholar] [CrossRef]
- Atheer Awad, B.W.A. 3D and 4D Printing in Digital Healthcare. In 3D & 4D Printing Methods for Pharmaceutical Manufacturing and Personalised Drug Delivery; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–23. [Google Scholar]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D printing promotes the development of drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef] [PubMed]
- Litman, T. Personalized medicine—Concepts, technologies, and applications in inflammatory skin diseases. APMIS 2019, 127, 386–424. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.; Hui, H.W.; Kumar, S.; Douroumis, D. Personalised paediatric chewable Ibuprofen tablets fabricated using 3D micro-extrusion printing technology. Int. J. Pharm. 2022, 626, 122135. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Zastrow, M. 3D printing gets bigger, faster and stronger. Nature 2020, 578, 20–23. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Shen, W.; Fan, X.; Jia, M.; Fu, G.; Chi, X.; Liang, X.; Zhang, Y. A Bibliometric Analysis of Cardioembolic Stroke From 2012 to 2022. Curr. Probl. Cardiol. 2023, 48, 101537. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Jäger, H. Current Status in the Utilization of Biobased Polymers for 3D Printing Process: A Systematic Review of the Materials, Processes, and Challenges. ACS Appl. Bio Mater. 2021, 4, 325–369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, W.; Zhu, X. Three-dimensional printing: The potential technology widely used in medical fields. J. Biomed. Mater. Res.—Part A 2020, 108, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single- and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef] [PubMed]
- Pistone, M.; Racaniello, G.F.; Arduino, I.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Rizzi, R.; Franco, M.; Lopedota, A.; Denora, N. Direct cyclodextrin-based powder extrusion 3D printing for one-step production of the BCS class II model drug niclosamide. Drug Deliv. Transl. Res. 2022, 12, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- Panraksa, P.; Zhang, B.; Rachtanapun, P.; Jantanasakulwong, K.; Qi, S.; Jantrawut, P. ‘Tablet-in-Syringe’: A Novel Dosing Mechanism for Dysphagic Patients Containing Fast-Disintegrating Tablets Fabricated Using Semisolid Extrusion 3D Printing. Pharmaceutics 2022, 14, 443. [Google Scholar] [CrossRef]
- Elbadawi, M.; Gustaffson, T.; Gaisford, S.; Basit, A.W. 3D printing tablets: Predicting printability and drug dissolution from rheological data. Int. J. Pharm. 2020, 590, 119868. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, J.; Meda, A.; Osei-Yeboah, F.; Peterson, M.L.; Repka, M.; Zhan, X. Development of a quantitative method to evaluate the printability of filaments for fused deposition modeling 3D printing. Int. J. Pharm. 2020, 588, 119760. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Bonnefille, M.; Aranyos, A.; Mitchell, J.C.; Douroumis, D. Taste masking of paracetamol by hot-melt extrusion: An in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 433–442. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- Boniatti, J.; Januskaite, P.; da Fonseca, L.B.; Viçosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Ré, M.I. Direct powder extrusion 3d printing of praziquantel to overcome neglected disease formulation challenges in paediatric populations. Pharmaceutics 2021, 13, 1114. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, D.T.; Ana, S.E.; José, B.F. A micro-extrusion 3D printing platform for fabrication of orodispersible printlets for pediatric use. Int. J. Pharm. 2021, 605, 120854. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Stogiannari, M.; Janeczko, S.; Khoshan, M.; Lin, Y.; Isreb, A.; Habashy, R.; Giebułtowicz, J.; Peak, M.; Alhnan, M.A. Towards point-of-care manufacturing and analysis of immediate-release 3D printed hydrocortisone tablets for the treatment of congenital adrenal hyperplasia. Int. J. Pharm. 2023, 642, 123072. [Google Scholar] [CrossRef] [PubMed]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I spy with my little eye: A paediatric visual preferences survey of 3d printed tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dumpa, N.; Bandari, S.; Durig, T.; Repka, M.A. Fabrication of Taste-Masked Donut-Shaped Tablets via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS PharmSciTech 2020, 21, 243. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Cellink. Cellink BIOX-Meet the Printer; Cellink: Gothenburg, Sweden, 2022. [Google Scholar]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. A Proof of Concept for 3D Printing of Solid Lipid-Based Formulations of Poorly Water-Soluble Drugs to Control Formulation Dispersion Kinetics. Pharm. Res. 2019, 36, 102. [Google Scholar] [CrossRef]
- Kirkgoz, T.; Guran, T. Primary adrenal insufficiency in children: Diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Hanawa, T.; Ozawa, A.; Matsumoto, T.; Yoshikawa, N.; Harada, T.; Iwahashi, K.; Yamatani, A. Stability Study of Baclofen in an Oral Powder Form Compounded for Pediatric Patients in Japan. Children 2022, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Madathilethu, J.; Roberts, M.; Peak, M.; Blair, J.; Prescott, R.; Ford, J.L. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ Paediatr. Open 2018, 2, e000198. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.; Sweis, D.; Planner, C.; Yeung, V.; Collier, J.; Haines, L.; Wong, I.C.K. Interventions to reduce dosing errors in children: A systematic review of the literature. Drug Saf. 2007, 30, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Öblom, H. 3D-Printed Drugs for Children—Are We Ready Yet? AAPS PharmSciTech 2017, 18, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. Simple equation for decproption of solute release I. Fickian and non-fickian release from non-sweliable devices in the for of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrocortisone (accessed on 1 March 2024).
- Svensson, A.; Neves, C.; Cabane, B. Hydration of an amphiphilic excipient, Gelucire® 44/14. Int. J. Pharm. 2004, 281, 107–118. [Google Scholar] [CrossRef]
- Agarwal, S.; Murthy, R.S.R.; Harikumar, S.L.; Garg, R. Quality by Design Approach for Development and Characterisation of Solid Lipid Nanoparticles of Quetiapine Fumarate. Curr. Comput. Aided Drug Des. 2019, 16, 73–91. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Islam, M.T.; Halsey, S.; Amin, D.; Douroumis, D. Novel Controlled Release Polymer-Lipid Formulations Processed by Hot Melt Extrusion. AAPS PharmSciTech 2016, 17, 191–199. [Google Scholar] [CrossRef]
- Ayyoubi, S.; van Kampen, E.E.M.; Kocabas, L.I.; Parulski, C.; Lechanteur, A.; Evrard, B.; De Jager, K.; Muller, E.; Wilms, E.W.; Meulenhoff, P.W.C.; et al. 3D printed, personalized sustained release cortisol for patients with adrenal insufficiency. Int. J. Pharm. 2023, 630, 122466. [Google Scholar] [CrossRef]
- Parulski, C.; Bya, L.A.; Goebel, J.; Servais, A.C.; Lechanteur, A.; Evrard, B. Development of 3D printed mini-waffle shapes containing hydrocortisone for children’s personalized medicine. Int. J. Pharm. 2023, 642, 123131. [Google Scholar] [CrossRef]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the Children’s Acceptability of Oral Formulations (CALF) Study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef] [PubMed]

| Excipients Ratio | Nozzle Temp. (°C) | Plate Temp. (°C) | Infill Density (%) | Layer Height (mm) | Pressure (kPa) | Speed (mm/s) |
|---|---|---|---|---|---|---|
| (70/30) Gelucire® /Precirol® | 60.0 | 9.0 | 60.0 | 1.5 | 44.0 | 4.0 |
| (60/40) Gelucire® Precirol® | 65.0 | 9.0 | 60.0 | 1.5 | 59.0 | 4.0 |
| (50/50) Gelucire®/Precirol® | 70.0 | 9.0 | 60.0 | 1.5 | 74.0 | 4.0 |
| Excipients Ratio | Width (mm) | Height (mm) | Weight (mg) |
|---|---|---|---|
| (70/30) 70% Gelucire® 44/14 and 30% Precirol® ATO 5 | 6.23 ± 1.03 | 2.5 ± 0.255 | 25 ± 3.08 |
| (60/40) 60% Gelucire® 44/14 and 40% Precirol® ATO 5 | 6.35 ± 1.21 | 2.5 ± 0.230 | 25 ± 3.25 |
| (50/50) 50% Gelucire® 44/14 and 50% Precirol® ATO 5 | 6.14 ± 1.11 | 2.5 ± 0.250 | 25 ± 3.13 |
| Formulation | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Y0 | Κ0 | R2 | Y1 | R2 | ΚH | R2 | ΚHP | n | |
| HCT-SF | * | * | * | * | * | * | * | * | * | * |
| Formulation 70/30 | 0.89 | 24.06 | 0.17 | 0.80 | 35.44 | 0.94 | 4.58 | 0.92 | 2.37 | 0.63 |
| Formulation 60/40 | 0.95 | 17.37 | 0.16 | 0.87 | 28.16 | 0.96 | 3.95 | 0.95 | 1.86 | 0.64 |
| Formulation 50/50 | 0.85 | 15.30 | 0.12 | 0.74 | 23.49 | 0.91 | 3.02 | 0.90 | 2.23 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protopapa, C.; Siamidi, A.; Kolipaka, S.S.; Junqueira, L.A.; Douroumis, D.; Vlachou, M. In Vitro Profile of Hydrocortisone Release from Three-Dimensionally Printed Paediatric Mini-Tablets. Pharmaceutics 2024, 16, 385. https://doi.org/10.3390/pharmaceutics16030385
Protopapa C, Siamidi A, Kolipaka SS, Junqueira LA, Douroumis D, Vlachou M. In Vitro Profile of Hydrocortisone Release from Three-Dimensionally Printed Paediatric Mini-Tablets. Pharmaceutics. 2024; 16(3):385. https://doi.org/10.3390/pharmaceutics16030385
Chicago/Turabian StyleProtopapa, Chrystalla, Angeliki Siamidi, Siva Satyanarayana Kolipaka, Laura Andrade Junqueira, Dennis Douroumis, and Marilena Vlachou. 2024. "In Vitro Profile of Hydrocortisone Release from Three-Dimensionally Printed Paediatric Mini-Tablets" Pharmaceutics 16, no. 3: 385. https://doi.org/10.3390/pharmaceutics16030385








